Abstract
Usually, all newborns demonstrate high serum unconjugated bilirubin (UCB) level. UCB may induce adverse effects in the central nervous system. We aimed to evaluate the cytotoxic effects of UCB and the protective effects of docosahexaenoic acid (DHA) on astrocyte cell cultures. The viability of astrocyte cells decreased after UCB treatment in a dose-dependent manner. Pre-incubation of DHA prevents the cells from UCB-mediated neurotoxicity. Our results shown that UCB leads to inhibition of antioxidant enzymes superoxide dismutase (SOD), catalase and GPx activity and induction of apoptosis. But only 4-h pretreatment of DHA can suppress of UCB-mediated inhibition of antioxidant enzymes SOD, catalase and GPx activity and induction of apoptosis in astrocytes. Our results strongly indicated that DHA has a protective effect on UCB-mediated neurotoxicity through inhibition apoptosis and antioxidant enzymes activity of SOD, CAT and GPx in rat primer astrocyte cell line
Introduction
Despite recent improvements in neonatal care, the central nervous system damage due to unconjugated bilirubin (UCB) is still being an important problem. Almost, all newborns demonstrate high serum UCB level, a form of hyperbilirubinaemia clinically called neonatal jaundice which is a benign and transient phenomenon. However, under certain conditions, UCB may induce adverse effects in the central nervous system because of the neurotoxic effect of hyperbilirubinCitation1. Neurotoxicity of UCB is a vast clinical spectrum ranging from sensorineural hearing loss and choreoathetoid cerebral palsy, seizures or death from kernicterus, to mild mental retardation and subtle cognitive disturbance as mentioned bilirubin encephalopathyCitation2. Neurotoxicity caused by UCB includes more than one mechanism and most of these mechanisms are related with oxidative stress and apoptosisCitation3–5. It is known that physiological or mildly increased concentrations of UCB have protective effects against oxidative stressCitation6. However, moderate to severe hyperbilirubinaemia may lead to UCB deposition in the brain through a characteristic pattern, with intense coloration of the basal ganglia, medulla oblangata and cerebellum (kernicterus)Citation7. Nevertheless, there are still knowledge gaps regarding to neurologic features of UCB-induced brain injuryCitation5.
The neurons seem to be more susceptible than astrocytes to UCB-induced cell death by a mechanism that appears to involve oxidative stressCitation3,Citation4,Citation8,Citation9. Neural and glial cells are the main targets for UCB toxicity. UCB toxicity to neurons has been reported in brain sections, cultured cell lines, and isolated nerve terminalsCitation9. Recent studies have described that astrocyte functions are compromised following UCB exposureCitation9–11. Astrocytes are glial cells that provide metabolic support to neurons and contribute to formation of the blood-brain barrier. Previous studies suggest that these cells are the main transporters of UCB from blood to neurons and are involved in the release of waste products from UCB-damaged neuronsCitation12,Citation13. The astrocytes may be the first cells that come into contact with UCB in blood-brain barrier failure. This raises the question of the sensitivity of astrocytes to UCBCitation14.
The nervous system is highly enriched in long-chain polyunsaturated fatty acids (LC-PUFAs). Fish and maternal milk are the major dietary sources of LC-PUFA such as docosahexaenoic acid (DHA)Citation15,Citation16. DHA, in particular, is the most abundant n-3 LC-PUFA in the brain and it is essential for the normal brain functionCitation17,Citation18. Accretion of DHA in the central nervous system occurs actively during the developmental periodCitation19. While neurons are highly enriched with DHA, they cannot produce it because of lack of desaturase activity; only astrocytes have the capacity to synthesize DHACitation20. Astrocytes are in close contact with neurons and readily release DHA into the extracellular fluid under basal and stimulated conditions, thus providing a source for neuronal DHACitation21,Citation22. Considering astroglia support neurons by providing neurotrophic factors, the supply of DHA by astrocytes can also be trophicCitation23. DHA is an especially promising therapeutic or preventive intervention because it may simultaneously exert beneficial effects on several of the injury cascades contributing to perinatal brain injury, including free radicals, inflammatory cytokines, bioactive lipid mediators, and apoptosisCitation21,Citation24–26. Berman et al. (2009) showed that DHA had neuroprotective and restorative effects in their animal modelsCitation27. Although the role of DHA in bilirubin-induced brain injury is unknown, DHA is known to have neuroprotective effects against oxidative stress and apoptosis. Therefore, this study was designed to evaluate the effects of DHA and UCB on astrocyte cell culture, and to investigate protective effects of DHA on bilirubin neurotoxicity.
Material and methods
Cell cultures
This study was approved by the Local Ethical Committee for Experimental Research at Pamukkale University. Astrocyte cell cultures were prepared from brains of 1-day-old Wistar albino rat pups by a modification of the shake off method of Cole and de VellisCitation28,Citation29. Briefly, after decapitation, brains were retrieved and meninges were completely trimmed. The brains were minced and dissociated mechanically and then sieved through a nylon mesh (pore size of 70 µm; Millipore). Cells were spin at 1500 rpm/min for 5 min in a benchtop centrifuge, and the cell pellets were resuspended in Dulbecco’s Modified Eagle Medium/F12 (DMEM/F12) (DMEM/Ham’s F-12 1:1, 500 mL, Biochrom, Berlin, Germany), containing 10% heat inactivated foetal bovine serum (FBS) (Hyclone, 100 mL, Thermo Scientific, Cromlington, UK), 500 µL gentamisin (Gentamisin, 10 mg/10 mL, Sigma-Aldrich, St Louis, USA) and 5 mL fungisone (Gibco Antibiotic–Antimycotic, 25 µg/mL amphoterisin B, 100×/100 mL, Invitrogen, New York, USA). The resuspended cells were seed in 75-cm2 flasks previously coated with 10 µg/mL of poly-d-lysine (PDL) (Sigma-Aldrich, St Louis, USA) for primary neuron cell cultures. Cells were incubated in a humidified CO2 incubator at 37°C, CO2 5% and humidify 95% with changes of medium every 3 days. After 8–10 days of culture, macrophages and loosely attached cells were removed from the astrocyte monolayer by shaking cultures at 150 rpm for 1 h. Then oligodendrocytes present on the top of a confluent monolayer of astrocytes were dislodged by shaking at 150 rpm for 24 h on orbital shaker. The medium containing floating cells, microglias and oligodendrocytes were removed to different flasks. At the bottom of flasks, astrocyte cells were collected and the cell pellets were resuspended in astrocyte medium (DMEM/F12 containing 500 µL gentamisin, 5 mL fungisone, 15% FBS, 5 mL l-glutamine (Gibco l-Glutamine-200 mM, 100×/100 mL, Invitrogen, New York, USA)) and 500 µL insulin (Human Insulin <rh>, 0.5 mg/mL, Biochrom, Berlin, Germany). The method for the preparation of astrocytes has been estimated to yield cultures with purity approximating 95% astrocytes and for experiments, these astrocyte cell cultures were used. Stock UCB solution was prepared in 0.1 N NaOH and stored in the dark at 4°C until use. Stock UCB solution was further diluted with astrocyte medium under sterile conditions and added to cultures at various concentrations.
Determination of UCB and DHA concentrations and astrocyte cell viability
Cells were seeded in 96-well plates (3 × 104 cells/well). After 24 h, the cells treated with UCB the following concentrations: 1000 µM, 800 µM, 600 µM, 500 µM, 300 µM, 200 µM, 100 µM, 80 µM, 60 µM, 50 µM, 30 µM, 20 µM, 10 µM, 8 µM, 4 µM, 2 µM, 1 µM, 0.1 µM and 0.01 µM for 72 h. Astrocyte cells also treated with DHA with the following concentrations: 100 µM, 80 µM, 60 µM, 50 µM, 30 µM, 20 µM, 15 µM, 8 µM, 6 µM, 5 µM, 3 µM, 1 µM and 0.1 µM to examine DHA toxic or not on cells. Absorbance (A), which was proportional to cell viability, was measured by luminometric method. Cell viability was measured using the following calculation: Cell viability (%) = 100 × A1/A0, where A1 (72nd hour) and A0 (0th hour) are the absorbances obtained from treated cells and untreated cells, respectively.
Evaluation of apoptosis
Cells were treated with IC50 values of bilirubine before or after 4-h treatment of DHA to examine any protective effects. After 24 h incubation, cells were washed PBS and tripsinized. Deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labelling (TUNEL) method was used for determination of apoptosis. For TUNEL staining, ApopTag plus Peroxidase in Situ Apoptosis Detection (Millipore) kit was used in accordance with the manufacturer’s instructions. At least six random microscopic fields were counted per sample, and mean values were expressed as the percentage of apoptotic nucleiCitation30.
Evaluation of SOD, CAT and GPx enzyme activities
Cells were treated with IC50 values of UCB before or after 4-h treatment of DHA for examine any protective effects. After 24 h incubation, cells were washed PBS and tripsinized. And then we examined the effects of UCB and DHA on superoxide dismutase (SOD), CAT and GPx enzymes activity. SOD activity measurement method was based on the principle in which xanthine reacts with xanthine oxidase to generate superoxide radicals which react with tetrazolium salt to form a red formazon dye. The SOD activity is measured by the degree of inhibition of this reaction. For SOD activity measurement, SOD Assay Kit (Cayman) was usedCitation31.
Catalase activity was measured according to the method of Wheeler et al. 1990Citation32. The method is based on the reaction of the enzyme with methanol in the presence of an optimal concentration of hydrogen peroxide. For catalase activity measurement, catalase Assay Kit (Cayman) was used.
GPx activity was based on the method of Paglia and ValentineCitation33. The principle of the method was as follows: GPx catalyses the oxidation of glutathione by cumene hydroperoxide. In the presence of glutathione reductase and NADPH, the oxidized glutathione is immediately converted to the reduced form with a concomitant oxidation of NADPH to NADP+. The decrease in absorbance of NADPH was measured at 340 nm. For GPx activity measurement, GPx Assay Kit (Cayman) was used.
Statistical evaluation
Statistical analyses were performed by Systat statistical software (version 17.0 for Windows; Statistical Packages for Social Sciences Inc, Chicago, IL, U.S.A.). The Kruskal−Wallis and Mann−Whitney U test analysis were used for data analyses. Statistical significance was taken at p < 0.05. All data are presented as the mean ± SD.
Results
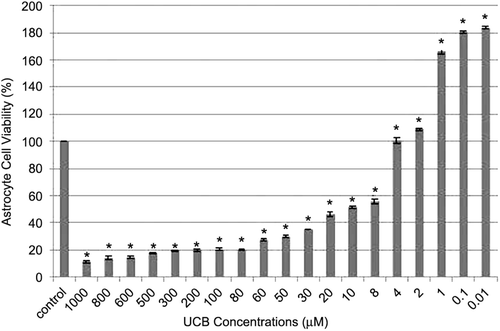
The astrocyte cell viability in the control group was considered to be 100% at 72nd hour, while the astrocyte cell viability percentage was found to be 11.5 ± 0.9, 13.9 ± 1.3, 14.6 ± 1, 17.8 ± 0.5, 19.6 ± 0.3, 20 ± 1.07, 20.4 ± 0.85, 20.4 ± 0.1, 27.5 ± 0.85, 30.1 ± 0.59, 35.4 ± 0.8, 46.4 ± 1.44, 51.6 ± 1.8, 55.7 ± 1.6, 102 ± 2.08, 109.1 ± 0.85, 166.3 ± 1.8, 180.6 ± 0.64 and 183.6 ± 0.8, in the 1000, 800, 600, 500, 300, 200, 100, 80, 60, 50, 30, 20, 10, 8, 4, 2, 1, 0.1, and 0.01 µM UCB groups, respectively (). IC50 of UCB for astrocyte cells was determined as 10 µM and this concentration was used in future experiments.
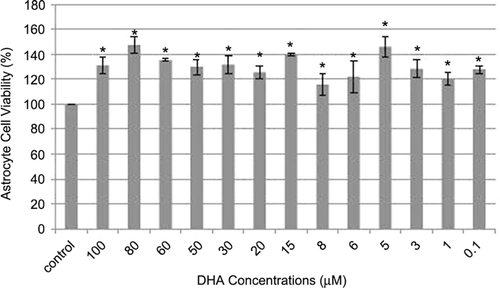
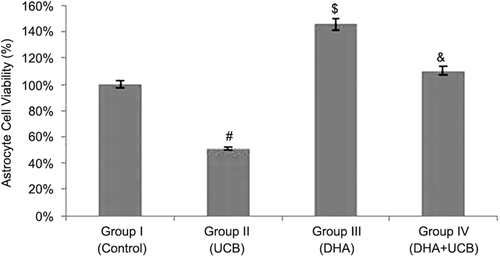
We also examined the effects of DHA on astrocyte cell viability. In , it is clearly shown that astrocyte cell viability was increased after DHA treatment in all concentrations. Therefore, we can not calculate IC50 values of DHA for astrocyte cells and the most effective concentration of DHA was determined as 5 µM and, then, this concentration was used in future experiments for examine the protective effects of DHA on astrocyte cells from UCB toxicity. Because we want to examine any protective effects of DHA on UCB toxicity in astrocyte cells, we pretreated cells for 4 h with DHA then add UCB (10 µM). Astrocyte cell viability was significantly decreased after 10 µM UCB treatment (51 ± 1.8%) when compared with untreated control group (p < 0.001). On the contrary, astrocyte cell viability was found to be significantly increased in the DHA pretreated cells (110 ± 6.3%) (p < 0.001). After 72 h incubation, 10 µM UCB leads to the decrease of astrocyte cells viability, but only 4-h pretreatment of DHA enough for the terminate of UCB toxicity on astrocyte cells ().
Figure 3. Alterations of the astrocyte cell viability in the groups (%). #Decrease of the cell viability of the group II compared to the group I, III and IV (p < 0.001). $Increase of the cell viability of the group III compared to the group I, II and IV (p < 0.001). &Increase of the cell viability of the group IV compared to the group I and II (p < 0.001).
Because UCB has oxidant effects and DHA has antioxidant ability we aim to examine the effects of UCB and DHA on SOD, CAT and GPx enzyme activities in astrocyte cells. Therefore, we incubated cells with 10 µM UCB, DHA 5 µM or preincubated DHA for 4 h then add UCB. After 24 h incubation, cells were washed PBS twice and then lysed. We used these lysates for determining of antioxidant enzyme activity.
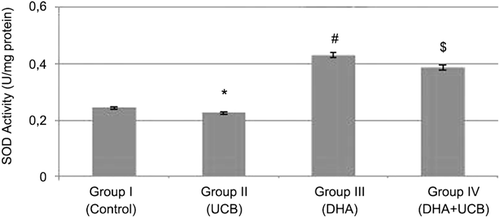
The SOD activity was found to be 0.24 ± 0.05 U/mg protein in the untreated control group, 0.22 ± 0.02 U/mg protein in UCB treated cells, 0.42 ± 0.0007 U/mg protein in the DHA treated cells and 0.38 ± 0.02 U/mg protein in the pretreated DHA then UCB treated cells. The SOD activity in the DHA treated cells were significantly increased compared to the control (p < 0.001). Furthermore, 4-h pretreatment of DHA (5 µM) leads to the significantly increased SOD activity when compared with only UCB treated cells (p < 0.001) ().
Figure 4. Evaluation of the SOD activity in the groups. *Decrease of the SOD activity in the group II compared to the groups I, III and IV (p < 0.05, p < 0.001 and p < 0.01, respectively). #Increase of the SOD activity in the group III compared to the group I, II and IV (p < 0.01, p < 0.001 and p < 0.05, respectively). $Increase of the SOD activity in the group IV compared to the group I and II (p < 0.01).
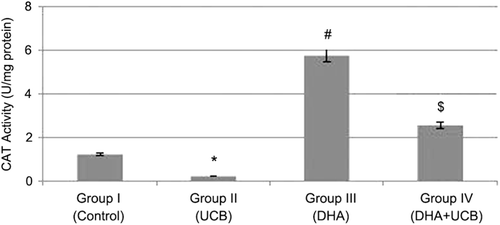
The catalase (CAT) activity was found to be 1.2 ± 0.01 U/mg protein in the untreated control group, 0.22 ± 0.004 U/mg protein in the UCB treated cells, 5.75 ± 0.002 U/mg protein in the DHA treated cells and 2.5 ± 0.004 U/mg protein in the pretreated DHA then UCB treated cells. Although the CAT activity was significantly inhibit in the UCB treated cells, in only DHA treated or pre DHA then UCB treated cells, this CAT activity was significantly increased (p < 0.05). Furthermore, pretreatment of DHA significantly terminate the negative effect of UCB on the catalase enzyme activity in astrocyte cells (p < 0.001) ().
Figure 5. Evaluation of the CAT activity in the groups. *Decrease of the CAT activity in the group II compared to the group I, III and IV (p < 0.05, p < 0.001 and p < 0.001, respectively). #Increase of the CAT activity in the group III compared to the group I, II and IV (p < 0.001). $Increase of the CAT activity in the group IV compared to the group I and II (p < 0.001).
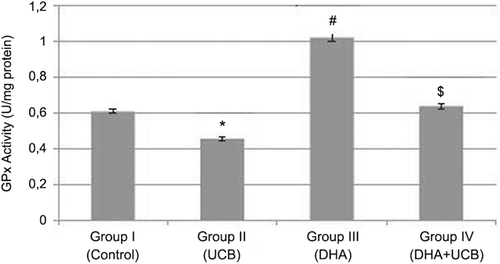
The glutathione peroxidase (GPx) activity was found to be 0.6 ± 0.015 U/mg protein in the untreated control group, 0.44 ± 0.010 U/mg protein in the UCB treated cells, 1.02 ± 0.011 U/mg protein in the DHA treated cells and 0.64 ± 0.011 U/mg protein in the pre DHA then UCB treated cells. Although the GPx activity was significantly inhibited in the UCB treated cells compared with untreated control, DHA treated and pre DHA then UCB treated cells (p < 0.05) ().
Figure 6. Evaluation of the GPx activity in the groups. *Decrease of the GPx activity in the group II compared to the group I, III and IV (p < 0.001). #Increase of the GPx activity in the group III compared to the group I, II and IV (p < 0.001). $Increase of the GPx activity in the group IV compared to the group I, and II (p < 0.01).
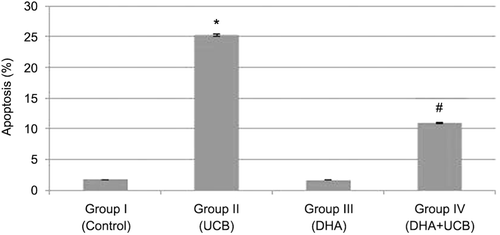
Because we observed that UCB has toxic effects on astrocyte cells and DHA can astrocyte protect cells from this UCB toxicity, we want to examine UCB toxicity come from induction of apoptosis or not and there is also any protective effects of DHA UCB mediated induction of apoptosis on astrocyte cells. Therefore, we treated astrocyte cells UCB (10 µM), DHA (5 µM) and 4 h pretreated with DHA then UCB for 24 h. After the end of incubation time cell washed, and induction of apoptosis was determined with TUNEL assay. In , it is clearly shown that UCB-induced apoptosis (25.27 ± 2.1), and pretreatment of DHA leads to decrease of UCB-induced apoptosis (10.9 ± 0.5) (p < 0.001). This means DHA has protective effects on UCB-induced apoptosis in astrocyte cells.
Discussion
UCB is a pigment resulting from the degradation of heme protein. Deposition of this pigment in the central nervous system is the major factor causing UCB encephalopathy during severe neonatal hyperbilirubinaemiaCitation34. The cytotoxic effect of UCB has been investigated mostly in neuronal and astroglial cells to dateCitation35. Although UCB is a well-known neurotoxin, the cellular mechanisms mediating its toxicity remain poorly understood. It is hoped that clarification of toxicity mechanisms may yield novel ideas for future therapeutic approachesCitation36. Various toxic effects to astrocyte cells involving the inhibition of DNA and protein synthesis, impairment of neurotransmitter synthesis, release and uptake, inhibition of several mitochondrial enzymes and protein phosphorylation have been accounted for UCB neurotoxicity and most of these mechanisms are a result of oxidative stressCitation11,Citation34. The results of the present study are the first in vitro demonstration on the astroglial cell culture that DHA has a protective effect against bilirubin neurotoxicity.
Moderate degrees of hyperbilirubinaemia turned out to be associated with a significant increase in minor neurologic dysfunction throughout the first year of lifeCitation37. Neural and astrocyte cells are the main targets for UCB toxicity. Recent studies have shown toxic and apoptotic effects of UCB on astrocytesCitation9,Citation14. Tastekin et al. (2006) demonstrated that UCB was highly toxic in 10−3 M concentration, with cell death of about 90%Citation36. Cell death was rather stable between 10−4 and 10−8 M concentration, causing cell death between 47 and 37%. In the present study, we demonstrated that UCB was highly toxic in 10−3 M concentration, with cell death of about 90%. Even though high concentration of UCB increased cell death of astrocyte cells, low concentration of UCB (4−0.01 µM) increases the astrocyte cell viability. These findings supports the previous studies that UCB has a dual effect on astrocyte cells, being neuroprotective at lower concentrations, but neurotoxic at higher concentrations. Furthermore, the present study also demonstrated that IC50 of the UCB was 10 µM on astrocyte cells, as showed by Berns et al. (2009) and Tastekin et al. (2006)Citation36,Citation38.
Researchers have investigated the effects of fatty acids, especially DHA on several physiological systems for the past 25 yearsCitation26,Citation39. These includes heart disease, cancer, immune problems, neuronal functions, aging and “other” hard to categorize problems such as migraine headaches, malaria and sperm infertilityCitation40. Berman et al. (2009) have shown that DHA pretreatment provides effective neuroprotection to the neonatal rat brain, improving functional testing to near-normal levels and reducing brain volume loss, particularly in the hippocampus, in perinatal hypoxia-ischemia animal modelCitation27. Furthermore, DHA is reported to play a neuroprotective role against oxidative stress in photoreceptors and to be important both for the maturation of retinal photoreceptors and for preventing photoreceptor apoptosis in the developing retinaCitation25,Citation41,Citation42. Shimazawa et al. (2009) also demonstrated that DHA had neuroprotective effects against oxidative stress in retinal ganglion cellsCitation26.
Several studies showed that DHA has protective effects in concentrations ranging between 2 and 6.7 µM. The higher concentrations than 10 µM were found to be causing photoreceptor cell deathCitation43. In the present study, it was found that no any concentration of DHA between 0.1 and 100 µM had toxic effects on astrocyte cells.
Recently, many agents have been investigated in UCB neurotoxicity. Tastekin et al. (2006) have demonstrated that l-carnitine has neuroprotective effect against UCB neurotoxicity not also all concentrations but only 10−4 MCitation36. Zhang et al. (2010) showed that taurine pretreatment reduced UCB-mediated apoptotic cell death in primary neuron cultures in a concentration-dependent mannerCitation1. Furthermore, minocycline, an anti-inflammatory and anti-apoptotic semisynthetic tetracycline has been shown that it prevents hyperbilirubinaemia-induced acute UCB neurotoxicity in jaundiced Gunn rat pupsCitation44. The present study showed that DHA increased astrocyte cells viability in all concentrations and had also a protective effect against UCB neurotoxicity.
Nerve cell injury induced by UCB has been suggested by two mechanisms: apoptosis and necrosis. Falcao et al. (2007) demonstrated that neurons which were exposed to UCB, dead by apoptosisCitation5. Also in a recent study, the authors have provided evidence that low, clinically relevant concentrations of UCB promote delayed and programmed neuronal death reflecting an apoptotic phenomenonCitation45. Moreover, Kumral et al. (2005) showed that UCB-induced cytotoxicity and apoptotic astrocyte death in a concentration and time dependent mannerCitation14. In this study, apoptosis was found to be 25% at 72nd hour in 10 µM UCB demonstrated for IC50. Hence, the present study also confirmed findings of previously studies.
The protective effects of DHA against apoptosis have been showed in vitro retinal photoreceptors studiesCitation25,Citation42. Furthermore in these studies, the effectivity of DHA in therapeutic range has been reported between 2 and 6.7 µM concentrations. Our results indicate that DHA had no any apoptotic effect and it has also protective effect against UCB-mediated apoptosis.
Although UCB was suggested a powerful antioxidant and a modulator of cell growth, recent studies have provided strong evidence in the last decade that, UCB neurotoxicity in part due to oxidative stress, which leads to DNA damage and cell growth reductionCitation46. The organism’s defence system against oxidative stress and reactive oxygen species includes non-enzymatic mechanisms (vitamin A, E and C) and enzymes such as SOD, CAT and GPxCitation47. Brito et al. (2004) have demonstrated that UCB induces oxidative stress in cortical synaptosomal membrane systems, by means of enhanced formation of free radical, protein oxidation and lipid peroxidationCitation3. Also, in another study, authors have shown that UCB induces a dose-dependent increase in neuronal death in parallel with the oxidation of cell components and a decrease in the intracellular glutathione contentCitation4. Bracci et al. (1988) showed that antioxidant enzymes of erythrocyte were significantly lower in cord blood and on the 4th day of life in babies with high bilirubinemia compared to less jaundiced babiesCitation48. Recently, Dani et al. (2003) have shown a decrease plasma bilirubin level and oxidative stress concominant with an increase in plasma antioxidant capacity in the preterm infantsCitation49. In the present study, here we show that UCB inhibits the SOD, CAT and GPx enzyme activities in the astrocyte cell culture compared to the untreated control group.
Hossain et al. (1999) have reported the antioxidative effects of DHA in aged and hypercholesterolemic ratsCitation50. Authors have suggested that DHA may play an important role in antioxidative defence mechanism by enhancing the cerebral activities of CAT, GPx and glutathione. Afterward, many studies were reported that DHA increase the antioxidant enzyme activities including SOD, CAT and GPx in the different cell lines such as retinal ganglion cells, cerebellar astrocytes and neurons cellsCitation23,Citation25,Citation26. In an ischemia-reperfusion animal model study, Bas et al. (2007) have reported that DHA increases SOD activity in rat hippocampusCitation24. Additionally, Ozen et al. (2008) have shown the increased CAT activity on cerebral ischemia in rat prefrontal cortex by DHA pretreatmentCitation51. In another animal study, Choi-Kwon et al. (2004) have demonstrated that the SOD, CAT, and GPx cerebral activities increased due to DHA treatmentCitation52. A recent study investigated the effects of n-3 polyunsaturated fatty acids in the prevention and treatment of neurological diseases, authors showed no any toxic effect of DHA in glial cells. Moreover, it was reported that low concentrations of DHA leads to increase GPx and CAT activitiesCitation53. In the present study, we demonstrated that DHA not only significantly increased SOD, CAT and GPx activities in the astrocyte cell culture but also prevented to UCB-mediated inhibition of these antioxidant enzymes activities.
In conclusion, we conclude that the neuroprotective effect of DHA against UCB neurotoxicity observed in this study is primarily because of the increasing the cell viability and the antioxidant enzyme activities of SOD, CAT and GPx and the inhibition of apoptosis. To our knowledge, this study is the first to demonstrate the neuroprotective effects of DHA against UCB neurotoxicity. Further research is needed to confirm our findings and to clarify the future mechanisms responsible for the protective effects of DHA on UCB neurotoxicity.
Acknowledgment
The authors thank Pamukkale University Animal Research Laboratory for their help with experimental techniques.
Declaration of interest
This study was supported by Scientific Research Foundation (No: 2010TPF009) of Pamukkale University.
References
- Zhang B, Yang X, Gao X. Taurine protects against bilirubin-induced neurotoxicity in vitro. Brain Res 2010;1320:159–167.
- Wong R, DeSandre G, Sibley E, Stevenson D. (2006). Neonatal jaundice and liver disease. In: Fanaroff and Martin’s Neonatal-Perinatal Medicine Diseases of the Fetus and Infant. Philadelphia: Mosby Elsevier. Volume 2. pp. 1419–1465.
- Brito MA, Brites D, Butterfield DA. A link between hyperbilirubinemia, oxidative stress and injury to neocortical synaptosomes. Brain Res 2004;1026:33–43.
- Brito MA, Rosa AI, Falcão AS, Fernandes A, Silva RF, Butterfield DA et al. Unconjugated bilirubin differentially affects the redox status of neuronal and astroglial cells. Neurobiol Dis 2008;29:30–40.
- Falcão AS, Silva RF, Pancadas S, Fernandes A, Brito MA, Brites D. Apoptosis and impairment of neurite network by short exposure of immature rat cortical neurons to unconjugated bilirubin increase with cell differentiation and are additionally enhanced by an inflammatory stimulus. J Neurosci Res 2007;85:1229–1239.
- Doré S, Takahashi M, Ferris CD, Zakhary R, Hester LD, Guastella D et al. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci USA 1999;96:2445–2450.
- Hansen TW. Pioneers in the scientific study of neonatal jaundice and kernicterus. Pediatrics 2000;106:E15.
- Notter MF, Kendig JW. Differential sensitivity of neural cells to bilirubin toxicity. Exp Neurol 1986;94:670–682.
- Silva RF, Rodrigues CM, Brites D. Rat cultured neuronal and glial cells respond differently to toxicity of unconjugated bilirubin. Pediatr Res 2002;51:535–541.
- Rhine WD, Schmitter SP, Yu AC, Eng LF, Stevenson DK. Bilirubin toxicity and differentiation of cultured astrocytes. J Perinatol 1999;19:206–211.
- Rodrigues CM, Solá S, Silva RF, Brites D. Aging confers different sensitivity to the neurotoxic properties of unconjugated bilirubin. Pediatr Res 2002;51:112–118.
- Chen HC, Tsai DJ, Wang CH, Chen YC. An electron microscopic and radioautographic study on experimental kernicterus. I. Bilirubin transport via astroglia. Am J Pathol 1969;56:31–58.
- Chen HC, Wang CH, Tsan KW, Chen YC. An electron microscopic and radioautographic study on experimental kernicterus. II. Bilirubin movement within neurons and release of waste products via astroglia. Am J Pathol 1971;64:45–66.
- Kumral A, Genc S, Genc K, Duman N, Tatli M, Sakizli M et al. Hyperbilirubinemic serum is cytotoxic and induces apoptosis in murine astrocytes. Biol Neonate 2005;87:99–104.
- Larque E, Demmelmair H, Koletzko B. Perinatal supply and metabolism of long-chain polyunsaturated fatty acids: importance for the early development of the nervous system. Ann N Y Acad Sci 2002;967:299–310.
- Rodriguez-Palmero M, Koletzko B, Kunz C, Jensen R. Nutritional and biochemical properties of human milk: II. Lipids, micronutrients, and bioactive factors. Clin Perinatol 1999;26:335–359.
- Kim HY. Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem 2007;282:18661–18665.
- Uauy R, Calderon F, Mena P. Essential fatty acids in somatic growth and brain development. World Rev Nutr Diet 2001;89:134–160.
- Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci USA 1989;86:2903–2907.
- Moore SA, Yoder E, Murphy S, Dutton GR, Spector AA. Astrocytes, not neurons, produce docosahexaenoic acid (22:6 omega-3) and arachidonic acid (20:4 omega-6). J Neurochem 1991;56:518–524.
- Kim HY, Edsall L, Garcia M, Zhang H. The release of polyunsaturated fatty acids and their lipoxygenation in the brain. Adv Exp Med Biol 1999;447:75–85.
- Moore SA. Polyunsaturated fatty acid synthesis and release by brain-derived cells in vitro. J Mol Neurosci 2001;16:195–200; discussion 215.
- Kaur P, Heggland I, Aschner M, Syversen T. Docosahexaenoic acid may act as a neuroprotector for methylmercury-induced neurotoxicity in primary neural cell cultures. Neurotoxicology 2008;29:978–987.
- Bas O, Songur A, Sahin O, Mollaoglu H, Ozen OA, Yaman M et al. The protective effect of fish n-3 fatty acids on cerebral ischemia in rat hippocampus. Neurochem Int 2007;50:548–554.
- Rotstein NP, Politi LE, German OL, Girotti R. Protective effect of docosahexaenoic acid on oxidative stress-induced apoptosis of retina photoreceptors. Invest Ophthalmol Vis Sci 2003;44:2252–2259.
- Shimazawa M, Nakajima Y, Mashima Y, Hara H. Docosahexaenoic acid (DHA) has neuroprotective effects against oxidative stress in retinal ganglion cells. Brain Res 2009;1251:269–275.
- Berman DR, Mozurkewich E, Liu Y, Barks J. Docosahexaenoic acid pretreatment confers neuroprotection in a rat model of perinatal cerebral hypoxia-ischemia. Am J Obstet Gynecol 2009;200:305.e1–305.e6.
- Cole R, de Vellis J. (1989). A dissection and tissue culture manual of thenervous system. In: Alan R. Liss. A Dissection and Tissue Culture Manual of the Nervous System. >Alan R. Liss Inc. New York. pp. 121–133.
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 1980;85:890–902.
- Negoescu A, Lorimier P, Labat-Moleur F, Drouet C, Robert C, Guillermet C et al. In situ apoptotic cell labeling by the TUNEL method: improvement and evaluation on cell preparations. J Histochem Cytochem 1996;44:959–968.
- Maier CM, Chan PH. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist 2002;8:323–334.
- Wheeler CR, Salzman JA, Elsayed NM, Omaye ST, Korte DW Jr. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal Biochem 1990;184:193–199.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967;70:158–169.
- Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbilirubinemia. N Engl J Med 2001;344:581–590.
- Genc S, Genc K, Kumral A, Baskin H, Ozkan H. Bilirubin is cytotoxic to rat oligodendrocytes in vitro. Brain Res 2003;985:135–141.
- Tastekin A, Gepdiremen A, Ors R, Buyukokuroglu ME, Halici Z. Protective effect of L-carnitine against bilirubin-induced neuronal cell death. Brain Dev 2006;28:436–439.
- Soorani-Lunsing I, Woltil HA, Hadders-Algra M. Are moderate degrees of hyperbilirubinemia in healthy term neonates really safe for the brain? Pediatr Res 2001;50:701–705.
- Berns M, Toennessen M, Koehne P, Altmann R, Obladen M. Ibuprofen augments bilirubin toxicity in rat cortical neuronal culture. Pediatr Res 2009;65:392–396.
- Gorjão R, Azevedo-Martins AK, Rodrigues HG, Abdulkader F, Arcisio-Miranda M, Procopio J et al. Comparative effects of DHA and EPA on cell function. Pharmacol Ther 2009;122:56–64.
- Stillwell W. Docosahexaenoic acid: a most unusual fatty acid. Chem Phys Lipids 2008;153:1–2.
- Birch DG, Birch EE, Hoffman DR, Uauy RD. Retinal development in very-low-birth-weight infants fed diets differing in omega-3 fatty acids. Invest Ophthalmol Vis Sci 1992;33:2365–2376.
- Rotstein NP, Aveldaño MI, Barrantes FJ, Roccamo AM, Politi LE. Apoptosis of retinal photoreceptors during development in vitro: protective effect of docosahexaenoic acid. J Neurochem 1997;69:504–513.
- Rotstein NP, Politi LE, Aveldaño MI. Docosahexaenoic acid promotes differentiation of developing photoreceptors in culture. Invest Ophthalmol Vis Sci 1998;39:2750–2758.
- Geiger AS, Rice AC, Shapiro SM. Minocycline blocks acute bilirubin-induced neurological dysfunction in jaundiced Gunn rats. Neonatology 2007;92:219–226.
- Grojean S, Koziel V, Vert P, Daval JL. Bilirubin induces apoptosis via activation of NMDA receptors in developing rat brain neurons. Exp Neurol 2000;166:334–341.
- Deganuto M, Cesaratto L, Bellarosa C, Calligaris R, Vilotti S, Renzone G et al. A proteomic approach to the bilirubin-induced toxicity in neuronal cells reveals a protective function of DJ-1 protein. Proteomics 2010;10:1645–1657.
- Turgut M, Basaran O, Cekmen M, Karatas F, Kurt A, Aygün AD. Oxidant and antioxidant levels in preterm newborns with idiopathic hyperbilirubinaemia. J Paediatr Child Health 2004;40:633–637.
- Bracci R, Buonocore G, Talluri B, Berni S. Neonatal hyperbilirubinemia. Evidence for a role of the erythrocyte enzyme activities involved in the detoxification of oxygen radicals. Acta Paediatr Scand 1988;77:349–356.
- Dani C, Martelli E, Bertini G, Pezzati M, Filippi L, Rossetti M et al. Plasma bilirubin level and oxidative stress in preterm infants. Arch Dis Child Fetal Neonatal Ed 2003;88:F119–F123.
- Hossain MS, Hashimoto M, Gamoh S, Masumura S. Antioxidative effects of docosahexaenoic acid in the cerebrum versus cerebellum and brainstem of aged hypercholesterolemic rats. J Neurochem 1999;72:1133–1138.
- Ozen OA, Cosar M, Sahin O, Fidan H, Eser O, Mollaoglu H et al. The protective effect of fish n-3 fatty acids on cerebral ischemia in rat prefrontal cortex. Neurol Sci 2008;29:147–152.
- Choi-Kwon S, Park KA, Lee HJ, Park MS, Lee JH, Jeon SE et al. Temporal changes in cerebral antioxidant enzyme activities after ischemia and reperfusion in a rat focal brain ischemia model: effect of dietary fish oil. Brain Res Dev Brain Res 2004;152:11–18.
- Leonardi F, Attorri L, Benedetto RD, Biase AD, Sanchez M, Tregno FP et al. Docosahexaenoic acid supplementation induces dose and time dependent oxidative changes in C6 glioma cells. Free Radic Res 2007;41:748–756.