Abstract
A series of new quinolone-3-carboxylic acids as HIV-1 integrase inhibitors featuring a fluorine atom at C-5 position were synthesized and evaluated for their antiviral activity in C8166 cell culture. These newly synthesized compounds showed anti-HIV activity against wild-type virus with an EC50 value ranging from 29.85 to 0.032 μΜ. The most active compound 4e exhibited activity against wild-type virus and the mutant virus A17 with an EC50 value of 0.032 and 0.082 μΜ, respectively. Preliminary structure–activity relationship of these 5-fluoroquinolone-3-carboxylic acids was also investigated.
Introduction
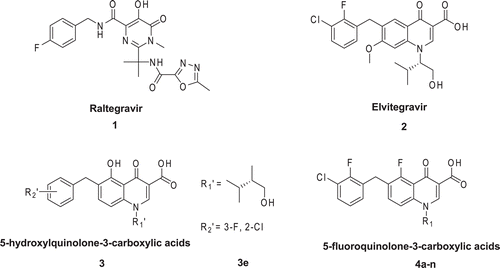
Insertion of proviral DNA into the host chromosome is a crucial step in the HIV replication cycle. This process is catalyzed by virally encoded and packaged enzyme, namely HIV integrase (IN)Citation1,Citation2. The recent approval of Raltegravir ()Citation3–5 by FDA and the encouraging late-stage clinical trials with Elvitegravir ()Citation6,Citation7 have validated IN as a novel HIV therapeutic target.
Elvitegravir and its derivatives, firstly reported by Sato et al. in 2006, have emerged as promising therapeutic IN inhibitors, due to their high activity and favorable pharmacokinetic propertyCitation8. Subsequent chemical modifications of their quinolone core focused on N-1, C-6, 7 and 8 position have been introduced in order to obtain more potent and selective IN inhibitorsCitation9–11. However, very few modifications have been carried out at the C-5 position.
Considering the above facts, we have focused our attention on the introduction of the substituent at the C-5 position. Very recently, we identified a series of 5-hydroxylquinolone-3-carboxylic acids () with low micromolar to submicromolar EC50 values against HIV-1 virusCitation12,Citation13. Among these 5-hydroxylquinolone analogues, the most active compound 3e characterized by a hydroxymethyl moiety at the 1S-position of the isobutyl group exhibited 0.13 μM EC50 valueCitation12. As part of our ongoing effort to find potent anti-HIV 5-substitued-quinolone analogues, as well as the incorporation of fluorine into the target molecules could affect a variety of properties (e.g., enhanced binding interactions, metabolic stability, changes in physical properties, and selective reactivities) due to its special nature such as the high electronegativity, small size, and its van der Waals radius closer to that of oxygenCitation14–16, a series of new 5-fluoroquinolone-3-carboxylic acids () were synthesized and evaluated as potential IN inhibitors.
Chemistry
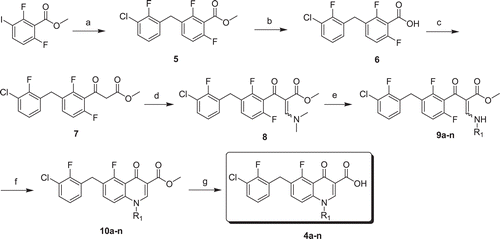
Two series of 5-fluoroquinolone-3-carboxylic acids (N-alkyl-substituted compounds 4a–e and N-aryl-substituted compounds 4f–n) were synthesized similar to our previously reported protocolCitation12,Citation13 as depicted in . Compound 8 were prepared starting from 2, 6-difluoro-3-iodobenzoate according to reported proceduresCitation12. Condensation of 8 with N, N-dimethylformamide dimethyl acetal and followed by substitution with appropriate amines led to acrylates 9a–n, which were then cyclized using 1,8-Diazabicycloundec-7-ene as a base to furnish quinolone esters 10a–n. Ester hydrolysis of 10a–n with satd aq LiOH afforded the target compounds.
Results and discussion
Biological evaluation
All the target compounds were evaluated by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assayCitation17,Citation18 for cytotoxicity and antiviral activity in C8166 cells infected with the wild-type HIV-1 (LAI strain IIIB) and HIV-1 mutant virus (A17). Elvitegravir and 3eCitation12 were included as the reference compounds. The results, expressed as EC50, CC50 and SI, are illustrated in .
Table 1. Anti-HIV-1 activity and cytotoxicity of compounds 4a–n in C8166 cellsa.
The newly synthesized quinolone-3-carboxylic acids 4a–n exhibited EC50 values against HIV-1 IIIB in the range of 0.032–29.85 μM. Compound 4a, bearing a propyl group at N-1 position of quinolone core, showed antiviral activity with 13.44 μM EC50 value. The replacement of straight chain alkyl group of 4a with branched (4b) or cyclic one (4c) led to an improvement of antiviral activity against wild-type HIV-1 IIIB. Compound 4d with an i-butyl group at N-1 position showed more potent than i-propyl counterparts (4b). The introduction of a hydroxymethyl group at the 1S-position of butyl moiety significantly further enhanced its potency. Compound 4e was the most active of this new series and was more potent than the reference compound 3e. These results are consistent with the SAR for the 5-hydroxylquinolone analogues that the replacement of straight chain alkyl group with branched ones caused an increase in antiviral activity and the introduction of a hydroxymethyl group at the 1S-position of butyl moiety resulted in significant improvement of activityCitation12.
For the N-aryl-substituted compounds 4f–n,p-chloro compound 4h showed higher activity against both wild-type HIV-1 IIIB and HIV-1 mutant virus A17 than o-chloro (4f) and m-chloro (4g) compounds. Since the effect of the introduction of a withdrawing group at the para position was marked, a fluoro (4i), a bromo (4j), or a trifluoromethyl (4k) was introduced. Among these compounds, only 4k exhibited slightly increased activity against HIV-1 IIIB compared to 4h. The introduction of a methyl group at the para position resulted in 4m, which showed less potent than 4h; however, the introduction of dual methyl group at ortho position (4n) led to comparable activity to 4h.
Molecular modeling calculations
In an attempt to investigate the binding model of our newly synthesized compounds with IN, molecular docking study was performed.
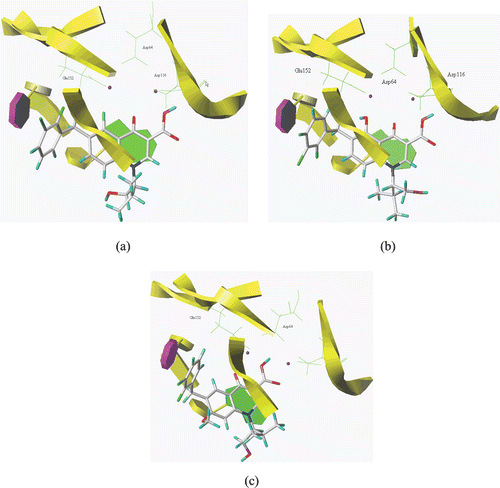
Compound 4e, which displayed the most activity against both wild-type HIV-1 and the mutant virus A17, was docked into our previously constructed model of the HIV-1 IN catalytic core domain (CCD)/viral DNA complexCitation12 using SURFLEX-DOCK SYBYLX 1.1. For comparison, the binding modes of Elvitegravir and 3e were also investigated. All the molecules were energy minimized by the conjugated gradient method with Gasteiger-Hückel charge until a convergence value of 0.01 kcal/(Å mol), using the Tripos force field. After the hydrogen atoms were added to the HIV-1 IN CCD/DNA complex, atomic charges were recalculated by Kollman all-atom for the protein and Gasteiger-Hückel for ligand. The protomol generated using a threshold of 0.50 and bloat of zero (default values). Other parameters were set as defaults for Surflex-Dock. The docking results showed that 4-ketone and 3-carboxylate in compound 4e could form Mg2+ chelation with HIV-1 IN (). The quinolone ring exhibit π–π stacking interaction with A17, like 3eand Elvitegravir do ( and ). No interaction could be detected between the substituent on N-1 or C-5 fluoro atom and IN. Although the anti-HIV activity of 3e () might involve a two-metal chelating mechanismCitation12, 4e showed more potent against HIV-1 IIIB than 3e, this might due to the incorporation of fluorine into 4e favorite its physicochemical properties.
Conclusions
In conclusion, we designed and synthesized a series of new 5-fluoroquinolone-3-carboxylic acids. All the compounds showed moderate to good activity against wild-type virus with an EC50 value ranging from 29.85 to 0.032 μM. Compound 4e was identified as the most active compound of this new series (EC50 = 0.032 μM, SI = 1295.90) associated with high activity against HIV-1 mutant strain A17 (EC50 = 0.082 μM). Preliminary structure–activity relationship of the newly synthesized quinolone analogues was also investigated.
Experimental
General procedures
Melting points were measured on a WRS-1 digital melting point apparatus and are uncorrected. Citation1H NMR and Citation13C NMR spectra on a Brucker AV 400 MHz spectrometer were recorded in CDCl3. Chemical shifts are reported in δ(ppm) units relative to the internal standard tetramethylsilane. Mass spectra were obtained on an Agilent MS/5975 mass spectrometer. All chemicals and solvents used were of reagent grade and were purified and dried by standard methods before use. All air-sensitive reactions were run under a nitrogen atmosphere. All the reactions were monitored by thin layer chromatography on pre-coated silica gel G plates at 254 nm under a UV lamp using ethyl acetate/hexane as eluents. Flash chromatography separations were obtained on silica gel (300–400 mesh).
General procedure for the preparation of 9a–n
A mixture of 2-(3-(3-Chloro-2-fluorobenzyl)-2,6-difluoro benzoyl)-3-(dimethylamino) acrylate 8 (618 mg, 1.5 mmol) and appropriate amines (1.8 mmol) in tetrahydrofuran (15 mL) was stirred at 50°C for 5–10 min and then concentrated under reduced pressure. The resulting residue 9a–n was used directly for the next step without further purification.
General procedure for the preparation of 10a–n
A mixture of 9a–n (1.0 mmol) and K2CO3 (2.5 mmol) in DMF (15 mL) was stirred at 60°C overnight, filtered and poured into ice-water. The mixture was extracted by dichloromethane (5 mL × 3). The combined organic solution was washed with brine (5 mL × 2), dried over MgSO4 and concentrated under reduced pressure. The residue was purified by column chromatography (silica gel, petroleum ether/ethyl acetate 5/1 to 3/1, v/v) to give the desired compound 10a–n.
General procedure for the preparation of 4a–n
To a solution of satd aq LiOH (5 mL) in dioxane (8 mL) was added 10a–n (1.2 mmol). After being stirred at 50°C for 3 h, the mixture was cooled, poured into ice-water and acidified with 4 M HCl to pH ~2. The resulting precipitate was collected by filtration, washed by water and ethanol, dried to give the desired compound 4a–n.
6-(3-chloro-2-fluorobenzyl)-5-fluoro-4-oxo-1-propyl-1,4-dihydroquinoline-3-carboxylic acid (4a)
Yield 75%. Mp 192–193°C. 1H NMR (CDCl3) δ1.01–1.05 (t, 3 H, J= 7.2 Hz, CH3), 1.92–1.97 (m, 2 H, CH2), 4.14 (s, 2 H, CH2), 4.22–4.26 (t, 2 H, J= 7.2 Hz, CH2), 7.01–7.04 (t, 1 H, J = 8.0 Hz, ArH), 7.17–7.21 (m, 1 H, ArH), 7.26–7.30 (m, 1 H, ArH), 7.32–7.35 (d, J = 8.8 Hz, 1 H, ArH), 7.65–7.66 (m, 1 H, ArH), 8.65 (s, 1 H, CH), 14.84 (s, 1 H, COOH). MS (ESI) m/z 392 [M+H]+. Anal. Calcd for C20H16ClF2NO3: C 61.31, H 4.12, N 3.58, found: C 61.59, H 4.36, N 3.32.
6-(3-chloro-2-fluorobenzyl)-5-fluoro-1-isopropyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4b)
Yield 82%. Mp 227–229°C. 1H NMR (CDCl3) δ1.63–1.65 (d, 6 H, J= 6.0 Hz, 2CH3), 4.15 (s, 2 H, CH2), 4.94–4.96 (m, 1 H, CH), 7.01–7.05 (t, 1 H, J = 8.0 Hz, ArH), 7.18–7.22 (m, 1 H, ArH), 7.26–7.30 (m, 1 H, ArH), 7.47–7.49 (d, J = 8.8 Hz, 1 H, ArH), 7.67–7.71 (m, 1 H, ArH), 8.85 (s, 1 H, CH), 14.93 (s, 1 H, COOH). MS (ESI) m/z 392 [M+H]+. Anal. Calcd for C20H16ClF2NO3: C 61.31, H 4.12, N 3.58, found: C 61.52, H 3.86, N 3.35.
6-(3-chloro-2-fluorobenzyl)-1-cyclopropyl-5-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4c)
Yield 80%. Mp 239–240°C. 1H NMR (CDCl3) δ1.18–1.19 (d, 2 H, J= 3.2 Hz, CH2), 1.39–1.40 (d, 2 H, J= 3.2 Hz, CH2), 3.54–3.57 (m, 1 H, CH), 4.15 (s, 2 H, CH2), 7.00–7.04 (t, 1 H, J = 8.0 Hz, ArH), 7.17–7.21 (m, 1 H, ArH), 7.26–7.30 (m, 1 H, ArH), 7.67–7.71 (m, 1 H, ArH), 7.83–7.85 (d, J = 8.8 Hz, 1 H, ArH), 8.80 (s, 1 H, CH), 14.72 (s, 1 H, COOH). MS (ESI) m/z 390 [M+H]+. Anal. Calcd for C20H14ClF2NO3: C 61.63, H 3.62, N 3.59, found: C 61.85, H 3.38, N 3.32.
6-(3-chloro-2-fluorobenzyl)-5-fluoro-1-isobutyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4d)
Yield 82%. Mp 152–154°C. 1H NMR (CDCl3) δ0.99–1.01 (d, 6 H, J= 6.4 Hz, 2 CH3), 2.24–2.27 (m, 1 H, CH), 4.05–4.07 (d, 2 H, J= 6.4 Hz, CH2), 4.14 (s, 2 H, CH2), 7.00–7.04 (t, 1 H, J = 8.0 Hz, ArH), 7.18–7.21 (m, 1 H, ArH), 7.26–7.28 (d, 1 H, J= 6.8 Hz,ArH), 7.29–7.32 (m, 1 H, ArH), 7.65–7.66 (m, 1 H, ArH), 8.65 (s, 1 H, CH), 14.84 (s, 1 H, COOH). MS (ESI) m/z 406 [M+H]+. Anal. Calcd for C21H18ClF2NO3: C 62.15, H 4.47, N 3.45, found: C 62.41, H 4.68, N 3.22.
(S)-6-(3-chloro-2-fluorobenzyl)-5-fluoro-1-(1-hydroxy-3-methylbutan-2-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4e)
Yield 70%. Mp 95–97°C. 1H NMR (CDCl3) δ 0.71–0.73 (d, 3 H, J = 6.4 Hz, CH3), 1.11–1.12 (d, 3 H, J = 6.0 Hz, CH3), 2.34 –2.35 (m, 1 H, CH), 3.74–3.77 (brs, 1 H, OH), 4.04–4.11 (m, 4 H, 2 CH2), 4.43–4.44 (m, 1 H, CH), 6.93–6.97 (t, 1 H, J = 8.0 Hz, ArH), 7.09–7.12 (m, 1 H, ArH), 7.19–7.22 (m, 1 H, ArH), 7.46–7.48 (d, 1 H, J = 8.8 Hz, ArH), 7.55–7.57 (m, 1 H, ArH), 8.78 (s, 1 H, CH), 15.10 (s, 1 H, COOH). MS (ESI) m/z 436 [M+H]+. Anal. Calcd for C22H20ClF2NO4: C 60.63, H 4.63, N 3.21, found: C 60.40, H 4.36, N 3.45.
6-(3-chloro-2-fluorobenzyl)-1-(2-chlorophenyl)-5-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4f)
Yield 87%. Mp 236–238°C. 1H NMR (CDCl3) δ 4.11–4.20 (dd, J = 15.2, 21.6 Hz, 2 H, CH2), 6.65–6.67 (d, J= 8.8 Hz, 1 H, ArH), 7.02–7.06 (t, J = 8.0 Hz, 1 H, ArH), 7.19–7.23 (m, 1 H, ArH), 7.27–7.31 (m, 1 H, ArH), 7.49–7.54 (m, 2 H, ArH), 7.57–7.61 (m, 1 H, ArH), 7.63–7.67 (m, 1 H, ArH), 7.70–7.73 (m, 1 H, ArH), 8.65 (s, 1 H, CH), 14.66 (s, 1 H, COOH); MS (ESI) m/z 460 [M+H]+. Anal. Calcd for C23H13Cl2F2NO3: C 60.02, H 2.85, N 3.04, Found: C 59.81, H 3.11, N 3.28.
6-(3-chloro-2-fluorobenzyl)-1-(3-chlorophenyl)-5-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4g)
Yield 89%. Mp 215–217°C. 1H NMR (CDCl3) δ 4.06 (s, 2 H, CH2), 6.79–6.81 (d, J = 8.8 Hz, 1 H, ArH), 6.83–7.05 (m, 4 H, ArH), 7.20–7.35 (m, 3 H, ArH), 7.45–7.47 (d, J = 8.8 Hz, 1 H, ArH), 8.81 (s, 1 H, CH), 13.24 (s, 1 H, COOH); MS (ESI) m/z 460 [M+H]+. Anal. Calcd for C23H13Cl2F2NO3: C 60.02, H 2.85, N 3.04, Found: C 60.31, H 2.66, N 3.25.
6-(3-chloro-2-fluorobenzyl)-1-(4-chlorophenyl)-5-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4h)
Yield 90%. Mp >250°C. 1H NMR (CDCl3) δ 4.16 (s, 2 H, CH2), 6.84–6.86 (d, J = 8.8 Hz, 1 H, ArH), 7.03–7.07 (t, J = 7.6 Hz, 1 H, ArH), 7.20–7.24 (m, 1 H, ArH), 7.31–7.33 (m, 1 H, ArH), 7.36–7.39 (d, J = 8.8 Hz, 2 H, ArH), 7.50–7.56 (m, 1 H, ArH), 7.64–7.66 (d, J = 8.8 Hz, 2 H, ArH), 8.73 (s, 1 H, CH), 14.64 (s, 1 H, COOH); MS (ESI) m/z 460 [M+H]+. Anal. Calcd for C23H13Cl2F2NO3: C 60.02, H 2.85, N 3.04, Found: C 60.27, H 3.09, N 2.78.
6-(3-chloro-2-fluorobenzyl)-5-fluoro-1-(4-fluorophenyl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4i)
Yield 93%. Mp 225–227°C.1H NMR (CDCl3) δ 4.14 (s, 2 H, CH2), 6.83–6.85 (d, J = 8.8 Hz, 1 H, ArH), 7.01–7.03 (t, J = 8.0 Hz, 1 H, ArH), 7.18–7.22 (m, 1 H, ArH), 7.26–7.30 (m, 1 H, ArH), 7.33–7.37 (m, 2 H, ArH), 7.44–7.47 (m, 2 H, ArH), 7.51–7.55 (m, 1 H, ArH), 8.70 (s, 1 H, CH), 14.62 (s, 1 H, COOH); MS (ESI) m/z 444 [M+H]+. Anal. Calcd for C23H13ClF3NO3: C 62.25, H 2.95, N 3.16, Found: C 62.47, H 3.19, N 2.89.
6-(3-chloro-2-fluorobenzyl)-1-(4-bromophenyl)-5-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4j)
Yield 88%. Mp >250°C. 1H NMR (CDCl3) δ 4.15 (s, 2 H, CH2), 6.85–6.87 (d, J = 8.8 Hz, 1 H, ArH), 7.02–7.06 (t, J = 8.0 Hz, 1 H, ArH), 7.19–7.23 (t, J= 7.2 Hz, 1 H, ArH), 7.28–7.30 (m, 1 H, ArH), 7.32–7.34 (d, J = 8.4 Hz, 2 H, ArH), 7.52–7.56 (t, J = 8.0 Hz, 1 H, ArH), 7.80–7.82 (d, J =8.4 Hz, 2 H, ArH), 8.71 (s, 1 H, CH), 14.62 (s, 1 H, COOH). MS (ESI) m/z 504 [M+H]+. Anal. Calcd for C23H13BrClF2NO3: C 54.73, H 2.60, N 2.78, Found: C 54.47, H 2.36, N 2.49.
6-(3-chloro-2-fluorobenzyl)-5-fluoro-4-oxo-1-(4-(trifluoromethyl)phenyl)-1,4-dihydroquinoline-3-carboxylic acid (4k)
Yield 85%. Mp >250°C. 1H NMR (CDCl3) δ 4.17 (s, 2 H, CH2), 6.81–6.84 (d, J = 8.8 Hz, 1 H, ArH), 7.03–7.07 (t, J= 8.0 Hz, 1 H, ArH), 7.20–7.24 (m, 1 H, ArH), 7.31–7.33 (m, 1 H, ArH), 7.53–7.57 (m, 1 H, ArH), 7.60–7.62 (d, J = 8.4 Hz, 2 H, ArH), 7.95–7.97 (d, J = 8.4 Hz, 2 H, ArH), 8.73 (s, 1 H, CH), 14.56 (s, 1 H, COOH). MS (ESI) m/z 494 [M+H]+. Anal. Calcd for C24H13ClF5NO3: C 58.37, H 2.65, N 2.84, Found: C 58.59, H 2.38, N 2.59.
6-(3-chloro-2-fluorobenzyl)-5-fluoro-4-oxo-1-m-tolyl-1,4-dihydroquinoline-3-carboxylic acid (4l)
Yield 89%. Mp 218–220°C. 1H NMR (CDCl3) δ 2.48 (s, 3 H, CH3), 4.15 (s, 2 H, CH2), 6.90–6.92 (d, 1 H, J = 8.8 Hz, ArH), 7.02–7.05 (t, 1 H, J= 8.0 Hz, ArH), 7.18–7.22 (m, 3 H, ArH), 7.27–7.30 (m, 1 H, ArH), 7.44–7.46 (d, 1 H, J = 8.0 Hz, ArH), 7.49–7.52 (m, 2 H, ArH), 8.74 (s, 1 H, CH), 14.78 (s, 1 H, COOH); MS (ESI) m/z 440 [M+H]+. Anal. Calcd for C24H16ClF2NO3: C 65.54, H 3.67, N 3.18, Found: C 65.82, H 3.43, N 3.45.
6-(3-chloro-2-fluorobenzyl)-5-fluoro-4-oxo-1-p-tolyl-1,4-dihydroquinoline-3-carboxylic acid (4m)
Yield 90%. Mp 230–232°C. 1H NMR (CDCl3) δ 2.51 (s, 3 H, CH3), 4.14 (s, 2 H, CH2), 6.89–6.91 (d, 1 H, J = 8.8 Hz, ArH), 7.01–7.05 (t, 1 H, J = 8.0 Hz, ArH), 7.18–7.22 (m, 1 H, ArH), 7.26–7.28 (m, 1 H, ArH), 7.28–7.30 (d, 1 H, J = 7.6 Hz, ArH), 7.43–7.44 (d, 1 H, J = 7.6 Hz, ArH), 7.48–7.52 (m, 1 H, ArH), 8.73 (s, 1 H, CH), 14.78 (s, 1 H, COOH); MS (ESI) m/z 440 [M+H]+. Anal. Calcd for C24H16ClF2NO3: C 65.54, H 3.67, N 3.18, Found: C 65.28, H 3.39, N 3.42.
6-(3-chloro-2-fluorobenzyl)-1-(2,6-dimethylphenyl)-5-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4n)
Yield 85%. Mp 200–202°C. 1H NMR (CDCl3) δ 2.01 (s, 6 H, 2CH3), 4.16 (s, 2 H, CH2), 6.64–6.67 (d, J = 8.8 Hz, 1 H, ArH), 7.04–7.07 (t, J = 8.0 Hz, 1 H, ArH), 7.22–7.32 (m, 4 H, ArH), 7.42–7.46 (m, 1 H, ArH), 7.49–7.53 (m, 1 H, ArH), 8.62 (s, 1 H, CH), 14.81 (s, 1 H, COOH); MS (ESI) m/z 454 [M+H]+. Anal. Calcd for C25H18ClF2NO3: C 66.16, H 4.00, N, 3.09, Found: C 66.42, H 3.73, N 3.31.
Declaration of interest
This project was funded by China Postdoctoral Science Foundation (20100470625). The work was also supported in part by grants from the National Natural Science Foundation of China (20872018), from Chinese Academy of Sciences (KSCX2-YW-R-185), from the Eleventh Five-Year Key Scientific and Technological Program of China (2009ZX09501-029), and from National Basic Research Program of China (2009CB5223006).
References
- Craigie R. HIV integrase, a brief overview from chemistry to therapeutics. J Biol Chem 2001;276:23213–23216.
- Hare S, Vos AM, Clayton RF, Thuring JW, Cummings MD, Cherepanov P. Molecular mechanisms of retroviral integrase inhibition and the evolution of viral resistance. Proc Natl Acad Sci USA 2010;107:20057–20062.
- Cabrera C. Raltegravir, an HIV-1 integrase inhibitor for HIV infection. Curr Opin Investig Drugs 2008;9:885–898.
- Anker M, Corales RB. Raltegravir (MK-0518): a novel integrase inhibitor for the treatment of HIV infection. Expert Opin Investig Drugs 2008;17:97–103.
- Hatano H, Hayes TL, Dahl V, Sinclair E, Lee TH, Hoh R et al. A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis 2011;203:960–968.
- Klibanov OM. Elvitegravir, an oral HIV integrase inhibitor, for the potential treatment of HIV infection. Curr Opin Investig Drugs 2009;10:190–200.
- Mathieu M, Nick V, Kasthuraiah M, Alena N, Zhang XM, David R, Christophe M, Yves P. Elvitegravir overcomes resistance to raltegravir induced by integrase mutation Y143. AIDS 2011;25:1175–1178.
- Sato M, Motomura T, Aramaki H, Matsuda T, Yamashita M, Ito Y et al. Novel HIV-1 integrase inhibitors derived from quinolone antibiotics. J Med Chem 2006;49:1506–1508.
- Pasquini S, Mugnaini C, Tintori C, Botta M, Trejos A, Arvela RK et al. Investigations on the 4-quinolone-3-carboxylic acid motif. 1. Synthesis and structure-activity relationship of a class of human immunodeficiency virus type 1 integrase inhibitors. J Med Chem 2008;51:5125–5129.
- Sato M, Kawakami H, Motomura T, Aramaki H, Matsuda T, Yamashita M et al. Quinolone carboxylic acids as a novel monoketo acid class of human immunodeficiency virus type 1 integrase inhibitors. J Med Chem 2009;52:4869–4882.
- Nagasawa JY, Song J, Chen H, Kim HW, Blazel J, Ouk S et al. 6-Benzylamino 4-oxo-1,4-dihydro-1,8-naphthyridines and 4-oxo-1,4-dihydroquinolines as HIV integrase inhibitors. Bioorg Med Chem Lett 2011;21:760–763.
- He QQ, Gu SX, Liu J, Wu HQ, Zhang X, Yang LM et al. Structural modifications of quinolone-3-carboxylic acids with anti-HIV activity. Bioorg Med Chem 2011;19:5039–5045.
- He QQ, Zhang X, Wu HQ, Gu SX, Ma XD, Yang LM et al. Synthesis and biological evaluation of HQCAs with aryl or benzyl substituents on N-1 position as potential HIV-1 integrase inhibitors. Bioorg Med Chem 2011;19:5553–5558.
- Purser S, Moore PR, Swallow S, Gouverneur V. Fluorine in medicinal chemistry. Chem Soc Rev 2008;37:320–330.
- Kirk KL. Fluorination in medicinal chemistry: methods, strategies, and recent developments. Org Process Res Dev 2008;12:305−321.
- Hagmann WK. The many roles for fluorine in medicinal chemistry. J Med Chem 2008;51:4359–4369.
- Zheng YT, Zhang WF, Ben KL, Wang JH. In vitroimmunotoxicity and cytotoxicity of trichosanthin against human normal immunocytes and leukemia-lymphoma cells. Immunopharmacol Immunotoxicol 1995;17:69–79.
- Pannecouque C, Daelemans D, De Clercq E. Tetrazolium-based colorimetric assay for the detection of HIV replication inhibitors: revisited 20 years later. Nat Protoc 2008;3:427–434.