Abstract
New furanone derivatives incorporating the indolin-2-one moiety 3 were prepared via the Perkin reaction of isatins 1 with aroylpropionic acids 2 under conventional conditions or microwave irradiation. A series of functionally heterocyclic derivatives (e.g., pyridazines, pyrroles, and sulfonamides) incorporating the indolin-2-one moiety was achieved via reaction of 3 with different reagents under microwave irradiation conditions. The newly synthesized compounds were characterized on the basis of FTIR, 1H, 13C NMR and mass spectral studies. Some of the new synthesized compounds were screened for antibacterial activity against Gram-positive bacteria (Staphylococcus aureus and Bacillus cereus), Gram-negative bacteria (Escherichia coli and Shigilla flexneri) and antifungal activity against Aspergillus flavus and Candida albicans. Compound 8 j was equipotent to chloramphenicol in inhibiting the growth of E. coli minimum inhibitory concentration (MIC 2.5 μg/mL). Compound 8j may possibly be used as a lead compound for developing a new antibacterial agents. The antibacterial activity is expressed as the corresponding MIC (μg/mL) values.
Introduction
Among a wide variety of compounds that have been explored for developing pharmaceutically important antimicrobial agents, unsaturated γ-lactones have played an important role. Moreover, furanone ring derivatives have acquired a special place in natural chemistry and in heterocyclic chemistry, as the furanone frequently encountered structural motif in many pharmacologically relevant compounds. They are active constituents of many natural and synthetic compounds exhibiting pronounced biological activities, such as cytotoxicCitation1,Citation2, antifungalCitation3,Citation4, antibacterial, anti-inflammatory, analgesicCitation5–7, cardiotonicCitation8, cyclo-oxygenase-2 (COX-2) inhibitoryCitation9,Citation10, antiviralCitation11, and antibiotic activityCitation12.
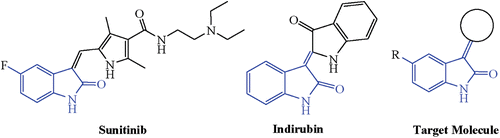
Indirubin identified as the active compound with increased emergence of bacterial resistance to antibiotic therapy, has created an urgent need for the development of new antibacterial agents ().
Since various kinases are involved in the growth of microorganisms and because many oxindoles are kinase inhibitorsCitation6,Citation12–16, a variety of 3-substituted indolin-2-ones have been utilized as anticancer drugs or drug candidatesCitation17–21. A representative member of this class is Sunitinib (SU11248, SutentTM; Pfizer, Cairo, Egypt) which is currently used in the clinics as a multi-targeting tyrosine kinase inhibitor with antiangiogenic activity Citation22,Citation23. Promoted by the above biological and chemical activities and in connection with our synthetic program aimed at the synthesis and bioassay of several poly-functional heteroannulated ring systems of expected bioresponse, samples of heterocycles incorporating indolin-2-one moiety were synthesized and assayed.
As part of our ongoing studies concerning the preparation of potential biologically active compoundsCitation24–29 and bioactive heterocycles based on oxindole moietyCitation30–35, we report herein a convenient microwave-enhanced, high speed, fast, and economic way for the synthesis of new 2(3H)-furanones, for use as key intermediates for the synthesis of new pyridazine, pyrrol, and sulfonamide derivatives.We investigated their antimicrobial activity in order to get new compounds that could be optimized as potent antimicrobial agents.
Results and discussion
Chemistry
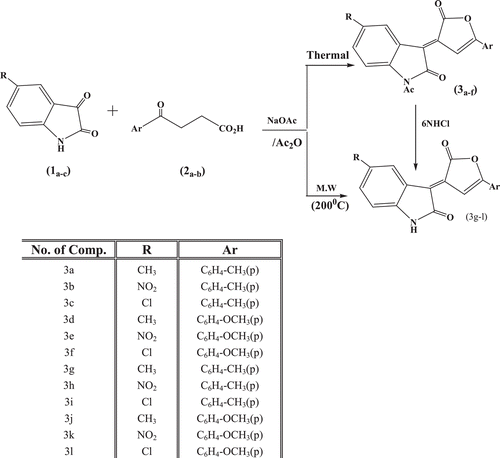
1-Acetyl-3-[(5-(4-alkylphenyl)-2-oxofuran-3(2H)-ylidene)]-5-alkyl-indolin-2-ones (3a–f) were achieved from the reaction of isatin derivatives (1a–c) with β-aroyl propionic acids (2a,b) under conventional Perkin conditions according to the method reported in our previous workCitation30. However, in some cases of microwave irradiation, to afford the formation of 5-alkyl-3-[(2-oxo-5-(4-alkylphenyl)furan-3(2H)-ylidene)]indolin-2-ones (3g–l) was achieved. Compounds (3g–l) were also obtained by the action of 6N HCl on compounds (3a–f). The synthetic route used to synthesize compounds (3a–l) is outlined in .
(3a–l) were established on the basis of IR, mass spectrum, 1HNMR, and 13CNMR spectral data. The IR spectra of compounds (3g–l) revealed absorption bands at 3,425–3,397 (NH), while compounds (3a–f) displayed no frequencies for a NH group. The IR spectra of compounds (3a–l) revealed absorption bands at 1,793–1,729 (lactone, C=O).
Structures
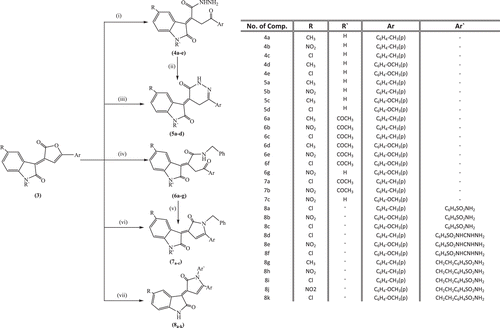
The syntheses of poly functionally heterocyclic (e.g., pyridazine, pyrrole, and sulfonamide) derivatives incorporating indolin-2-one moiety were achieved from investigating the reactivity of compounds (3a–l), towards nucleophiles such as hydrazine hydrate and benzylamine. When compounds (3g–j,l) were stirred with hydrazine hydrate in ethanol at room temperature, the products were 2-[(5-alkyl-2-oxoindolin-3-ylidene)-4-oxo-4-(4-alkylphenyl)] butanehydrazides (4a–e). The structures of (4a–e) were established from IR, mass, 1HNMR, and 13CNMR spectra. The 1HNMR spectra of compounds (4a–e) displayed the NH protons of the NH2 group which disappeared on addition of D2O. Cyclization of 2-[(5-Alkyl-2-oxoindolin-3-ylidene)-4-oxo-4-(4-alkylphenyl)]butane hydrazides (4a–e) can occurred with microwave irradiation in ethanol to give 5-alkyl-3-[(oxo-6-(4-alkylphenyl)-2,3-dihydropyridazin-4-(5H)-ylidene)]indolin-2-ones (5a–d).The structures of the products were confirmed by IR, mass,1HNMR, and 13CNMR spectra. The 1HNMR spectra of compounds (5a–e) lack any absorption bands responsible for the NH2 group. Also, 5-methyl-3-[(oxo-6-(4-methoxyphenyl)-2,3-dihydropyridazin-4(5H)-ylidene)] indolin-2-one (5a) was formed from microwave irradiation of a mixture of the furanone derivative (3g) and hydrazine hydrate in alkaline ethanol .
Scheme 2. Synthesis of the target compounds 4, 5, 6, 7, and 8. Reagents and conditions: (i) NH2NH2,EtOH, Stirr.,(R.T.), (ii) EtOH, M.W., (iii) NH2NH2, EtOH, M.W., (iv) Stirr, (R.T.) or M.W., NH2CH2Ph, EtOH, (v) CH3COOH, HCl reflux or M.W., (vi)NH2CH2Ph, CH3COOH, HCl, M.W., (vii) appreciate sulfonamide, CH3COOH, M.W.
The 13CNMR spectrum of 5-methyl-3-[(oxo-6-(4-methoxyphenyl)-2,3-dihydro pyridazin-4(5H)-ylidene)]indolin-2-one (5a) showed bands at 176.38, 160.19, 141.7, 137.54, 135, 130.09, 129.95, 127.64, 125.87, 125.15, 123.98, 116.02, 108.86, 102.02, 100.19, 35.82, 20.86 and 20.69.
The reaction of 1-acetyl-5-alkyl-3-[(2-oxo-5-(4-alkylphenyl)furan-3(2H)-ylidene)] indolin-2-ones (3a–f) and 3-[(5-(4-methoxyphenyl)-2-oxofuran-3(2H)-ylidene)-5-nitro] indolin-2-one (3k) with benzylamine were carried out in ethanol by stirring at room temperature or by refluxing or by microwave irradiation for 3 min at 85°C under the before mentioned conditions. All reactions gave the same products, that is 2-[(1-acetyl-5-alkyl-2-oxo-indolin-3-ylidene)]-N-benzyl-4-oxo-4(4-alkylphenyl)butaneamide (6a–f) or N-benzyl-4-[(4-methoxyphenyl)-2-(5-nitro-2-oxo-indolin-3-ylidene)]-4-oxo-butaneamide (6g) respectively. Compounds (6a–g) were characterized by their IR spectra of which showed absorption bands of the NH group at 3,293.82–3,291.89 cm−1. 1HNMR spectra of (6a–g) showed the NH signal at 4.77-4.27 which disappeared by the addition of D2O. The amide derivatives (6a,c,d) can be cyclized by refluxing for 3 h or by microwave irradiation for 3 min at 120°C in acetic acid and hydrochloric acid to yield the corresponding pyrrolones, 1-alkyl-3-[(1-benzyl-2-oxo)-5-(4-alkylphenyl)-1,2-dihydropyrrol-3-ylidene)-5-alkyl]-indolin-2-one (7a–c). 1HNMR spectra of (7a,b) showed a lack of frequencies for NH groups. The IR spectrum of (7c) revealed absorption bands for C=O at 1,672.95 and 1,642.58 cm−1. Further support for the structure of these compounds was obtained by IR, 13CNMR and mass spectra.
1-Acetyl-3-[(1-benzyl-2-oxo-5(4-tolyl)-1,2-dihydropyrrol-3-ylidene)-5-chloro]indolin-2-one (7a), was obtained in one step via reaction of 1-acetyl-5-[chloro-3-(2-oxo-5-p-tolylfuran-3(2H)-ylidine)]indolin-2-one (3c) with benzylamine in acetic acid and hydrochloric acid using microwave irradiation for 3 min at 120°C. M.p and mixed m.p experiment confirmed successive synthesis.
A series of sulfonamide derivatives were synthesized by the reaction of 3-[5-(4-alkylphenyl)-2-oxofuran-3(2H)-ylidene]5-alkylindolin-2-ones (3i,k,l) with different sulfonamides such as sulfanilamide, sulfaguanidine, and (3g–i,k,l) with 4-(2-aminoethyl) benzene sulfonamide, in the presence of acetic acid under microwave irradiation for 3 min and at 120°C, to give the corresponding sulfonamide derivatives (8a–k). The IR spectra of compounds (8a–k) were characterized by the presence of absorption bands of SO2 at 1,402-1,300.75 cm−1, and lack of any absorption bands corresponding to a lactone. Further support for the structure of these compounds was obtained from IR, 1HNMR, and mass spectra.
Biological evaluation (in vitro antimicrobial measurement)
The in vitro antimicrobial potency of some of the newly synthesized compounds was compared with broad spectrum antibiotic namely chloramphenicol and nystatin against Gram-negative Escherichia coli (ATCC-25922) and S. flexeniri, gram-positive Staphylococcus aureus (ATCC-25923), Bacillus cereus, and fungi Aspergillus flavus and Candida albicans (ATCC 10231) strains.
The antibacterial activity of the synthesized compounds was tested using an agar well diffusion methodCitation36. The agar-diffusion method was used for the determination of the preliminary antibacterial and antifungal activity. Chloramphenicol and nystatin were used as reference drugs. The results were recorded for each tested compound as the average diameter of inhibition zones (IZ) of bacterial or fungal growth around the disks in mm. Compounds inhibiting the growth of one or more of the above microorganisms were further tested for their MIC and were determined by broth dilution techniqueCitation37. The MIC (mg/mL) and inhibition zone diameters values are recorded in . The IZ diameters values cited in between brackets are attributed to the tested original concentration (1 mg/mL) as a preliminary test. The results depicted in revealed that most of tested compounds displayed variable inhibitory effects on the growth of the tested Gram-positive and Gram-negative bacterial strains, and also against fungal strains. A close investigation of the MIC values indicates that sulfonamide derivative 8j (MIC 2.5 μg/mL) against E.coli, was equipotent with the reference and antifungal C. albicans, also depicted (MIC 5 μg/mL).
Table 1. Minimal inhibitory concentrations (MIC, µg/mL) and inhibition zones (mm) of some new synthesized compounds.
Materials and methods
Chemistry
All melting points are uncorrected and were determined on a Gallenkamp instrument. Infrared spectra of the new compounds were measured on a Perkin-Elmer spectrophotometer model 1430 using potassium bromide pellets and frequencies are reported in cm−1. The 1H NMR were measured on a Varian genini-300 MHZ spectrophotometer and chemical shifts (δ) are in ppm. The mass spectra (m/z) values were measured on mass spectrophotometer HP model GC MS-QPL000EX (Shimadzu) at 70 eV. Elemental analyses were carried out at the Microanalytical Centre, Cairo University, Egypt. Antimicrobial activity evaluations were carried out at the Basic Science Department, Faculty of Applied Medical Science, October 6th University, October City, Egypt. An Explorer Automated Microwave Synthesis Workstation (CEM) was used for synthesis of new compounds.
General procedure for synthesis of furanone derivatives via conventional Perkin reaction conditions (3a–f)
Furanone derivatives were prepared via condensation of finely powdered β-aroylpropionic acid (0.01 mol), fused sodium acetate (0.01 mol) and 5-alkylisatin (0.01 mol) in acetic anhydride (10 ml) heated on a hot plate until a clear solution was obtained. Heating was continued on a water bath until a solid separated out. The reaction mixture was cooled, filtered off and the precipitate was washed with water, petroleum ether (b.p. 40–60°C) and the solid product was crystallized from acetic acid to give (3a–f).
1-Acetyl-3-[(5-(4-tolyl)-2-oxofuran-3(2H)-ylidine)]-5-methylindolin-2-one (3a)
Reddish brown crystals, 74%, m.p. 262–264°C; IR:CH(alicyclic) at 2,930.31, C=O(lactone) at 1,729.83, C=O(amide) at 1,680.89, C=C at 1,618.95 cm−1; MS (EI) m/z 361 (M+ + 2), 1HNMR (DMSO, 300 MHz): δ 8.22-7.15 (m, 7H, 2Ar-H), 6.44 (s, 1H, hetero CH), 3.30-3.19 (s, 3H, COCH3), 2.68-2.60 (s, 6H, 2CH3).
1-Acetyl-3-[(5-(4-tolyl)-2-oxofuran-3(2H)-ylidine)]-5-nitroindolin-2-one (3b)
Reddish brown crystals, 72%, m.p. 272–274°C; IR: CH(alicyclic) at 2,924.52, C=O(lactone) at 1,730.8, C=O(amide) at 1,687.81, C=C at 1,621.84 cm−1; MS(EI) m/z 390 (M+), 1HNMR (DMSO, 300 MHz): δ 8.31-7.01 (m, 7H, 2Ar-H), 6.90-6.87 (s, 1H, hetero CH), 3.75 (s, 3H, COCH3), 2.49-2.36 (s, 3H, OCH3).
1-Acetyl-3-[(5-(4-tolyl)-2-oxofuran-3(2H)-ylidine)]-5-chloroindolin-2-one (3c)
Reddish brown crystals, 69%, m.p. 252–254°C; IR: CH(alicyclic) at 2,922.59, C=O(lactone) at 1,764.83, C=O(amide) at 1,714.41, C=C at 1,614.13 cm−1, MS(EI) m/z 378 (M+ − 1), 1HNMR (DMSO, 300 MHz): δ 8.13-7.28 (m, 7H, 2Ar-H), 6.44 (s, 1H, hetero CH), 3.35-2.65(s, 3H, COCH3), 2.60-2.36 (s, 3H, OCH3).
1-Acetyl-5-methyl-3-[(2-oxo-5-p-methoxyphenylfuran-3(2H)-ylidin)]indolin-2-one (3d)
Reddish brown crystals,70%, m.p. 279–281°C; IR: CH(alicyclic) at 2,916.63, C=O (lactone) at 1,776.12, C=O(amide) at 1,707.66, C=C at 1,606.41 cm−1; MS(EI) m/z 376 (M+ + 1), 1HNMR (DMSO, 300 MHz): δ 8.72-7.10 (m, 7H, 2Ar-H), 6.99 (s, 1H, hetero CH), 3.87-3.85 (s, 3H, OCH3), 3.37-3.30 (s, 3H, COH3), 3.25-3.24 (s, 3H,CH3).
1-Acetyl-5-nitro-3-[(2-oxo-5-p-methoxyphenylfuran-3(2H)-ylidin)]indolin-2-one (3e)
Reddish brown crystals, 72%, m.p. 290–292°C; IR: CH(alicyclic) at 2,925.14, C=O(lactone) at 1,775.15, C=O(amide) at 1,723.09, C=C at 1,609.31 cm−1; MS(EI) m/z 406 (M+), 1HNMR (DMSO, 300 MHz): δ 8.33-7.01 (m, 7H, 2Ar-H), 6.99 (s, 1H, hetero CH), 3.87-3.82 (s, 3H, OCH3), 3.81-3.32(s, 3H, COCH3).
1-Acetyl-5-chloro-3-[(2-oxo-5-p-methoxyphenylfuran-3(2H)-ylidin)]indolin-2-one (3f)
Reddish brown crystals,75%, m.p. 280–282°C; IR:CH(alicyclic) at 2,924.52, C=O(lactone) at 1,779.97, C=O(amide) at 1,708.62, C=C at 1,604.48 cm−1; MS(EI) m/z 395 (M+), 1HNMR (DMSO, 300 MHz): δ 8.24-7.02 (m, 7H, 2Ar-H), 6.64 (s, 1H, hetero CH ), 3.88-3.82 (s, 3H, OCH3), 3.39-2.66 (s, 3H, COCH3).
General procedure for the synthesis of furanone derivatives via microwave irradiation under Perkin reaction conditions (3g–l)
Furanone derivatives were prepared via condensation of finely powdered β-aroyl propionic acid (0.01 mol), fused sodium acetate (0.01 mol) and 5-alkylisatin (0.01 mol) in acetic anhydride (10 ml) via microwave irradiation at 200°C for 2 min. The reaction mixture was cooled, filtered off and the precipitate was washed with water and petroleum ether (b.p. 40–60°C). The solid product was crystallized from acetic acid to give (3g–l).
5-Methyl-3-[(2-oxo-5-(4-tolyl)furan-3(2H)-ylidine)]indolin-2-one (3g)
Reddish brown crystals,79%, m.p. 238–240°C; IR: NH at 3,425, CH(alicyclic) at 2,973.1-2,916.8, C=O(lactone) at 1,779.5,C=O(amide) at 1,712.5, C=C at 1,698.8 cm−1; MS(EI) m/z 314 (M+ − 3), 1HNMR (DMSO, 300 MHz): δ 10.75 (s, 1H, NH), 8.51-7.14 (m, 7H, 2Ar-H), 6.78 (s, 1H, hetero CH), 3.32, 3.19 (s, 6H, 2CH3).
5-Nitro-3-[(2-oxo-5-(4-tolyl)furan-3(2H)-ylidine)]indolin-2-one (3h)
Reddish brown crystals, 76%, m.p. 280–282°C; IR: NH at 3,397.96, CH(alicyclic) at 3,031.55–2,923.56, C=O(lactone) at 1,793.47, C=O(amide) at 1,722.12, C=C at 1,639.2 cm−1; MS(EI) m/z 350 (M+ + 2),1HNMR (DMSO, 300 MHz): δ 9.62 (s, 1H, NH), 8.39-7.33 (m, 7H, 2Ar-H), 6.51 (s, 1H, hetero CH ), 3.29 (s, 3H, CH3).
5-Chloro-3-[(2-oxo-5-(4-tolyl)furan-3(2H)-ylidine)]indolin-2-one (3i)
Reddish brown crystals, 80%, m.p. 270–272°C; IR: NH at 3,418.21, CH(alicyclic) at 2,924.52–2,845.3, C=O(lactone) at 1,775.15, C=O(amide) at 1,711.51, C=C at 1,604.48 cm−1; MS(EI) m/z 339 (M+ + 2), 1HNMR (DMSO, 300 MHz): δ 10.01 (s, 1H, NH), 8.21-7.31 (m, 7H, 2Ar-H), 6.42 (s, 1H, hetero CH), 3.30 (s, 3H, CH3), 13CNMR (DMSO, 300 MHz): 171.89, 163.48, 146.82, 146.40, 146.24, 138.40, 136.26, 130.09, 129.25, 129.04, 128.97, 127.24, 126.82, 124.58, 120.93, 112.72, 30.61.
5-Methyl-3-[(2-oxo-5-(4-methoxyphenyl)furan-3(2H)-ylidine)]indolin-2-one (3j)
Reddish brown crystals, 85%, m.p. 252–254°C; IR: NH at 3,424.5, CH(alicyclic) at 2,924.8, C=O(lactone) at 1,773, C=O(amide) at 1,694.3, C=C at 1,652.7 cm−1; MS(EI) m/z 330 (M+ + 2) 1HNMR (DMSO, 300 MHz): δ 10.71 (s, 1H, NH), 8.62-7.08 (m, 7H, 2Ar-H), 6.76 (s, 1H, hetero CH), 3.87-3.80 (s, 3H, OCH3), 3.35 (s, 3H, CH3).
5-Nitro-3-[(2-oxo-5-(4-methoxyphenyl)furan-3(2H)-ylidine)]indolin-2-one (3k)
Reddish brown crystals, 80%, m.p. 256–258°C; IR: NH at 3,422.7, CH(alicyclic) at 3,008.2-2,840.7, C=O(lactone) at 1,769.3, C=O(amide) at 1,720.1, C=C at 1,640.4 cm−1; MS(EI) m/z 363 (M+ − 1),1HNMR (DMSO, 300 MHz): 9.22 (s, 1H, NH), δ 7.91-7.00 (m, 7H, 2Ar-H), 6.41 (s, 1H, hetero CH), 3.86-3.82 (s, 3H, OCH3).
5-Chloro-3-[(2-oxo-(4-methoxyphenyl)furan-3(2H)-ylidine)]indolin-2-one (3l)
Reddish brown crystals, 82%, m.p. 230–232°C; IR: NH at 3,422.7, CH(alicyclic) at 3,065.2-2,839.1, C=O(lactone) at 1,787.9, C=O(amide) at 1,715.7, C=C at 1,650.4 cm−1; MS(EI) m/z 355 (M+ + 2) 1HNMR (DMSO, 300 MHz): δ 10.02(s, 1H, NH), 8.00-6.99 (m, 7H, 2ArH), 6.40-6.39 (s, 1H, hetero CH), 3.85-3.81 (s, 3H, OCH3).
General procedure for (4a–e)
Hydrazide derivatives were prepared via stirring (3g–j,l) (0.01 mol), (1 ml) of hydrazine hydrate in ethanol (20 ml) at room temperature until the color changed. The reaction mixture was filtered off. The solid product was crystallized from ethanol to give (4a–e).
2-[(5-Methyl-2-oxoindolin-3-ylidine)]-4-oxo-4-(4-tolyl)butane hydrazide (4a)
Pale brown crystals, 75%, m.p. 210–212°C; IR: NH2 at 3,334.5-3,272.9, NH at 3,187.6, CH (alicyclic) at 3,032-2,862.2, C=O(ketone) at 1,712.5-1,682.5, C=O(amide) at 1,620.4, C=C at 1,520.66 cm−1 MS(EI) m/z 349 (M+), 1HNMR (DMSO, 300 MHz): δ 10.55 (s, 1H, NH), 10.19 (t, 1H, NH), 7.47-6.71 (m, 7H, 2Ar-H), 4.67-4.54 (d, 2H, NH2), 4.16 (s, 2H, CH2), 3.48-3.30 (s, 6H, 2CH3), 13CNMR (DMSO, 300 MHz): 176.04, 168.98, 150.05, 140.67, 140.67, 140.48, 139.94, 136.73, 130.93, 129.40, 128.99, 126.92, 125.85, 121.15, 108.72, 48.19, 35.70, 20.75.
2-[(5-Nitro-2-oxoindolin-3-ylidine)]-4-oxo-4-(4-tolyl)butanehydrazide (4b)
Pale brown crystals,70%, m.p. 110–112°C; IR: NH2 at 3,408.57–3,359.5, NH at 3,238.09, CH(alicyclic) at 2,971.77–2,926.45, C=O(ketone) at 1,647.88, C=O(amide) at 1,615.41, C=C at 1,558.2 cm−1; MS(EI) m/z 380 (M+), 1HNMR (DMSO, 300 MHz): δ 9.64 (s, 1H, NH), 9.24 (t, 1H, NH), 7.65-7.05(m, 7H, 2Ar-H), 6.62 (d, 2H, NH2), 4.19 (s, 2H, CH2), 3.56-3.31 (s, 3H, CH3).
2-[(5-Chloro-2-oxoindolin-3-ylidine)]-4-oxo-4-(4-tolyl)butanehydrazide (4c)
Pale brown crystals,75%, m.p. 140–142°C; IR: NH2 at 3,410.49–3,317.4, NH at 3,222.2, CH(alicyclic) at 2,971.52–2,962.45, C=O(ketone) at 1,647.48, C=O(amide) at 1,615.44, C=C at 1,515.78 cm−1; MS(EI) m/z 369 (M+), 1HNMR (DMSO, 300 MHz): δ 10.22 (s, 1H, NH), 9.19 (t, 1H, NH),7.67-6.89 (m, 7H, 2Ar-H), 6.86 (d, 2H, NH2), 4.19 (s, 2H, CH2), 3.48 (s, 3H, CH3).
2-[(5-Methyl-2-oxoindolin-3-ylidine)]-4-oxo-4-(4-methoxyphenyl)-butane hydrazide (4d)
Pale brown crystals,73%, m.p. 162–164°C; IR: NH2 at 3,315.7-3,283.6, NH at 3,186.1, CH(alicyclic) at 3,035.1-2,839.3, C=O(ketone) at 1,702.6, C=O(amide) at 1,609.8, C=C at 1,513.1 cm−1; MS(EI) m/z (M+), 1HNMR (DMSO, 300 MHz): δ 9.99 (s, 1H, NH), 9.63-9.31 (t, 1H, NH), 7.54-6.74 (m, 7H, 2Ar-H), 6.35 (d, 2H, NH2), 4.23 (s, 2H, CH2), 3.81-3.69 (s, 3H, OCH3), 3.30-3.05 (s, 3H, CH3).
2-[(5-Chloro-2-oxoindolin-3-ylidine)]-4-oxo-4-(4-methoxyphenyl)-butane hydrazide (4e)
Pale brown crystals, 75%, m.p. 180–182°C; IR: NH2 at 3,443.2-3,348.7, NH at 3,261.58, CH(alicyclic) at 2,961.16, C=O(ketone) at 1,688.37, C=O(amide) at 1,617.02, C=C at 1,512.88 cm−1; MS(EI) m/z 385 (M+), 1HNMR (DMSO, 300 MHz): δ 10.2 (s, 1H, NH), 9.82 (s, 1H, NH), 7.27-6.88 (m, 7H, 2Ar-H), 6.56-6.53 (d, 2H, NH2), 4.36 (s, 2H, CH2), 3.86-3.81 (s, 3H, OCH3).
General procedure for the synthesis of (5a–d)
Pyridazine derivatives were prepared via reaction of hydrazide derivatives (4a,b,d,e) (0.01 mol) and ethanol (10 ml) by microwave irradiation at 150°C for 3 min. The reaction mixture was cooled, filtered off and the precipitate was crystallized from ethanol to give (5a–d).
5-Methyl-3-[(3-oxo-6-(4-tolyl)-2,3-dihydropyridazin-4(5H)-ylidene)]indolin-2-one (5a)
Orange crystals, 70%, m.p. 220–222°C; IR: NH(broad band) at 3,301-3,247.54, CH(aliphatic) at 3,021.91–2,849, C=O at 1,704.76–1,648.84, C=N at 1,549.52, C=C at 1,503.2 cm−1; MS(EI) m/z 331 (M+),1HNMR (DMSO, 300 MHz): δ 10.28 (s, 1H, NH(oxindole)), 9.48 (s, 1H, NH(pyridazine)), 7.77-6.73 (m, 7H, 2ArH), 4.64 (s, 2H, hetero CH2), 3.42 (s, 6H, 2CH3), 13CNMR (DMSO, 300 MHz): 176.38, 160.19, 141.70, 137.54, 135.00, 130.09, 129.95, 127.64, 125.87, 125.15, 123.08, 116.03, 108.86, 102.02, 100.19, 35.82, 20.86, 20.69.
5-Nitro-3-[(3-oxo-6-(4-tolyl)-2,3-dihydropyridazin-4(5H)-ylidene)]indolin-2-one (5b)
Pale brown crystals, 71%, m.p. 120–122°C; IR: NH(broad band) at 3,392.17, CH(aliphatic) at 2,973.7-2,924.5, C=O at 1,653.66–1,600, C=N at 1,545.96, C=C at 1,513.9 cm−1; MS(EI) m/z 363 (M+ + 1), 1HNMR (DMSO, 300 MHz): δ 9.72 (s, 1H, NH(oxindole)), 9.21 (s, 1H, NH(pyridazine)), 7.92-6.60 (m, 7H, 2ArH), 4.48 (s, 2H, hetero CH2), 3.30 (s, 3H, CH3).
5-Methyl-3-[3-oxo-(6-(4-methoxyphenyl)-2,3-dihydropyridazin-4(5H)-ylidine)]indolin-2-one (5c)
Orange crystals, 70%, m.p. 220–222°C; IR: NH(broad band) at 3,375-3,217.8, CH(aliphatic) at 2,933.3-2,855.5, C=O at 1,655.4, C=N at 1,608.6, C=C at 1,512.9 cm−1; MS(EI) m/z 347 (M+), 1HNMR (DMSO, 300 MHz): δ 9.99 (s, 1H, NH(oxindole), 9.31 (s, 1H, NH(pyridazine)), 7.70-6.35 (m, 7H, 2Ar-H), 4.34 (s, 2H, hetero CH2), 3.83-3.78 (s, 3H, OCH3), 3.40-3.37 (s, 3H, CH3).
5-Chloro-3-[3-oxo-(6-(4-methoxyphenyl)-2,3-dihydropyridazin-4(5H)-ylidine)]indolin-2-one (5d)
Pale brown crystals, 71%, m.p. 118–220°C; IR: NH(broad band) at 3,420.14–3,255.25, CH(aliphatic) at 2,922.59, C=O at 1,654.6, C=N at 1,541.81, C=C at 1,515.8 cm−1; MS(EI) m/z 367 (M+), 1HNMR (DMSO, 300 MHz): δ 10.21 (s, 1H, NH(oxindole)), 9.62 (s, 1H, NH(pyridazine)), 7.97-7.01 (m, 7H, 2ArH), 4.22 (s, 2H, hetero CH2), 3.30 (s, 3H, OCH3).
General procedure for the synthesis of 2-[(1-acetyl-5-alkyl-2-oxo-indolin-3-ylidene)]-N-benzyl-4-oxo-4(4-alkylphenyl)butanamides (6a–f), and N-benzyl-[4-(4-methoxyphenyl)-2-(5-nitro-2-oxo-indolin-3-ylidene)]-4-oxo-butan amide (6g)
A mixture of (3a–c,k) (0.01 mmol) in ethanol (20 ml) and benzyl amine (0.01 mmol) was refluxed for 3 h or stirred at room temperature for 3 h or microwave irradiated for 3 min at 85°C, The products were filtered off and recrystallized from ethyl alcohol to give the amide (6a–g).
2-[(1-Acetyl-5-methyl-2-oxo-Indolin-3-ylidene)]-N-benzyl-4-oxo-4(4-tolyl) butanamide (6a)
Pale brown crystals, 92%, m.p. 125–127°C; IR: NH at 3,292.86, CH(aliphatic) at 3,063.37–2,890.16, C=O(ketone) at 1,629.55, C=O(amide) at 1,563.06, C=C at 1,539.26 cm−1; MS(EI) m/z 467 (M+), 1HNMR (DMSO, 300 MHz): δ 7.79-7.20 (m, 12H, 3ArH), 4.77-4.49 (t, 1H, NH(amide)), 4.36-4.12 (d, 2H, NHCH2), 3.73 (s, 2H, CH2CO), 2.51-2.46 (s, 3H, COCH3), 2.48-2.47 (s, 3H, CH3).
2-[(1-Acetyl-5-nitro-2-oxo-indolin-3-ylidene)]-N-benzyl-4-oxo-4(4-tolyl)butanamide (6b)
Pale brown crystals, 87%, m.p. 110–111°C; IR: NH at 3,292.86, CH(aliphatic) at 3,064.33–2,890.16, C=O(ketone) at 1,629.55, C=O(amide) at 1,563.02, C=C at 1,538.26 cm−1; MS(EI) m/z 497 (M+ − 2), 1HNMR (DMSO, 300 MHz): Δ7.46-7.20 (m, 12H, 3ArH), 4.77 (t, 1H, NH(amide)), 4.23-4.12 (d, 2H, NHCH2), 3.73 (s, 2H, CH2CO), 2.51-2.49 (s, 3H, COCH3), 2.48-2.47 (s, 3H, CH3).
2-[(1-Acetyl-5-chloro-2-oxo-indolin-3-ylidene)]-N-benzyl-4-oxo-4(4-tolyl) butanamide (6c)
Pale brown crystals, 85%, m.p. 110–112°C; IR: NH at 3,292.86, CH(aliphatic) at 3,064.41–2,931.27, C=O(ketone) at 1,629.55, C=O(amide) at 1,563.02, C=C at 1,538.26 cm−1; MS(EI) m/z 486 (M+ + 2), 1HNMR (DMSO, 300 MHz): δ 7.46-7.20 (m, 12H, 3ArH), 4.77 (t, 1H, NH(amide), 4.23 (d, 2H, NHCH2), 3.73 (s, 2H, CH2CO), 2.51-2.5 (s, 3H, COCH3), 2.49 (s, 3H, CH3), 13CNMR (DMSO, 300 MHz):196.21, 171.21, 169.09, 165.09, 150.59, 149.03, 145.48, 140.19, 138.11, 137.79, 137.57, 137.41, 137.34, 137.01, 136.22, 135.79, 132.06, 130,121. 18,73.46, 63.08, 53.60, 49.76, 45.48.
2-[(1-Acetyl-5-methyl-2-oxo-indolin-3-ylidene)]-N-benzyl-4-(4-methoxyphenyl)-4-oxobutanamide (6d)
Pale brown crystals,90%, m.p. 120–121°C; IR: NH at 3,292.86, CH(aliphatic) at 3,064.33–2,895.98, C=O(ketone) at 1,630.52, C=O(amide) at 1,563.02, C=C at 1,533.26 cm−1; MS(EI) m/z 481 (M+ − 1),1HNMR (DMSO, 300 MHz): δ 7.32-7.17 (m, 12H, 3ArH), 4.27 (t, 1H, NH(amide)), 4.12 (d, 2H, NHCH2), 3.88 (s, 3H, OCH3), 3.73 (s, 2H, CH2CO), 2.51-2.49 (s, 3H, COCH3), 2.49-2.45 (s, 3H, CH3).
2-[(1-Acetyl-5-nitro-2-oxo-indolin-3-ylidene)]-N-benzyl-4-(4-methoxyphenyl)-4-oxobutanamide (6e)
Pale brown crystals, 89%, m.p. 118–120°C; IR: NH at 3,291.89, CH(aliphatic) at 3,061.44–2,896.54, C=O(ketone) at 1,628.59, C=O(amide) at 1,564.95, C=C at 1,539.73 cm−1; MS(EI) m/z 515 (M+), 1HNMR (DMSO, 300 MHz): δ 7.8-7.20 (m, 12H, 3ArH), 4.77-4.47 (t, 1H, NH(amide)),4.25-4.12 (d, 2H, NHCH2), 3.92-3.81 (s, 3H, OCH3), 3.72 (s, 2H, CH2CO), 2.51-2.49 (s, 3H, COCH3).
2-[(1-Acetyl-5-chloro-2-oxo-indolin-3-ylidene)]-N-benzyl-4-(4-methoxyphenyl)-4-oxobutanamide (6f)
Pale brown crystals, 88%, m.p. 100–102°C; IR: NH at 3,292.89, CH(aliphatic) at 3,063.48–2,898.12, C=O(ketone) at 1,629.59, C=O(amide) 1,565.92, C=C at 1,544.96 cm−1; MS(EI) m/z 502 (M+),1HNMR (DMSO, 300 MHz): Δ7.32-7.18 (m, 12H, 3ArH), 4.65-4.32 (t, 1H, NH(amide)), 4.13 (d, 2H, NHCH2), 3.80 (s, 3H, OCH3), 3.73 (s, 2H, CH2CO), 2,51-2.49 (s, 3H, COCH3).
N-benzyl-4-[(4-methoxyphenyl)-2-(5-nitro-2-oxo-indolin-3-ylidene)]-4-oxo-butan amide(6g)
Pale brown crystals, 90%, m.p. 100–102°C; IR: NH at 3,292.86, CH(aliphatic) at 3,063.37–2,894.15, C=O(ketone) at 1,631.48, C=O(amide) at 1,565.06, C=C at 1,538.2 cm−1; MS(EI) m/z 471(M+), 1HNMR (DMSO, 300 MHz):δ 7.47-7.18 (m, 12H, 3ArH), 4.77-4.65 (t, 1H, NH), 4.23 (s, 1H, NH), 4.13 (d, 2H, NHCH2), 3.73 (s, 2H, OCH3), 2.50 (s, 3H, CH2CO).
General procedure for the synthesis of 1-acetyl-3[-(1-benzyl-2-oxo)-5-p-tolyl-1,2-dihydropyrrol-3-ylidene)]-5-alkylindolin-2-one(7a,b) and3-[(1-benzyl-5-(4-methoxyphenyl)-2-oxo-1,2-dihydropyrrol-3-ylidine)]-5-nitroindolin-2-one (7c)
Method 1
A mixture of (6b,c,g) (0.01 mmol) and HCl/AcOH (1:1) was refluxed for 3 h or microwave irradiated for 3 min at 120°C. The solid product was filtered off and recrystallized from acetic acid to give the pyrrolone derivatives (7a–c).
Method 2: preparation of (7a)
Benzylamine (0.01 mmol), (3c) (0.01 mmol) and HCl/AcOH (1:1) were added together. The reaction mixture was reacted via microwave irradiation for 3 min at 120°C. The solid product was filtered off and recrystallized from a suitable solvent to give the pyrrolone derivative (7a).
1-Acetyl-3-[(1-benzyl-2-oxo)-5-p-tolyl-1,2-dihydropyrrol-3-ylidene)]-5-chloro indolin-2-one (7a)
White crystals, 82%, m.p. 286–288°C; IR: CH(aliphatic) at 3,000.69, C=O(ketone) at 1,676.68, C=O(amide) at 1,644.8, C=C at 1,593.88 cm−1; MS(EI) m/z 468 (M+), 1HNMR (DMSO, 300 MHz): δ 7.52-7.32 (m, 12H, 3ArH), 4.02 (s, 1H, hetero CH), 3.97 (s, 2H, N-CH2), 3.95-3.93 (s, 3H, COCH3), 2.50-2.49 (s, 3H, CH3).
1-Acetyl-3-[(1-benzyl-2-oxo)-5-p-tolyl-1,2-dihydropyrrol-3-ylidene)]-5-nitro-indolin-2-one (7b)
White crystals, 80%, m.p. 280–282°C; IR: CH(aliphatic) at 2,999.73, C=O(ketone) at 1,770.33, C=O(amide) at 1,683.55, C=C at 1,592.91 cm−1; MS(EI) m/z 474 (M+ − 5), 1HNMR (DMSO, 300 MHz): δ 7.54-7.34 (m, 12H, 3ArH), 4.22 s, 1H, hetero CH), 3.98 (s, 2H, NH2), 3.40 (s, 3H, COCH3), 2.49 (s, 3H, CH3). 13CNMR (DMSO, 300 MHz):172.2, 163.8, 161.4, 151.6, 144.1, 142.08, 141.8, 139.4, 138.2, 134.05, 132.06, 129.86, 128.9, 128.41, 128.23, 126.2, 125.8, 124.8, 123.6, 120.50, 100.2, 42.04, 38.94, 38.66.
3-[(1-Benzyl-5-(4-methoxyphenyl)-2-oxo-1,2-dihydropyrrol-3-ylidine)]-5-nitro- indolin-2-one (7c)
White crystals, 87%, m.p. 270–272°C; IR: NH at 3,216.13, CH(aliphatic) at 2,995.87, C=O(ketone) at 1,672.96, C=O(amide) at 1,642.58, C=C at 1,591.95 cm−1; MS(EI) m/z 450 (M+ − 3), 1HNMR (DMSO, 300 MHz): δ 7.52-7.36 (m, 12H, 3ArH), 4.02 (s, 1H, NH), 4.00 (s, 1H, hetero CH), 3.98-3.96 (s, 2H, N-CH2), 3.45 (s, 3H, OCH3).
General procedure for the synthesis of 4-[(3-(5-alkyl-2-oxindolin-3-ylidene)-5-(4-alkyl-phenyl)-2-oxo-2,3-dihydropyrrol-1-yl)] sulfonamides (8a–k)
A mixture of (3g,h,i,k,l),(0.01 mol), of the appropriate sulfonamide (0.01 mol), and fused sodium acetate (0.01 mol) in acetic acid (10 ml) were reacted via microwave irradiation for 3 min at 120°C, the reaction mixture was cooled, filtered off and the precipitate was washed with water, petroleum ether (b.p. 40–60°C). The solid product was crystallized from the suitable solvent to give (8a–i).
4-[(3-(5-Chloro-2-oxoindolin-3-ylidene)-5-(4-tolyl)-2-oxo-2,3-dihydro-pyrrol-1-yl)]benzene sulfonamide (8a)
Violet crystals, 69%, m.p. 219–220°C, IR: amino group NH(broadband) at 3,400-85 CH(aliphatic) at 2,924.5, C=O(amide) at 1,710.5, C=O(oxopyroline) at 1,601.5 and SO2 at 1,321.9 cm−1, MS(EI) m/z 492 (M+), 1HNMR (DMSO, 300 MHz): δ 10.89 (s, 2H, NH2), 9.48 (s, 1H, NH(oxindole)), 7.85-7.20 (m, 12H, 3Ar-H), 6.82 (s, 1H, CH(hetero)) and 2.49-2.39 (s, 3H, CH3).
4-[(3-(5-Naitro-2-oxindolin)-3-ylidene)-5-(4-methoxyphenyl)-2-oxo-2,3-dihydro-pyrrol-1-yl)] benzene sulfonamide (8b)
Violet crystals, 72%, m.p. 268–269°C, IR: NH(broadband) at 3,417.24, CH(aliphatic) at 2,921.63, C=O(amide) at 1,673.51, C=O(oxopyroline) at 1,608.34, and SO2 at 1,333.53 cm−1. MS(EI) m/z 519 (M+) 1HNMR (DMSO, 300 MHz): δ 11.42 (s, 2H, NH2), 10.32 (s, 1H, NH(oxindole)), 7.98-7.02 (m, 12H, 3Ar-H), 6.97-6.95 (s, 1H, CH(hetero)), and 3.85-3.77 (s, 3H, OCH3).
4-[(3-(5-Chloro-2-oxindolin-3-ylidene)-5-(4-methoxyphenyl)-2-oxo-2,3-dihydro-pyrrol-1-yl)] benzene sulfonamide (8c)
Violet crystals, 70%, m.p. 220–222°C, IR: NH(broadband) at 3,406.6, CH(aliphatic) at 2,929.3, C=O(amide) at 1,715.3, C=O(oxopyroline) at 1,602.56 and SO2 at 1,302.6 cm−1, MS(EI) m/z 508 (M+), 1HNMR (DMSO, 300 MHz): δ 10.88 (s, 2H, NH2), 10.02 (s, 1H, NHoxindole), 7.98-7.02 (m, 12H, 3Ar-H), 6.98-6.82 (s, 1H, CH(hetero)), and 3.89-3.79 (s, 3H, OCH3).
4-[(3-(5-Chloro-2-oxindolin-3-ylidene)-5-(4-tolyl)-2-oxo-2,3-dihydropyrrol-1-yl)] phenyl sulfonyl guanidine (8d)
Violet crystal, 65%, m.p. 240–242°C, IR: NH(broadband) at 3,433.6, CH(aliphatic) at 2,922.5, C=O(amide) at 1,734.08, C=O(oxopyroline) at 1,712.48, C=N at 1,617.98 and SO2 at 1,402 cm−1, MS(EI) m/z 535 (M+), 1HNMR: δ 10.84 (s, 2H, 2NH of NHCNHNH2), 10.08 (s, 2H, NH2), 7.87-7.18 (m, 12H, 3Ar-H), 6.73 (s, 1H, CH(hetero), and 2.49-2.29 (s, 3H, CH3).
4-[(3-(5-Nitro-2-oxindolin-3-ylidene)-5-(4-methoxyphenyl)-2-oxo-2,3-di-hydropyrrol-1-yl)] phenyl sulfonyl guanidine (8e)
Violet crystals, 64% , m.p. 273–274°C, IR: NH(broadband) at 3,433.64, CH(aliphatic) at 2,924.52, C=O(amide) at 1,721.52, C=O(oxopyroline) at 1,692.4, C=N at 1,612.2, and SO2 at 1,332.57 cm−1, MS(EI) m/z560 (M+), 1HNMR: δ 10.22 (s, 1H, NH(oxindole)), 10.19 (s, 2H, 2NH of NHCNHNH2), 9.99 (s, 2H, NH2), 7.93-6.79 (m, 12H, 3Ar-H), 6.69 (s, 1H, CH(hetero)), and 3.83-3.77 (s, 3H, OCH3).
4-[(3-(5-Chloro-2-oxindolin-3-ylidene)5-(4-methoxyphenyl)-2-oxo-2,3-dihydropyrrol-1-yl)]phenyl sulfonyl guanidine (8f)
Violet crystals, 60%, m.p. 200–202°C, IR: NH(broadband) at 3,376.75, CH(aliphatic) at 2,931.27, C=O(amide) at 1,734.07, C=O(oxopyroline) at 1,712.48, C=N at 1,603.52 and SO2 at 1,300.75 cm−1, MS(EI) m/z 550 (M+, 1HNMR): δ 10.88 (s, 1H, NH(oxindole)), 10.82 (s, 2H, 2NH NHCNHNH2), 10.02 (s, 2H, NH2), 7.95-6.84 (m, 12H, 3Ar-H), 6.48 (s, 1H, CH(hetero)), and 3.86-3.84 (s, 3H, OCH3).
4-[(2-(3-(5-Methyl-2-oxindolin-3-ylidene)-5-(4-tolyl)-2-oxo-2,3-di-hydropyrrol-1-yl) ethyl)]benzene sulfonamide (8g)
Violet crystals, 68%, m.p. 262–264°C, IR: NH of NH2 at 3,391.17–3,257.18, CH(aliphatic) at 2,925.48, C=O(amide) at 1,677.97, C=O(oxopyroline) at 1,584.24, and SO2 at 1,325.82 cm−1, MS(EI) m/z 498 (M+ + 1), 1HNMR: δ 10.60 (s, 1H, NH(oxindole)), 9.28 (s, 2H, NH2), 7.65-7.13 (m, 11H, 3Ar-H), 6.75-6.72 (s, 1H, CH(hetero)), 3.94-3.37 (t, 4H, 2CH2), and 3.31-3.25 (s, 6H, 2CH3). 13CNMR (DMSO): δ 169.99, 169.48, 152.79, 142.27, 142.14, 141.43, 140.09, 133.24, 132.46, 129.94, 129.56, 128.96, 127.97, 127.39, 125.91, 125.68, 121.53, 120.53, 109.32, 101.95, 41.61, 33.95, 20.99, 20.91.
4-[(2-(3-(5-Nitro-2-oxindolin-3-ylidene)-5-(4-tolyl)-2-oxo-2,3-dihydropyrrol-1-yl) ethyl)] benzene sulfonamide (8h)
Violet crystals, 73%, m.p. 255–256°C, IR: NH of NH2 at 3,405.67 (broad band), CH(aliphatic) at 2,925.48, C=O(amide) at 1,687.41, C=O(oxopyrolene) at 1,613.16 and SO2 at 1,336.43 cm−1, MS(EI) m/z 532 (M+), 1HNMR: δ 12.22 (s, 1H, NH(oxindole)), 11.41 (s, 2H, NH2), 8.24-7.02 (m, 11H, 3Ar-H), 6.99-6.63 (s, 1H, CH(hetero)), 4.09-3.76 (t, 4H, 2CH2) and 3.28-2.81 (s, 3H, CH3).
4-[(2-(3-(5-Chloro-2-oxindoline-3-ylidene)-5-(4-tolyl)-2-oxo-2,3-dihyd-ropyrro-l-1-yl) ethyl)]benzene sulfonamide (8i)
Violet crystals, 70%, m.p. 232–334°C, I.R. NH of NH2 at 3,423.03 (broad band), CH(aliphatic) at 2,925.48, C=O(amide) at 1,678.73, C=O(oxopyroline) at 1,612.2 and SO2 at 1,337.39, MS(EI) m/z 520 (M+), 1HNMR: δ 11.88 (s, 1H, NH(oxindole)), 10.82 (s, 2H, NH2), 8.00-7.09 (m, 11H, 3Ar-H), 6.86 (s, 1H, CH(hetero)), 4.09-3.68 (t, 4H, 2CH2), and 3.32 (s, 3H, CH3).
4-[(2-(3-(5-Nitro-2-oxindolin-3-ylidene)-5-(4-methoxyphenyl)-2-oxo-2,3-di-hydro-pyrrol-1-yl) ethyl)] benzene sulfonamide (8j)
violet crystal, 70%, m. p. 279–280°C, IR. NH of NH2 at 3,422.06 (broad band), CH(aliphatic) at 2,914.89, C=O(amide) at 1,652.83, C=O(oxopyroline) at 1,609.3, and SO2 at 1,334.5, MS(EI) m/z 548 (M+), 1HNMR: δ 11.4 (s, 1H, NHoxindole), 9.92 (s, 2H, NH2), 7.74-7.01 (m, 11H, 3Ar-H), 6.92 (s, 1H, CH(hetero)), 4.09-3.82 (t, 4H, 2CH2), and 3.78-3.29 (s, 3H, OCH3).
4-[(2-(3-(5-Chloro-2-oxindolin-3-ylidene)-5-(4-methoxyphenyl)-2-oxo-2,3-dihydro-pyrrol-1-yl)ethyl)]benzene sulfonamide (8k)
Violet crystals, 78%, m.p. 279–280°C, I.R. NH of NH2 at 3,437.49–3,347.82, CH(aliphatic) at 2,928.38, C=O(amide) at 1,715.56, C=O(oxopyroline) at 1,608.34, and SO2 at 1,376.93, MS(EI) m/z 534 (M+ − 1), 1HNMR: δ 10.86 (S, 1H, NH(oxindole)), 10.19 (s, 2H, NH2), 7.77-6.86 (m, 11H, 3Ar-H), 6.74-6.52 (s, 1H, CH(hetero)), 3.87-3.76 (t, 4H, 2CH2), and 3.31 (s, 3H, OCH3).
Antimicrobial evaluation
Most of the newly synthesized compounds in addition to the reference drugs Chloramphenicol and Nystatin were screened for their antibacterial and antifungal activities using the agar well diffusion techniqueCitation36. The microorganisms (reference and clinical isolates) used included Gram-negative E. coli (ATCC-25922) and S. flexeniri, Gram-positive Staphylococcus aureus (ATCC-25923), Bacillus cereus, and fungi Aspergillus flavus and Candida albicans (ATCC 10231). For the antibacterial assay, a standard inoculum (105 CFU/mL) was distributed on the surface of sterile nutrient agar plates by a sterile glass spreader. For the antifungal assay a loopful of a particular fungal strain was transferred to 3 mL of saline to get a suspension of the corresponding species. Then 0.1 mL of the spore suspension was distributed on the surface of sterile Sabouraud dextrose agar plates.
Six millimeter diameter wells were punched in the agar media and filled with 100 μL (500 μg/mL in DMSO) of the tested chemical compounds previously sterilized through 0.45 sterile membrane filterCitation38. The plates were kept at room temperature for 1 h and then incubated at 37°C for 24 h for bacteria and 30°C for 4 days for fungi.
The antimicrobial activities were evaluated by measuring the inhibition zone diameters (mm). Commercial antibiotic discs were used as positive reference standard to determine the sensitivity of the strains.
Determination of MIC of the synthesized compounds
Compounds inhibiting the growth of one or more of the above microorganisms were further tested for their MIC and were determined by broth dilution techniqueCitation37. The nutrient broth and the yeast extract broth media, which contained 1 mL of different concentrations of the tested compounds (2.5, 5, 10, 15, 20, 25 μg/mL) were inoculated with the microbial strains. The bacterial cultures were incubated for 24 h at 37°C, whereas the fungal ones were incubated at 30°C for 48 h. The growth was monitored spectrophotometrically. The lowest concentration required to arrest the microbial growth was regarded as MICs (μg/mL).
Conclusion
We have reported a convenient microwave-enhanced, high speed, short, and economic way for the synthesis of a new series of furanones and study their reactivity towards some nucleophiles with the hope for preparing new antimicrobial and antifungal agents. The data of MICs of the in vitro antimicrobial evaluation of the aroylpropionic acids 2a,b furanone derivatives 3a–f and the sulfonamide derivatives 8a–f against several pathogenic bacterial strains revealed that compound (8j) showed significant activity against bacterial species. Its activity was comparable to the reference chloramphenicol.
Declaration of interest
The authors declared no conflicts of interest.
References
- Albrecht A, Koszuk JF, Modranka J, Rózalski M, Krajewska U, Janecka A et al. Synthesis and cytotoxic activity of gamma-aryl substituted alpha-alkylidene-gamma-lactones and alpha-alkylidene-gamma-lactams. Bioorg Med Chem 2008;16:4872–4882.
- Moosavi-Movahedi AA, Hakimelahi S, Chamani J, Khodarahmi GA, Hassanzadeh F, Luo FT et al. Design, synthesis, and anticancer activity of phosphonic acid diphosphate derivative of adenine-containing butenolide and its water-soluble derivatives of paclitaxel with high antitumor activity. Bioorg Med Chem 2003;11:4303–4313.
- Levy LM, Cabrera GM, Wright JE, Seldes AM. 5H-furan-2-ones from fungal cultures of Aporpium caryae. Phytochemistry 2003;62:239–243.
- Vale-Silva LA, Buchta V, Vokurková D, Pour M. Investigation of the mechanism of action of 3-(4-bromophenyl)-5-acyloxymethyl-2,5-dihydrofuran-2-one against Candida albicans by flow cytometry. Bioorg Med Chem Lett 2006;16:2492–2495.
- Lattmann E, Dunn S, Niamsanit S, Sattayasai N. Synthesis and antibacterial activities of 5-hydroxy-4-amino-2(5H)-furanones. Bioorg Med Chem Lett 2005;15:919–921.
- Khan MS, Husain A. Syntheses and reactions of some new 2-arylidene-4-(biphenyl-4-yl)-but-3-en-4-olides with a study of their biological activity. Pharmazie 2002;57:448–452.
- Husain A, Khan MS, Hasan SM, Alam MM. 2-Arylidene-4-(4-phenoxy-phenyl)but-3-en-4-olides: synthesis, reactions and biological activity. Eur J Med Chem 2005;40:1394–1404.
- Leite L, Jansone D, Veveris M, Cirule H, Popelis Y, Melikyan G. 2-Arylidene-4-(4-phenoxy-phenyl)but-3-en-4-olides: Synthesis, reactions and biological activity. Eur J Med Chem 1999;34:859–865.
- Black WC, Brideau C, Chan CC, Charleson S, Cromlish W, Gordon R et al. 3,4-Diaryl-5-hydroxyfuranones: highly selective inhibitors of cyclooxygenase-2 with aqueous solubility. Bioorg Med Chem Lett 2003;13:1195–1198.
- Zarghi A, Praveen Rao PN, Knaus EE. Synthesis and biological evaluation of methanesulfonamide analogues of rofecoxib: Replacement of methanesulfonyl by methanesulfonamido decreases cyclooxygenase-2 selectivity. Bioorg Med Chem 2007;15:1056–1061.
- Hashem AI, Youssef AS, Kandeel KA, Abou-Elmagd WS. Conversion of some 2(3H)-furanones bearing a pyrazolyl group into other heterocyclic systems with a study of their antiviral activity. Eur J Med Chem 2007;42:934–939.
- Harvey SC. (1985). Ramington’s pharmaceutical science. Easton, PA: Mack Publishing Company, 1189.
- Shin SS, Byun Y, Lim KM, Choi JK, Lee KW, Moh JH et al. In vitro structure-activity relationship and in vivo studies for a novel class of cyclooxygenase-2 inhibitors: 5-aryl-2,2-dialkyl-4-phenyl-3(2H)furanone derivatives. J Med Chem 2004;47:792–804.
- Lau CK, Brideau C, Chan CC, Charleson S, Cromlish WA, Ethier D, Gauthier JY, Gordon R, Guay J, Kargman S, Li CS, Prasit P, Riendeau D, Therien M, Visco DM, Xu L. Synthesis and biological evaluation of 3-heteroarizoxy-4-phenyl-2(5H)-furanones as selective COX-2 inhibitors. Bioоrg Med Chem Lett 1999;9:3187–3192.
- Lattmann E, Kinchington D, Dunn S, Singh H, Ayuko WO, Tisdale MJ. Cytotoxicity of 3,4-dihalogenated 2(5H)-furanones. J Pharm Pharmacol 2004;56:1163–1170.
- Heindel ND, Minatelli JA. Synthesis and antibacterial and anticancer evaluations of alpha-methylene-gamma-butyrolactones. J Pharm Sci 1981;70:84–86.
- Mologni L, Rostagno R, Brussolo S, Knowles PP, Kjaer S, Murray-Rust J et al. Synthesis, structure-activity relationship and crystallographic studies of 3-substituted indolin-2-one RET inhibitors. Bioorg Med Chem 2010;18:1482–1496.
- Beauchard A, Laborie H, Rouillard H, Lozach O, Ferandin Y, Le Guével R et al. Synthesis and kinase inhibitory activity of novel substituted indigoids. Bioorg Med Chem 2009;17:6257–6263.
- Zhang W, Go ML. Functionalized 3-benzylidene-indolin-2-ones: inducers of NAD(P)H-quinone oxidoreductase 1 (NQO1) with antiproliferative activity. Bioorg Med Chem 2009;17:2077–2090.
- Wee XK, Yeo WK, Zhang B, Tan VB, Lim KM, Tay TE et al. Synthesis and evaluation of functionalized isoindigos as antiproliferative agents. Bioorg Med Chem 2009;17:7562–7571.
- Andreani A, Bellini S, Burnelli S, Granaiola M, Leoni A, Locatelli A et al. Substituted E-3-(3-indolylmethylene)-1,3-dihydroindol-2-ones with antitumor activity. In depth study of the effect on growth of breast cancer cells. J Med Chem 2010;53:5567–5575.
- Sun L, Liang C, Shirazian S, Zhou Y, Miller T, Cui J et al. Discovery of 5-[5-fluoro-2-oxo-1,2- dihydroindol-(3Z)-ylidenemethyl]-2,4- dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinase. J Med Chem 2003;46:1116–1119.
- Sun CL, Christensen JG, McMahon G. (2009). In: Li R, Stafford JA. eds. Kinase Inhibitor Drugs. Hoboken, New Jersey: John Wiley & Sons Inc., Chapter 1.
- Mohamed MI. Synthesis and antibacterial activity of some novel heterocycles. J Bulg Chem Commun 2004;36:241–248.
- Mohamed MI, Zaky HT, Kandile NG. Novel synthesis and antibacterial activity of some pyridazine derivatives. J Chinese Chem Soc 2004;51:963–968.
- Mohamed MI, Zaky HT, Mohamed HM, Kandile NG. Novel heterocyclic systems of pyridazines. Afinidad 2005;62:48–56.
- Mohamed MI. Synthesis and antimicrobial activity of new pyridazinyl sulfonamide derivatives. J Bulg Chem Commun 2007;39:152–158.
- Kandile NG, Mohamed MI, Zaky HT, Mohamed HM. Novel pyridazine derivatives: Synthesis and antimicrobial activity evaluation. Eur J Med Chem 2009;44:1989–1996.
- Kandile NG, Mohamed MI, Zaky HT, Mohamed HM. Silver nanoparticles effect on antimicrobial and antifungal activity of new heterocycles. Bull Korean. Chem Soc 2010;31:3530–3538.
- El Abbady AM, Omara MA, Kandile NG. Condensation of β-Aroylpropionic Acids With Isatin Under Perkin Conditions. Rev Roum Chem 1974;19:79–82.
- Hamad A-SS, Kandile NG, Zaky HT, Mohamed MI, Ahmed HM. Conversion of 2(3H)-furanones bearing indole nuclei into novel amide, pyrrolone, and imidazole derivatives. Heteroatom Chem 2003;14:434–442.
- Hamad A-SS, Kandile NG, Zaky HT, Mohamed MI, Ahmed HM. 2(3H)-Furanones as synthons for polyamides of 1,3-diazines and 1,3,5-triazines. Arkivok 2006;10:162–172.
- Zaky HT. New oxazolones as synthons for antimicrobial compounds. J Bulg Chem Commun 2007;39:134–141.
- Zaky HT. 3,1- Benzothiazine,benzimidazol,amide and quinazoline derivatives via 2(3H) Furanones. J Bulg Chem Commun 2006;38:248–254.
- Kandile NG, Zaky HT, Mohamed MI, Ismaeel HM, Ahmed NA.. Synthesis, characterization and in vitro antimicrobial evaluation of new compounds incorporating oxindole nucleus. J Enz Inhib Med Chem ,Early online 1-10 (2011).
- Ahmad S, Rathish IG, Bano S, Alam MS, Javed K. Synthesis and biological evaluation of some novel 6-aryl-2-(p-sulfamylphenyl)-4,5-dihydropyridazin-3(2H)-ones as anti-cancer, antimicrobial,and anti-inflammatory agents. J Enzyme. Inhib Med Chem 2010;25:266–271.
- Tadeg H, Mohammed E, Asres K, Gebre-Mariam T. Antimicrobial activities of some selected traditional Ethiopian medicinal plants used in the treatment of skin disorders. J Ethnopharmacol 2005;100:168–175.
- Karthikeyan SM, Prasad JD, Mahalinga M, Holla SB, Kumari SB. Antimicrobial studies of 2,4-dichloro-5-fluorophenyl containing oxadiazoles. Eur J Med Chem 2008;43:25–31.