Abstract
Serum paraoxonase 1 (EC 3.1.8.1, PON1), a calcium-associated enzyme, has an ability to hydrolyze organophosphate compounds. Related to this property, PON1 has a critical role in antioxidant mechanisms. It is well-known that the enzyme protects LDL from oxidation. In this study we investigated the in vitro inhibitory effects of some drugs. These drugs are oxytocin, dexamethasone, atropine sulphate, gentamicin sulphate, sulfadoxine-trimethoprim, furosemid, metamizole sodium and toldimfos sodium. The IC50 values obtained varied markedly from 0.014 to 507.72 mg/mL. According to our findings, most potent and significant inhibition was displayed by dexamethasone, atropine sulphate and furosemid.
Introduction
The serum A-esterase PON1, which is primarily synthesized in liver and secreted into blood, catalyses the hydrolysis of organophosphates (OP) that have been used in production of insecticides and neurotoxic gases, and thus the enzyme has great importance for xenobiotic metabolism in vivo and toxicological studiesCitation1.
PON1 plays an important physiological role in lipid metabolism by hydrolysis of oxidized lipids, in the form of lipid hydroperoxides, generated on lipoproteins such as High-density lipoprotein (HDL) and Low-density lipoprotein (LDL) and within this ability it is considered as an antioxidative/anti-inflammatory component of HDL. Thus it provides protection against the development of oxidative stress, though the exact mechanism by which PON1 reverse and prevent liver damage by exogenous antioxidants PON1 enzyme protects LDL, bad cholesterol, from oxidation and neutralizes radicals including hydrogen peroxide by its antioxidant propertyCitation2. It also hydrolyzes oxidized cholesteryl linoleate hydroperoxides in LDL and specific oxidized phospholipidsCitation3. According to its antioxidant role, PON1 concentration was also found to be inversely correlated with the development of atherosclerosis, and its reduced activity is associated with hypercholesterolemia, diabetes, and coronary vascular disease (CVD)4–6. Decreased activity of PON1 has been postulated as a risk factor for CVDCitation4–6.
Mitochondria are the important sites for production of reactive oxygen species (ROS) due to incomplete reduction of molecular oxygen to water as a consequence of electron leakage in the electron transport chainCitation7,Citation8. Increased oxygen consumption and oxidative phosphorylation result in increased production of ROSCitation9,Citation10. Ohara et al. reported that higher levels of plasma lipids are associated with increased production of ROSCitation11.
The oxygen analogs of a number of OPs (e.g. paraoxon, chlorpyrifos oxon, diazinon oxon) are hydrolyzed by PON1, which plays a central role in their detoxication and toxicityCitation12. While PON1 activity can be detected at low levels in most tissues, very high levels are present in rat liver and plasma, with plasma accounting for more than 50% of ‘whole body’ PON1 activityCitation13. Animal studies carried out to-date provide convincing evidence that PON1 status plays a major role in determining sensitivity or resistance to OP compounds processed through the P450/PON1 pathwayCitation14. Initial evidence was provided by the observation that injection of an ‘A-esterase concentrate’ from rabbit serum into rats, could protect them from the acute toxicity of paraoxon given by i.v. injectionCitation15. Further correlative evidence came from studies that showed animals with low PON1 levels were more sensitive to specific OP compounds than animals with high enzyme levels. For example, birds, which have very low to undetectable PON1 activity, are more sensitive than various mammals to the acute toxicity of paraoxon, diazinon oxon and pirimiphos oxonCitation16,Citation17. Likewise, rabbits, which have a seven-fold higher serum PON1 activity than rats, are four-fold more resistant to the acute toxicity of paraoxonCitation18.
It is known that the acute toxicity of OPs is influenced by age, with substantial evidence indicating that young animals are more sensitive than adultsCitation19,Citation20. The lower metabolic abilities of young animals appear to be a major determinant of their increased sensitivity to acute OP toxicityCitation20. For example, in cattle, the toxicity of chlorpyrifos is 30-fold higher in calves than in adult animals, while for disulfoton, which is not metabolized by PON1, there was only a two-fold differenceCitation21.
In the present study, we investigate the differential in vitro inhibitory effects upon PON1 activity of some drugs used in cattle during infectious diseases or inflammatory-like conditions.
Materials and methods
Sepharose-4B, L tyrosine, 9-aminophenanthrene, paraoxon, protein assay reagents and all chemicals for electrophoresis were obtained from Sigma Chem. Co. (Milan/Italy). All other general chemicals used were analytical grade and obtained from various sources.
Paraoxonase purification from serum
Bovine serum was isolated from fresh bovine blood collected in a dry tube. The blood samples were centrifuged at 1500 rpm for 15 min and the serum was collected. Different ammonium sulphate intervals, 0–100%, were determined for serum paraoxonase enzyme. Upon each stage, it was spinned at 15 000 rpm for 30 min and the total protein concentration and paraoxonase enzyme activity were determined in the each ammonium sulphate intervals. The precipitation intervals for paraoxonase enzyme were 60–80%. The precipitate was collected by centrifugation at 15000 rpm for 20 min, and redissolved in 100 mM Tris–HCl buffer (pH 8.0). The crude PON1 was then subjected to hydrophobic interaction chromatography. The PON1 solution was adjusted to 1M ammonium sulphate, and then loaded onto a hydrophobic gel column comprised of Sepharose-4B-L-tyrosine-9-aminophenanthrene which was synthesized as previously described by us for this enzyme purificationCitation22. The purification table was given in .
Paraoxonase enzyme assay
PON1 enzyme activity towards paraoxon as a substrate was quantified spectrophotometrically by the method described by Gan et al.Citation23 The reaction was followed for 2 min at 37°C by monitoring the appearance of p-nitrophenol at 412 nm on a Biotek (Winooski, VT, United States) automated recording spectrophotometer. The final substrate concentration during enzyme assay was 2 mM, and all rates were determined in duplicate and corrected for the non-enzymatic hydrolysis. A molar extinction coefficient (ϵ) of 17,100 M−1cm−1 for p-nitrophenol at pH 8.0 in 100 mM Tris–base buffer was used for the calculation. One unit of PON1 activity is defined as 1 μmol of p-nitrophenol formed per minute under the above assay conditions.
In vitro inhibition studies
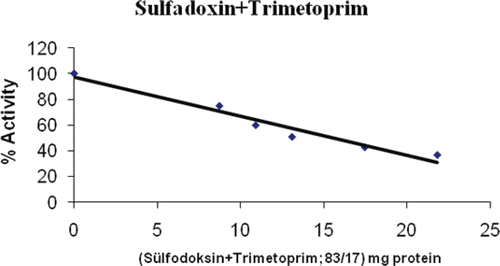
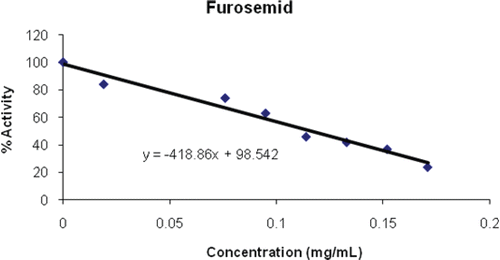
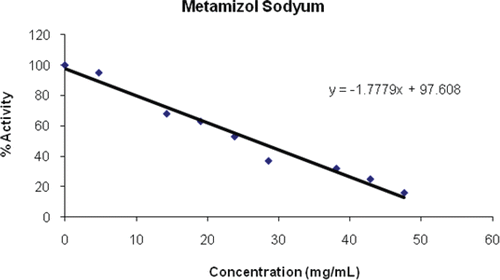
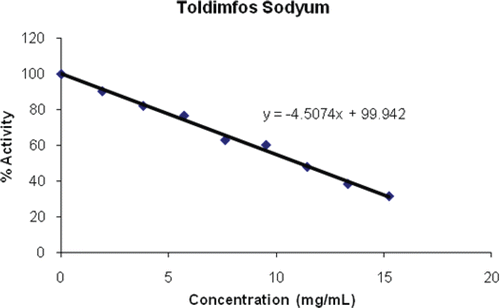
For the inhibition studies, PON1 activity was assayed by following the hydration of paraoxon in the presence of five different concentrations of various drugs. The drugs investigated were oxytocin, dexamethasone, atropine sulphate, gentamicin sulphate, sulfadoxine-trimethoprim, furosemid, metamizole sodium and toldimfos sodium. They were obtained from a local pharmacy in Balikesir /Turkey. Control PON1 activity was accepted as 100%, and the concentration of each drug causing 50% inhibition (IC50 values) of activity were calculated from inhibition curves (– and ).
Table 1. IC50 values of drugs on PON1.
Results and discussion
Oxidative stress affects PON1 activity, and there is an inverse relationship between lipid peroxidation and PON1Citation24. Dairy cows are highly susceptible to oxidative stress which commonly occurs in late pregnancy and early lactation. During the transition period, increased production of reactive oxygen species is associated to processes of metabolic adaptation to a low-energy balance. An increased capability of milk production is associated with the changes of metabolic and energy homeostasis related to this, the blood concentration of PON1 can be relatively stable throughout the lifespan of an individualCitation25,Citation26 though it can be influenced by disease state, diet, and other environmental factors. For example, PON1 levels fall after serious insults such as atherosclerosis, myocardial infarctionCitation27,Citation28.
Several factors affect the fate of drugs in animalsCitation29. Oxytocin, dexamethasone, atropine sulphate, gentamicin sulphate, sulfadoxin-trimethoprim, furosemid, metamizole sodium and toldimfos sodium drugs are all used in the treatment of cattleCitation30. In our study the differential inhibitory effects of these drugs upon PON1 were determined in vitro for a better understanding of peroxidative damage and its relation to serum PON 1.
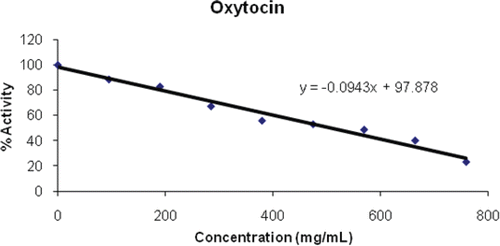
Oxytocin is a posterior pituitary hormone that acts directly on smooth muscle to produce rhythmic contractions and with this ability it is closely involved in lactationCitation30. Because of this property, oxytocin is often injected into cattle that are under stress or unable to produce much milk to enhance milk productionCitation31. However, some research indicates that administering oxytocin to stimulate uterine contractions in pregnant cows can lead to increased early embryonic deathCitation32. The present results indicate that oxytocin does not affect the activity of serum PON1 as it showed a very high IC50 of 507.72 mg/mL ( and ).
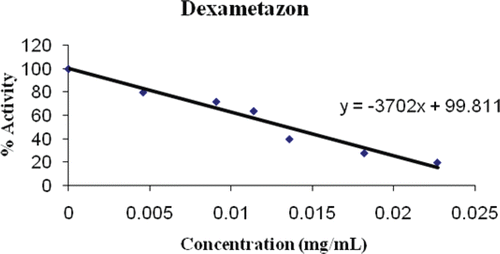
Dexamethasone is a synthetic analogue of prednisolone, having similar but more potent anti-inflammatory therapeutic action and diversified hormonal and metabolic effects. Experimental animal studies have revealed it possesses greater anti-inflammatory activity than many steroids. It is used topically on the eye after cataract operations, on the skin for eczema and psoriasis, as well as intra-articularly for arthritis and osteoarthritisCitation33. In this study, a strong inhibition of PON1 was observed (an IC50 of 0,014 mg/mL; and ) with dexamethasone. PON1 plays an important physiological role in lipid metabolism, as it can hydrolyze oxidized lipids in the form of lipid hydroperoxides generated on lipoproteins such as HDL and LDL, thus providing protection against the development of oxidative stress. Usage of this drug could therefore result in lowered PON1 activity in cattle, which could potentially enhance production of reactive oxygen species (ROS), due to incomplete reduction of molecular oxygen to water as a consequence of electron leakage in electron transport chain.
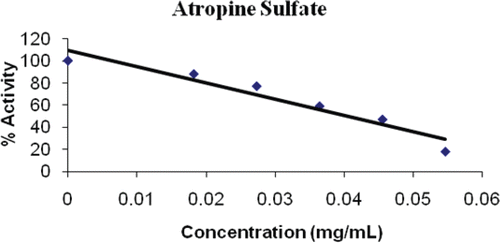
A much more potent inhibition of PON1 was obtained with atropine sulphate with an IC50 of 0.041 mg/mL (, ). Atropine is a plant alkaloid that has been used extensively in most animal species as an anticholinergic agent, and atropine sulphate is used as an antidote in the treatment of organophosphate insecticide poisoning of cattle, horses and sheep, and in the treatment of nerve agent casualties requiring artificial respirationCitation34. As both atropine sulphate and PON1 are recognized to have a role in the metabolism of xenobiotics, through hydrolysis of the toxic oxon metabolites of organophosphorus compounds providing limited protection against chronic exposure to OPCitation35, this drug inhibition of PON1 is highly significant.
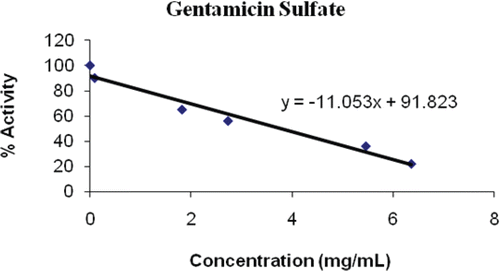
Gentamicin sulphate is an aminoglycoside antibiotic with a wide antibacterial spectrum that is commonly used in veterinary practiceCitation36. Although there is considerable information on the pharmacokinetics of this antibiotic in domestic animalsCitation37, the Food and Drug Administration (FDA) recently issued warning letters to veterinarians for their illegal use of gentamicin sulfateCitation38. The FDA has not approved gentamicin for use in cattle because of the propensity for these drugs to be retained in kidney tissue for long periods. But aminoglycoside nephrotoxicity is a significant problem that limits the clinical use of this important class of antibiotics. Insight into the mechanisms of aminoglycoside entry into tubular cells and subsequent cytotoxic events could provide important clues toward reducing the toxicity of these agentsCitation39. Thus it could be also related with paraoxonase activity because of this enzyme relation with xenebiotic metabolism as well. In our study we found an IC50 value of 3.784 mg/mL for PON1 inhibition by showed no better inhibition with comparing other drugs at the above (, ).
Sulfadoxine-trimethoprim is a potent antibacterial drug, and the reservoir of trimethoprim in the extravascular drug compartment, which is available for potentiation of the more slowly eliminated sulfadoxine, probably explains the high and prolonged antibacterial activity of the combination in ruminantsCitation40. In the literature, penicillin, oxytetracycline, and a trimethoprimsulfadoxine combination were compared as first choice antibiotics for the treatment of acute bovine respiratory disease in weaned beef calvesCitation41. The selection of an appropriate antibiotic and the evaluation of its success in the treatment of respiratory disease is an important consideration. Initial field trials with a potentiated sulfonamide suggested a high level of efficacy in the treatment of bacterial diseases of cattle and pigsCitation42. In our study there was no significant inhibitory effect of this drug on PON1 activity (IC50 of 15.636 mg/mL; , ).
Furosemid is a loop diuretic used in the treatment of congestive heart failure and edema. In cases of edema involving cardiac insufficiency, the continued use of heart stimulants such as digitalis or its glycosides is indicatedCitation43. It has also been used to prevent thoroughbred and standard bred race horses from bleeding through the nose during races. As with many diuretics, it can cause dehydration and electrolyte imbalance, including loss of potassium, calcium, sodium, and magnesiumCitation44. Furosemid had an effective inhibition of PON1 with an IC50 of 0.235 mg/mL (, ).
Metamizole sodium as a non-steroidal, anti-inflammatory drug commonly used in many countries as a powerful painkiller, for pain originating from spasms of smooth muscles and fever reducerCitation45. Metamizole preparations are indicated for use in horses, cattle and swine as an adjunct to therapy in many inflammatory conditions of musculoskeletal and locomotor systems. It is better known under the names Dipyrone, Analgin, Novalgin, and Melubrin. Here in this study, this drug gave a poor inhibition of PON1 with an IC50 of 26.777 mg/mL ( and ).
Toldimfos sodium is the sodium salt of 4-dimethylamino-2-methyl-phenyl-phosphinous acid, a derivative of phosphoric acid. It is indicated for the treatment and prophylaxis of diseases which arise in connection with parturition and the peri-partum period, developmental and nutritional disorders in young animals, disorders of bone growth and tetasy or paresis caused by disorders of calcium, magnesium and phosphorous metabolismCitation46. Here we found only a poor inhibition of serum PON1 (an IC50 value of 11.08 mg/mL), compared to the effects of dexamethasone, atropine sulphate and furosemid ( and ).
In the literature there are also some studies about PON1 purification and its interaction with sulfonamides, Supuran and his collegues had 302-fold purification with a final specific activity of 4775 U/mg and a yield of 32% with using six sulfonamides dose-dependently decreased the activity of hPON1 with inhibition constants in the millimolar-micromolar range by using several purification stepsCitation47. But in this present study we had 1690 fold purification and a 14922.8 U/mg specific activity by using a hydrophobic gel column comprised of Sepharose-4B-L-tyrosine-9-aminophenanthrene in a single step. Also we determined the inhibition effects of some cattle drugs on PON1 level. Another study had showed evaluation in vitro and in vivo effects of the intravenous anesthetics, etomidate, propofol, and ketamine, on the activity of human serum paraoxonase (hPON1Citation48). and hPON1 was purified in three steps with a high specific activity, and anesthetics inhibited PON1 activity. Comparing their results of our study, the purification fold of PON 1 is higher than their method. Beydemir et al also had another different study with PON 1 purification using very simple methods and investigation of the interactions between the enzyme and some commonly used antibioticsCitation49. The drugs were determined to decrease the enzyme activity at quite different concentrations. We also used gentamicine sulfate for having a comparison on PON1 level that is why recently this kind of studies have more attractions for a better understanding of drug metabolism. According to our findings, most potent and significant inhibition was displayed by dexamethasone, atropine sulphate and furosemide. But in their study some of the antibiotics exhibited different inhibitory effects on hPON1.
Conclusions
PON1 has been reported to be involved in drug metabolism and is used for drug inactivationCitation50. In this study, PON1 is purified by an hydrophobic gel column comprised of Sepharose-4B-L-tyrosine-9-aminophenanthren (). The efficacy of drug inhibition of PON1 activity was very variable across the panel of drugs tested with IC50 values varying between 0.014–507.72 mg/mL. When listed in decreasing order of inhibitory potential the drugs are: dexamethasone (0.014 mg/mL), atropine sulphate (0.041 mg/mL), furosemid (0.235 mg/mL), gentamicin sulphate (3.784 mg/mL), toldimfos sodium (11.08 mg/mL), sulfadoxine-trimetoprim (15.636 mg/mL), metamizol sodium (26.777 mg/mL) and oxytocin (507.72 mg/mL) (). Within these results, it is clear that significant inhibition was observed with dexamethasone, atropine sulphate and furosemid.
Table 2. Summary of the purification of bovine serum paraoxonase.
Turk et alCitation51 demonstrated that serum PON1 activity is reduced in early postpartum dairy cows, and oxidative stress is considered to contribute to various disorders in this period thus long periods with lower concentrations of PON1 activity, which defends the animal against oxidative stress, may increase the risk of oxidative damage, particularly at the lipoprotein level. Beydemir et alCitation52 reported that dexamethasone inhibited in vitro human PON1 activity. They found that IC50 value was 1.106 mM for dexamethasone. When this study was compared to our results, both the results were given similar inhibition values. The results showed that dexamethasone is a potent inhibitor. So, the drug must be used carefully and the dosage closely monitored to decrease side effects. In addition, Sinan et alCitation53 performed some experiments about the in vitro effects of sodium ampicillin, ciprofloxacin, and clindamycin phosphate on PON1 purified from human serum. The study found that sodium ampicillin, ciprofloxacin, and clindamycin phosphate were potent inhibitors for human serum PON1, and IC50 values were 27.51 mM, 0.313 mM and 0.902 mM, respectively. Having a comparison to our values, it is seen these drugs had more inhibition effects than our results.
The objective of this study was to determine the relationship between serum PON1 activity level and cattle drugs for a better understanding of peroxidative damage in relation to serum PON1 activity and how various drug treatments might compromise these processes which may cause some side effects on health problems, inflammatory conditions, plasma metabolic measures, and milk yield on bovines. The ability of some cattle drugs to significantly inhibit PON1 activity in vitro indicate that further studies, especially in vivo, are needed to better understand the potential inter-relationships between drug treatments, PON1 activity, liver function and oxidative stress, and subsequent effects on animal health.
Acknowledgement
This work was carried out in the Balikesir University Research Center of Applied Sciences (BURCAS) Balikesir/Turkey.
Declaration of interest
The authors report no conflict of interest.
References
- Hassett C, Richter RJ, Humbert R, Chapline C, Crabb JW, Omiecinski CJ, Furlong C. Characterisation of cDNA clones encoding rabbit and human serum paraoxonase: The mature protein retains its signal sequence. Biochemistry 1991;30:10141–10149.
- Aldridge WN. Serum esterases 2. An enzyme hydrolysing diethyl p-nitrophenyl phosphate (E 600) and its identity with the A-esterase of mammalian sera. Biochem J 1953;53:117–124.
- Draganov D, La Du BN. Pharmacogenetics of paraoxonases: A brief review. Naunyn Schmiedebergs Arch Pharmacol 2004;369:78–88.
- Aviram M, Rosenblat M, Bisgaier C, et al. Paraoxonase inhibits highdensity lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest 1998;101:1581–90.
- Laplaud PM, Dantoine T, Chapman MJ. Paraoxonase as a risk marker for cardiovascular disease: Facts and hypotheses. Clin Chem Lab Med 1998;36:431–441.
- Abbott CA, Mackness MI, Kumar S, Boulton AJ, Durrington PN. Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler Thromb Vasc Biol 1995;15:1812–1818.
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem j 1973;134:707–716.
- Nohl H, Gille L, Staniek K. The mystery of reactive oxygen species derived from cell respiration. Acta Biochim Pol 2004;51:223–229.
- Videla LA. Energy metabolism, thyroid calorigenesis, and oxidative stress: Functional and cytotoxic consequences. Redox Report 2000;5:265–275.
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev 2001;81:1097–1142.
- Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Investig 1993;91(6):2546–2551.
- Walker CH, Mackness MI. “A” esterases and their role in regulating the toxicity of organophosphates. Arch Toxicol 1987;60:30–33.
- Pellin MC, Moretto A, Lotti M, Vilanova E. Distribution and some biochemical properties of rat paraoxonase activity. Neurotoxicol Teratol 1990;12:611–614.
- Costa LG, Li WF, Richter RJ, Shih DM, Lusis A, Furlong CE. The role of paraoxonase (PON1) in the detoxification of organophosphates and its human polymorphism. Chem Biol Interact 1999;119–120: 429–438..
- Main AR. The role of A-esterase in the acute toxicity of paraoxon, TEPP, and parathion. Can j Biochem Physiol 1956;34:197–216.
- Machin AF, Anderson PH, Quick MP, Waddell DF, Skibniewska KA and Howells LC. The metabolism of diazinon in the liver and blood of species of varying susceptibility to diazinon poisoning. Xenobiotica 1976;6:104.
- Brealey CJ, Walker CH and Baldwin BC. A-esterase activities in relation to the differential toxicity of pirimiphos-methyl to birds and mammals. Pest Sci 1980;11:546–554.
- Costa LG, Richter RJ, Murphy SD, Omenn GS, Motulsky AG and Furlong CE.(1987). Species differences in serum paraoxonase correlate with sensitivity to paraoxon toxicity. In: Costa LG, Galli CL and Murphy SD. Editors, Toxicology of Pesticides: Experimental Clinical and Regulatory Perspectives Heidelberg: Springer, 93–107.
- Brodeur J, Dubois KP. Comparison of acute toxicity of anticholinesterase insecticides to weanling and adult male rats. Proc Soc Exp Biol Med 1963;114:509–511.
- Benke GM, Murphy SD. The influence of age on the toxicity and metabolism of methyl parathion and parathion in male and female rats. Toxicol Appl Pharmacol 1975;31:254–269.
- Virgo BB.(1984). Pesticides and the neonate. In: Kacew S and Reasor MJ. Editors, Toxicology and the Newborn. Amsterdam:Elsevier, 252–267.
- Gençer N, Arslan O. Purification human PON1Q192 and PON1R192 isoenzymes by hydrophobic interaction chromatography and investigation of the inhibition by metals. j Chromatogr b Analyt Technol Biomed Life Sci 2009;877:134–140.
- Gan KN, Smolen A, Eckerson HW, La Du BN. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos 1991;19:100–106.
- Aviram M, Rosenblat M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med 2004;37:1304–1316.
- Butler WR. Nutritional interactions with reproductive performance in dairy cattle. Anim Reprod Sci 2000;60–61:446–457.
- Lucy MC. Mechanisms linking nutrition and reproduction in postpartum cows. Reprod Suppl 2003;61:415–427.
- Mackness B, Durrington PN, Mackness MI. Human serum paraoxonase. Gen Pharmacol 1998;31:329–336.
- Costa LG, Richter RJ, Li WF, Cole T, Guizzetti M, Furlong CE. Paraoxonase (PON 1) as a biomarker of susceptibility for organophosphate toxicity. Biomarkers 2003;8:1–12.
- Ferré NJ, Camps E, Prats E, Vilella A, Figuera PL and Joven J. Serum paraoxonase activity: A new additional test for the improved evaluation of chronic liver damage. Clin Chem 2002;48:261–268.
- Gibson GG & Skett P. Introduction to Drug Metabolism. 2nd Edn. Chapman & Hall, London, 1994.
- Mlynarczuk J, Wrobel MH, Kotwica J. Effect of environmental pollutants on oxytocin synthesis and secretion from corpus luteum and on contractions of uterus from pregnant cows. Toxicol Appl Pharmacol 2010;247:243–249.
- Weiss D, Dzidic A, Bruckmaier RM. Effect of stimulation intensity on oxytocin release before, during and after machine milking. j Dairy Res 2003;70:349–354.
- Maciel SM, Chamberlain CS, Wettemann RP, Spicer LJ. Dexamethasone influences endocrine and ovarian function in dairy cattle. j Dairy Sci 2001;84:1998–2009.
- Short CE. (1987). Anticholinergics. In: Short CE, ed. Veterinary Anesthesia Baltimore: Williams & Wilkins, 8–15.
- Broomfield CA, Maxwell DM, Solana RP, Castro CA, Finger AV and Lenz DE. Protection of butyrylcholinesterase against organophosphorus poisoning in non-human primates. J Pharmacol and Exp Therapeut Vol 1991;259:633–638.
- Elsheikh HA, Osman IA, Ali BH. Comparative pharmacokinetics of ampicillin trihydrate, gentamicin sulphate and oxytetracycline hydrochloride in Nubian goats and desert sheep. j Vet Pharmacol Ther 1997;20:262–266.
- Haddad NS, Ravis WR, Pedersoli WM, Carson RL Jr. Pharmacokinetics of single doses of gentamicin given by intravenous and intramuscular routes to lactating cows. Am j Vet Res 1986;47:808–813.
- Available at: http://www.avma.org/onlnews/javma/aug04/040801b.asp. Accessed on 1 August 2004.
- Girton RA, Sundin DP, Rosenberg ME. Clusterin protects renal tubular epithelial cells from gentamicin-mediated cytotoxicity. Am j Physiol Renal Physiol 2002;282:F703–F709.
- White G, Piercy DW, Gibbs HA. Use of a calf salmonellosis model to evaluate the therapeutic properties of trimethoprim and sulphadiazine and their mutual potentiation in vivo. Res Vet Sci 1981;31:27–31.
- Gerald D, Mechor G, Kee J, Eugene D. Janzen Comparison of penicillin, oxytetracycline, and trimethoprim-sulfadoxine in the treatment of acute undifferentiated bovine respiratory disease. Can Vet J 1988;29:438–443.
- Rehm WF, White G. A field trial with trimethoprim and sulfadoxine in bacterial diseases of cattle and pigs. Vet Rec 1970;87:39–42.
- Timmerman RJ, Springman FR, Thoms RK. Evaluation of furosemide, a new diuretic agent. Curr Therapeut Res 1964;6:88–94.
- Rais-Bahrami K, Majd M, Veszelovszky E, Short B. Use of furosemide and hearing loss in neonatal intensive care survivors. Am J Perinatol 2004;21:329–32.
- Anthony Wong in WHO. Pharmaceuticals Newsletter 2002; p15.
- Available at: www.emea.europa.eu/pdfs/vet/mrls/071799en.pdf. Accessed December 1999
- Ekinci D, Sentürk M, Beydemir S, Küfrevioğlu O.I, Supuran CT An alternative purification method for human serum paraoxonase 1 and its interactions with sulfonamides. Chem Biol Drug Des 2010;76:552–55.
- Alici HA, Ekinci D, Beydemir S Intravenous anesthetics inhibit human paraoxonase-1 (PON1) activity in vitro and in vivo. Clin Biochem 2008;41:1384–1390.
- Ekinci D, Beydemir S. Evaluation of the impacts of antibiotic drugs on PON 1; A major bioscavenger against cardiovascular diseases. European J Pharmacol 2009;617:84–89.
- Biggadike K, Angell RM, Burgess CM, Farrell RM, Hancock AP, Harker AJ, Irving WR, Ioannou C, Procopiou PA, Shaw RE, Solanke YE, Singh OM, Snowden MA, Stubbs RJ, Walton S, Weston HE. Selective plasma hydrolysis of glucocorticoid gammalactones and cyclic carbonates by the enzyme paraoxonase: An ideal plasma inactivation mechanism. J Med Chem 2000; 43:19–21.
- Turk R, Juretic D, Geres D, Turk N, Rekic B, Simeon-Rudolf V and Svetina A. Serum paraoxonase activity and lipid parameters in the early postpartum period of dairy cows. Res Vet Sci 2004;76:57–61.
- Beydemir Ş, Ekinci D and Ates O. In Vitro effects of dexamethasone on human serum paraoxonase-i (pon1) activity. hacettepe. J Biol & Chem 2009;37:197–205.
- Sinan S, Kockar F, Gençer N, Yıldırım H and Arslan O. Effects of some antibiotics on paraoxonase from human serum in vitro and from mouse serum and liver in vivo. Biol Pharm Bull 2006; 29:1559–1563.