Abstract
The plant-derived terpenoids are considered to be the most potent anticancer, anti-inflammatory and anticarcinogenic compounds known. Enzymatic biotransformation is a very useful approach to expand the chemical diversity of natural products. Recent enzymatic biotransformation studies on terpenoids have resulted in the isolation of novel compounds. 14-hydroxy methyl caryophyllene oxide produced from caryophyllene oxide showed a potent inhibitory activity against the butyryl cholinesterase enzyme, and was found to be more potent than parent caryophyllene oxide. The metabolites 3β,7β-dihydroxy-11-oxo-olean-12-en-30-oic acid, betulin, betulonic acid, argentatin A, incanilin, 18β glycyrrhetinic acid, 3,11-dioxo-olean-12-en-30-oic acid produced from 18β glycyrrhetinic acid were screened against the enzyme lipoxygenase. 3,11-Dioxo-olean-12-en-30-oic acid, was found to be more active than the parent compound. The metabolites 3β-hydroxy sclareol 18α-hydroxy sclareol, 6α,18α-dihydroxy sclareol, 11S,18α-dihydroxy sclareol, and 1β-hydroxy sclareol and 11S,18α-dihydroxy sclareol produced from sclareol were screened for antibacterial activity. 1β-Hydroxy sclareol was found to be more active than parent sclareol. There are several reports on natural product enzymatic biotransformation, but few have been conducted on terpenes. This review summarizes the classification, advantages and agents of enzymatic transformation and examines the potential role of new enzymatically transformed terpenoids and their derivatives in the chemoprevention and treatment of other diseases.
Introduction
In plants, terpenoids represent a chemical defence against environmental stress and provide a repair mechanism for wounds and injuries. Interestingly, effective ingredients in several plant-derived medicinal extracts are terpenoid compounds of monoterpenes, sesquiterpenoids, diterpenoids and triterpenoid groups. Terpenes have a widespread occurrence and can be found in all organisms, i.e., in prokaryotic as well as eukaryotic systems. However, most of the bioactive terpenes have been detected in higher plants. Whereas mono- and sesquiterpenes are predominantly found in essential oils of plant raw material, the higher terpenes, such as triterpenes, are mainly found in balsams and resinsCitation1–4.

From a biological perspective, it is assumed that the most important triterpenoid structures are the oleanane (2), ursane (1), lupine (3) and cycloartane–euphane (58a-58n) carbon skeletons. The global market for plant-derived drugs has been estimated to be approximately 30.69 billion USD for the last decade, with phytochemicals such as terpenes and steroids representing the most significant fraction with estimated annual sales of 12.4 billion USDCitation5. A large number of triterpenoids have been synthesized by structural modification of natural compounds for optimization of bioactivity. An increasing number of triterpenoids have been reported to exhibit cytotoxicity against a variety of cancer cells without manifesting any toxicity in normal cellsCitation6–8. They also demonstrate antitumour efficacy in preclinical animal models of cancerCitation7,Citation8. Ursolic acid (1) inhibits growth of tumor cells both in vitro and in vivo by decreasing proliferation of cells and inducing apoptosisCitation9. It was converted into oleanolic acid methyl ester via two intermediates, oleanolic acid, and ursolic acid methyl esterCitation10.
Cucurbitacin ICitation10 has been found to inhibit the proliferation of MDA-MB-468 human breast cancer cells that express constitutively activated STAT3Citation11 and also reduced the levels of phosphotyrosine of constitutively activated STAT3 and induced apoptosis in the same tumour modelCitation12. Cucurbitacin Q induces apoptosis more potently in MDA-MB-435 human breast cancer cells. Cucurbitacin D analog, 2-deoxycucurbitacin D as well as cucurbitacin D and 25-acetylcucurbitacin have exhibited potent cytotoxic activity against MCF-7 human breast cancer cellsCitation13. Cucurbitacin B, D, E and I, displayed antitumour effects with specific cyclooxygenase-2 (COX-2) enzyme inhibition; however, cucurbitacin B has been found to be the most potent compoundCitation14.
23,24-Dihydrocucurbitacin F and 23,24-dihydro-25-acetylcucurbitacin F have shown moderate cytotoxic activity against MCF-7 cellsCitation15. 23,24-Dihyderocucurbitacin B, possesses anti-cancer activity in a dose- and time-dependent manner against human breast cancer cell lines including Bcap37 and MCF-7. This triterpenoid exerts cell cycle arrest at G2/M phase and induces apoptosis in Bcap37 cells through mitochondria-dependent pathwayCitation16,Citation17.
Cucurbitacin B exhibited antiproliferative effects against all the cell lines tested. MDA-MB-435 was found to be the most sensitiveCitation18. Balsaminapentanol, balsaminol A, balsaminol B and cucurbalsaminol B have suppressed the growth of MCF-7 cells with varying potencyCitation19. Four dammarane triterpenoids showed weak anti-breast cancer activity in vitroCitation20. Three ergostane-type triterpenoidsCitation21 displayed potent cytotoxic effects against MCF-7 as well as MDA-MB-231 cellsCitation22. Tingenone and netzahualcoyonol, exhibited cytotoxicity against MDA-MB-231 cellsCitation23. Triterpenoids, celastrol and its methyl ester pristimerin are significantly active against the proliferation of MCF-7 cell linesCitation24. Mechanistic studies have revealed that pristimerin induces rapid apoptosis characterized by caspase activation, decrease of mitochondrial membrane potential and a rapid release of cytochromeCitation25.
Celastrol triterpenoid has shown to inhibit the growth and induce apoptosis of W256 cells as evidenced by caspase-3 activation and nuclear morphology. It also abolished interleukin-1β and tumour necrosis factor-α (TNF-α)-induced IκB phosphorylation, IκB kinase activation and prevented nuclear translocation of nuclear factor-kappa B (NF-κB) and DNA binding reduced the expression of matrix metalloproteinase-9 (MMP-9) and urinary plasminogen activator leading to inhibition of migration of W256 cellsCitation26. It has been reported to potentiate tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) induced cytotoxicity and apoptosis of T47D and MDA-MB-231 cells. It also downregulated the expression of cell survival proteins, namely cFILP, IAP-1, Bcl-2, Bcl-xL, survivin and XIAP and upregulated Bax expression. The cell surface expression of both the tumour necrosis factor-related apoptosis-inducing ligand receptors–death receptor 4 (DR4) and (DR5) have also been found to be induced by celastrol treatment. Sulphurenic acid and its analog 15α-acetyl-dehydrosulphurenic acid have also shown antitumour efficacy with both compounds being more potent against MDA-MB-231 cells as compared with MCF-7 cells. Limonoid was found to be cytotoxic against MCF-7 cellsCitation27,Citation28. Other limonoids, melianin B, melianin C and meliavolkinin are active in killing MCF-7 cellsCitation29.
Betulinic acid has shown antiproliferative activities toward MCF-7 as well as MDA-MB-231 cellsCitation30,Citation31. It also exhibited a remarkable cytotoxic effect against T47D cells with a simultaneous induction of apoptosis, decrease of Bcl-2 and cyclin D1 and increase of Bax gene expressionCitation32. Basu and colleaguesCitation33 have demonstrated lethal effects of betulinic acid in another breast cancer cell line SKBR3. Betulinic acid has been shown to induce early apoptotic events accompanied by flopping of phosphatidylserine on the outer lamella of the plasma membrane as evidenced by elegant fluorescent microscopy. The mitochondrial signalling pathway has been implicated in the observed apoptosis-inducing property of betulinic acid. Using nine breast cancer cell linesCitation34 have confirmed the antitumour effects of betulinic acid against mammary carcinoma with various degree of sensitivity. Betulin (53) inhibits the proliferation of T47D breast carcinoma cellsCitation35. Lupeol (3) has caused significant inhibition of growth of MDA-MB-231 cells. It has also been able to induce ERα expression in this ERα-negative breast cancer cell lineCitation36.
The extensive literature on the biotransformation of different types of terpenoid molecules were reviewed, which have verified that biotransformed metabolites suppressed the process of inflammation and cancer. Terpenoids have been a very promising chemotherapeutic agent for the treatment of HIV infection, cancers and other pharmacological studies37. Bioconversion of bioactive natural products produces metabolites with an enhanced bioactivity and low toxicityCitation38–40. Apart from the previous study, there are no detailed available data on the biotransformation of terpenes. This review examines the potential role of natural terpenoids and their biotransformed products. The substrates discussed in this review were xenobiotic in nature, which enhances the substrate flexibility towards the microbial enzymatic system.
Biotransformation
Biotransformation is defined as the use of biological systems to bring about structural changes in chemical compounds that are not their natural substrates. This distinguishes biotransformation from biosynthesis, which is concerned with the synthetic ability of biological systems in their normal habitat.
Classification of biotransformation
Biotransformation may be classified into two different categories as under:
The first type of transformation is “xenobiotic transformation” in which the substrate is completely alien to the microorganism.
The second type of transformation is “biogenetically directed”, and is also known as “analogue biosynthesis”, in which the substrate is a structural analogue of a biosynthetic intermediate. This biotransformation relies on the substrate flexibility of the enzymatic pathway responsible for the secondary metabolic biosynthesis.
Advantages of biotransformation
Biotransformation has a number of advantages when compared with the corresponding chemical methods. Many biotransformation not only are regio- and stereo specific, but are also enantiospecific, allowing the production of chiral products from racemic mixtures. The conditions for biotransformations are mild and in the majority of cases, they do not require the protection of pre-existing functional groups.
Furthermore, the features governing their regiospecificity differ from those controlling the chemical specificity, and indeed it is possible to obtain structural transformation at positions that are chemically unreactive. Biosynthetically-directed transformations are also used to obtain biosynthetic informations, the design of selective biosynthetic inhibitors. The dominant feature in any biotransformation is the topological relationship between the substrate and the active site of the enzyme. From a commercial point of view, some biotransformations can be cheaper and more direct than their chemical analogues, while the transformations proceed under conditions that are normally regarded as environmentally friendly.
Agents of biotransformation
A great challenge for a specific biotransformation of a certain substrate is to find the appropriate microorganism, so that, traditionally, one of the most widely used techniques is screening with different microbial strains. Nowadays, biotransformation of compounds is performed by employing following systems:
By using microbes (fungi and bacteria)
By using plant and animal cell cultures
By using animals
Use of fungi in biotransformation
Fungi are playing a prominent role in the catalysis of organic compounds and in the production of commercially and industrially important compounds, because of their ability to catalyze novel reactions. Fungi are commonly used in industry for production of fermented beverages, foods, physiologically active substances, solvents, organic acids, polysaccharides, antibiotics etcCitation41. Of the zygomycota, Mucor and Rhizopus are commonly used in industry. Rhizopus strains are important in citric acid production. Mucor strains make a significant number of important lipases, and catalyze the hydroxylation of a wide range of chemical compounds. In a survey of fungi with reference to their ability to hydroxylate the polycyclic aromatic hydro carbons naphthalene, 55 species of fungi were found to posses the same activityCitation42.
Microbial enzymes and their reactions
In these biotransformation processes, several reactions that are difficult to achieve by chemical means have been accomplished such as:
Microbial enzymes (produced by micro organisms) show a diverse range of reactions including the insertion of oxygen into C-H and C-C bonds, introduction of hydroxyl groups into remote positions of terpenes; selective cleavage of the side chains of tetracyclic terpenoids to produce C19 steroids; regioselective glycosidic transfer reactions; selective ring cleavage through a Baeyer-Villiger-type oxidation to render seco-triterpenoids, and carbon skeleton rearrangements involving a methyl group migration, addition of oxygen to alkene, hydrolysis or formation of amides, transferring of acyl or sugar units from one substrate to another, epoxide, ester and nitriles hydrogenation, hydration and elimination of small units, racemization, epimerization and isomerization reactions and formation of C-C, C-O, C-N, and C-S bondsCitation43. Reduction of carboxyl groups and hydroxylation at non active sites are among the most valuable reactions catalyzed by the micro organisms.
Reduction/hydroxylation reactions
The enzymatic reduction takes place with a regio and stereo chemical homogeneity that are difficult to achieve chemically. These enzymatic methods possess an enantioselectivity which in many cases can be predicted on the basis of empirical rules. The reduction of a carbonyl, particularly to generate a new chiral center, is one of the most widely used biotransformations.
When a compound is incubated with microorganisms, a hydroxyl group may be inserted at a site that is remote from any other functionalities. This ability of micro organisms to hydroxylate compounds at chemically inactive sites is a valuable synthetic tool. The intact organism is normally used for these transformations because many of the enzyme systems that are involved are membrane bound and are consequently difficult to obtain in a stable form. The value of microbiological hydroxylation lies in the fact that the sites of hydroxylation are often different from those at which normal chemical reactions occur.
Monooxygenase are enzymes responsible for the microbiological hydroxylation and transfer of one oxygen atom to the organic substrateCitation44. The catalytic cycle of the enzymes has been deduced largely from a study of camphor hydroxylating enzymes isolated from Pseudomonas putidaCitation45.
Use of plant cell cultures in biotransformations
It has been reported that the formation and the accumulation of some secondary metabolites does not normally occur in the cultured cells of higher plantsCitation46–49, and it is found to be difficult to harness this potential to organic synthesis or industrial processes. To overcome these problems, the biotransformation of exogenous substrates mainly by the micro organism has extensively been investigated. In addition, plant cultured cells are also used to transform substrate.
Many studies have recently been focussed on the ability of the plant cultured cells to transform xenobiotic compoundsCitation50–54. Plant cultured cells have the potential to be used as an excellent tool for the organic synthesis. The advantages of using the plant cultured cells as biocatalysts are: (i) The plant cultured cells can be grown in the laboratory. The cultured material is homogenous, and experiments can be performed and reproduced in all seasons. (ii) The plant cultured cells can accumulate high amounts of transformed products. (iii) The plant cultured cells can also be grown to an almost unlimited quantities of material in bioreactor.
Use of plant bacteria in biotransformations
Bacterial transformation involves changes in the structure of organic compounds through degradation pathways due to which many biologically inactive or compounds with low activities can be converted into more potent bioactive compoundsCitation55,Citation56.
Microbial enzymatic transformation of terpenes
The antitumour efficacy of several triterpenoids are currently being evaluated in phase I clinical trialsCitation8. Several reviews on microbial transformation of terpenoids have been published in recent yearsCitation57–60.
Microbial enzymatic transformation of some sesquiterpenes
The biotransformation of terpenoids is an important field of toxicology and xenobiochemistryCitation61. These biotransformations have been used as in vitro models to mimic and predict the mammalian metabolism of biologically active triterpenoids. Several sesquiterpenoids have displayed analeptic, anthelmentic, anti-inflammatory, antitumor, hypotensive and sedative activities.
Terpenoidal substrates showed a variety of stereoselective reactions mainly oxidation, reduction, C-C bond formation, esterification etc. with various fungal cultures. Hydroxylation was found to be the most common reaction in sesquiterpenes pyrethrosin (5), plectranthone (6)Citation62, costunolide (8)Citation38,Citation63 and partheniol (7) () afforded mainly their hydroxylated derivatives with various fungal strains. Published data related to the microbial enzyme transformation of terpenes showed higher susceptibility of this class of natural products towards the microbial enzymatic systemCitation64.
Ambrein oxidation and enzymatic transformation of ambrox
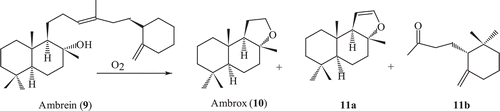
Ambrein (9) is the major constituent of the ambergris and possesses an amber-like odour. During drifting in the sea, ambrein is oxidatively decomposed by the action of sea water, air and sunlight to give rise to several odourous compounds ()Citation65 Microbial oxidation of ambrox has been carried out to obtain interesting metabolites, which were otherwise difficult to synthesize by using chemical methods.
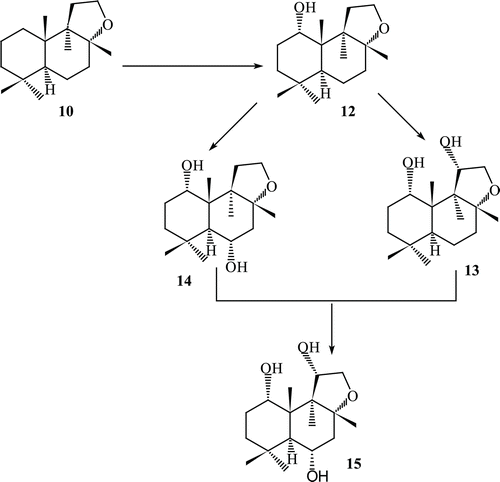
Fermentation of ambrox with Fusarium lini afforded many new mono, di- and tri-hydroxylated metabolites such as 1α-hydroxy ambrox (12), 1α,11α-dihydroxyambrox (13), 1α,6α-dihydroxy ambrox (14), 1α,6α-11α−trihydroxy ambrox (15). Enantio selective α-hydroxylation occurred at C-1, C-6 and C-11 ().
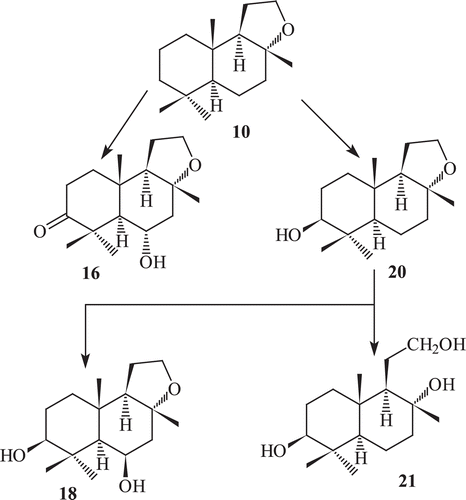
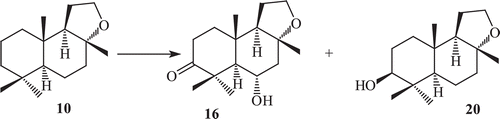
Incubation of ambrox with Rhizopus stolonifer, resulted in the formation of four metabolites, 3-oxoambrox (16), 3β-hydroxyambrox (17), 3β,6β-dihydroxyambrox (18) and its other cleaved product, tetranor-12,8-diol-labdane (21)Citation66 (). Fermentation of ambrox with Curvularia lunata yielded metabolites 3-oxoambrox and 3-oxoambrox (16), 3β-hydroxyambrox (20).
Metabolites 3-oxoambrox, 3β-hydroxyambrox (20) and tetranor-12,8-diol-labdane (21) have been earlier reported as metabolites of ambrox by Cephalosporium aphidicola (wild type), Aspergillus niger (IFO 4049), C. lunata and Aspergillus cellulose (IFO4040). Incubation of ambrox with Cunninghamella elegans, resulted in the formation of two metabolites 3-oxoambrox (16) and 3β-hydroxyambrox (17) ( and )Citation66–68. The oxygenated metabolites of ambrox did not show any effective odour, while its ether cleaved product tetranor-12,8-diol-labdane (21) exhibited strong sweet odour, which is totally different from amber-like odour.
Biotransformation of (−)-ambrox with cell suspension cultures of Actinidia deliciosa (Kiwifruit) yielded the regio and stereospecific oxygenated products 3-oxoambrox,3β-hydroxyambrox,1α-hydroxyambrox,3β,6β-dihydroxyambrox,1α,6β-dihydroxyambrox, and 1α,3β-dihydroxyambrox. Metabolites 1α,6β-dihydroxyambrox and 1α,3β-dihydroxyambrox were found to be new compoundsCitation68.
Microbial oxidations of sclareolide by C. elegans
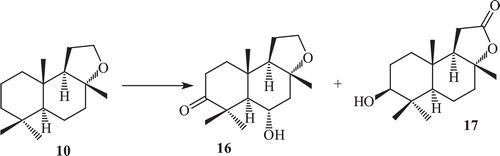
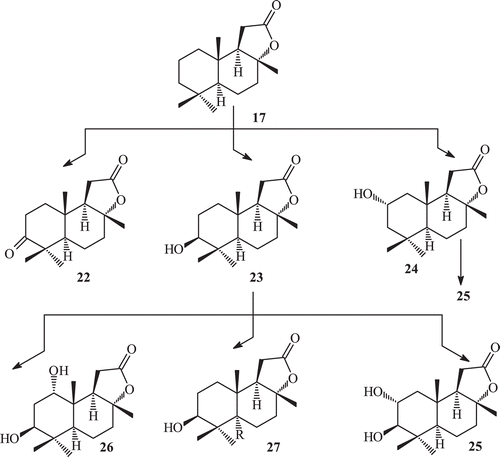
Sclareolide exhibits good phytotoxic and cytotoxic activities against human cancer cellCitation69. Sclareolide is structurally different from ambrox only by the presence of a keto group at C-12. The effect of the presence of a lactone moiety on the metabolism was studied by incubating sclareolide (17) with C. elegans. This yielded a new set of metabolites, 3-oxosclareolide (22), 3β-hydroxysclareolide (23), 2α-hydroxysclareolide (24), 2α,3β-dihydroxyepisclareolide (25), 1α,3β-dihydroxyepisclareolide (26) and 3β-hydroxyepisclareolide (27). This indicated that the lactone functionality directs the enantioselective hydroxylations at C-2 and C-1, and epimerization at C-8 positions ().
Metabolites 3-oxosclareolide (22), 3β-hydroxysclareolide (23) and 1α,3β-dihydroxyepisclareolide (26) have already been reported as fermented products of compound sclareolide by various fungi and have shown in vitro anti-cancer activities against human lungs, colon, skin and breastCitation70. All metabolites 22–27 showed significant phytotoxic activity against Lemna acquinoctialis Welv. except 2α,3β-dihydroxyepisclareolide (25).
Microbial enzymatic transformation of isolongifolen-4-one (30)
Longifolen is a tricyclic olefin and undergo a variety of rearrangements. Isolongifolen, isolongifolol and isolongifolen-4-one are the commonly rearranged products of 28. The microbial enzymatic transformation of (+)-isolongifolen-4-one by a number of fungi by means of a standard two-stage fermentation technique afforded (7R)-12-hydroxyisolongifolen-4-one, (7S)-13-hydroxyisolongifolen-4-one, (11R)-11 hydroxyisolongifolen-4-one, (10R)-10-hydroxyisolongifolen-4-one, and (9R)-9-hydroxyisolongifolen-4-one. The metabolites (7S)-13-hydroxyisolongifolen-4-one and (9R)-9-hydroxyisolongifolen-4-one showed potent tyrosinase inhibitory activity with an IC50 = 51 µMCitation71.
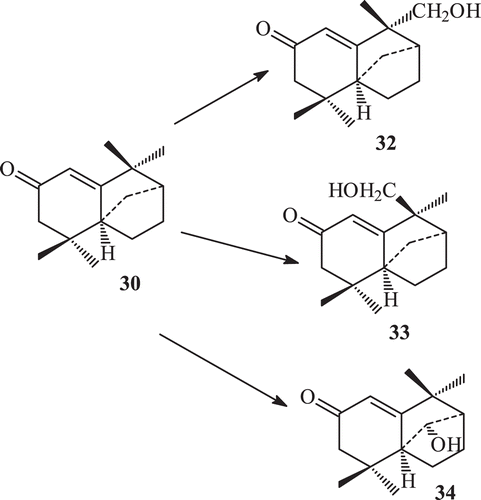
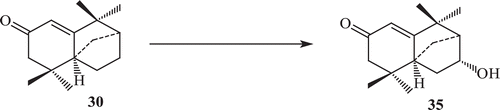
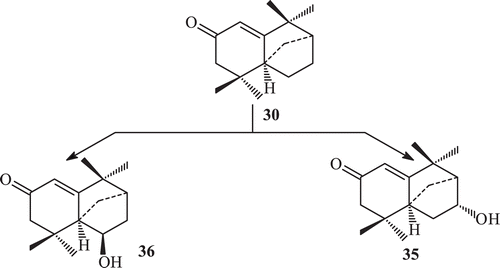
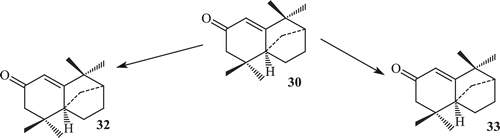
Derivatization of isolongifolen-4-one was carried out in order to find some derivatives with increased potency against tyrosinase. The transformation of isolongifolen-4-one with A. niger (ATCC 10549) was carried out and three new metabolites namely (7R)-12-hydroxyisolongifolin-4-one (32), (7S)-12-hydroxyisolongifolin-4-one (33), (11S) -9-hydroxyisolongifolin-4-one (34) were obtained (). Biotransformation of isolongifolen-4-one with C. aphidicola (IMI 68689) afforded compound (9S)-9-hydroxyisolongifolin-4-one. The metabolites (7R)-12-hydroxyisolongifolin-4-one, (7S)-12-hydroxyisolongifolin-4-one and (9S)-9-hydroxyisolongifolin-4-one showed enzyme inhibitory activityCitation71 (). The transformation of isolongifolen-4-one with F. lini (NRRL 68751) afforded (9S)-9-hydroxyisolongifolin-4-one (35) and (10R)-9-hydroxyisolongifolin-4-one (36) (). Metabolites (7R)-12-hydroxyisolongifolin-4-one (32) and (7S)-12-hydroxyisolongifolin-4-one (33) were also obtained by the transformation of isolongifolen-4-one (30) with R. stolonifer (ATCC 10404) ().
The major metabolites (7R)-12-hydroxyisolongifolin-4-one (32), (7S)-12-hydroxyisolongifolin-4-one (33) and (9S)-9-hydroxyisolongifolin-4-one (35) were obtained in good yields.
Microbial enzymatic transformation of isolongifolol
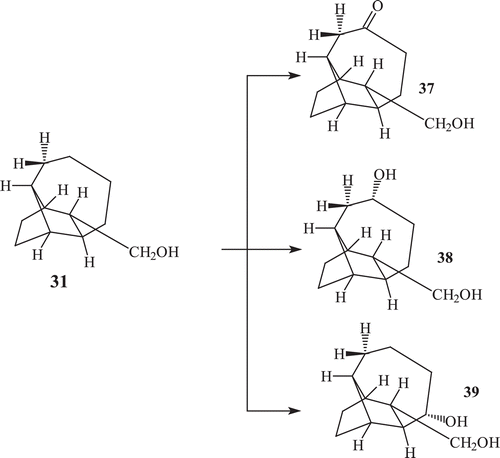
A total of 20–30 natural products are known which contain the bridged tricyclic sesquiterpenes skeleton isolated from plants and fungi and possess interesting biological activities. Very few reports on the effect of fungal enzymatic system towards natural products possessing bridged tricyclic sesquiterpenes skeleton are found in literatureCitation71–73. The incubation of bridged tricyclic sesquiterpene isolongifolol (31) with F. lini afforded its polar oxygenated metabolites named as, 10 oxo isolongifolol (37), 10α-hydroxy isolongifolol (38), 9α−hydroxy isolongifolol (39)Citation73 (). Incubation of compound isolongifolol with R. stolonifer afforded metabolites isolongifolol (38) and 9α−hydroxy isolongifolol (39).
Biotransformation of caryophyllene oxide
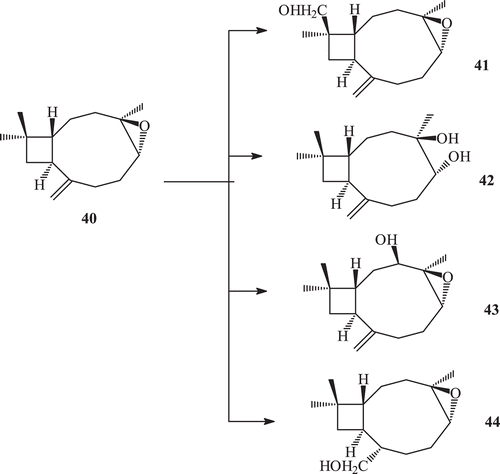
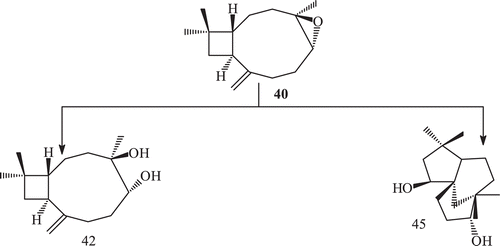
Caryophyllene oxide reported to have strong anti-inflammatory activityCitation71,Citation72. It also exhibits potent antimutagenic propertyCitation74. Caryophyllene oxide (40) was incubated with the cell suspension culture of Catharanthus roseus. It was observed that C. roseus cell culture can convert caryophyllene oxide into several metabolites 41–44 ()Citation73,Citation75.
Compound 41 was previously reported as a biotransformed product of compound 40 by Botrytis cinereaCitation76, while compound 42 was reported as a synthetic derivative of 5α-hydroxycaryophyll-8(13)-ene-3,4-epoxideCitation77. Metabolites 43 and 44 were found to be new metabolites ().
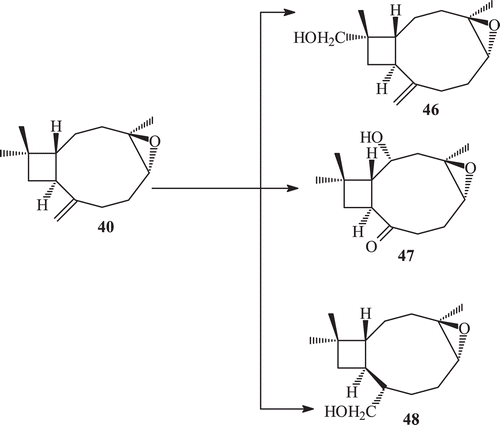
Fungal transformation of caryophyllene oxide (40) by a number of fungi afforded 15-hydroxymethyl caryophyllene oxide (41), 4β,5α-dihydroxy caryophyll-8 (13)-ene (42), clovane-5α,9β-diol (45), 14-hydroxy methyl caryophyllene oxide (46), 2β-hydroxy caryophyllane oxide-8-one (47), 8α-hydroxymethyl caryophyllane oxide (48), caryolane-5α,6β,13β-triol (49), 3β,8α-dihydroxy methyl caryophyllan oxide (50) and clovane-5α,9β-12-triol (51)Citation71,Citation73,Citation74,Citation77 ().
Scheme 15. Metabolism of (−) caryophyllene oxide 40 with Macrophomina phaseolina and Rhizopus stolonifer.
Scheme 16. Metabolism of (−) caryophyllene oxide 40 with Aspergillus niger, Fusarium lini and Gibbrella fujikuroi.
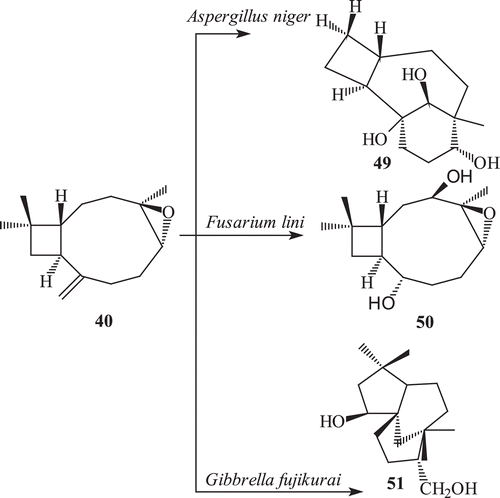
The compound 14-hydroxymethyl caryophyllene oxide (46) showed a potent inhibitory activity against the butyryl cholinesterase enzymeCitation75, with an IC50 = 10.9 µM. This was more potent than the parent caryophyllene oxide (IC50 = 208.4 µM). Compound 8α-hydroxy methyl caryophyllan oxide (48) and caryolane-5α,6β,13β-triol caryolane-5α,6β,13β-triol (49) also showed inhibitory activity against enzyme butyryl cholinesterase.
The compounds clovane 5α,9β-diol, 14-hydroxymethyl caryophyllene oxide and caryolane-5α,6β,13β-triol caryolane-5α,6β,13β-triol showed an enhancing activity of α−glucosidase enzyme, whereas compounds 8α-hydroxymethyl caryophyllene oxide (48) and 3β,8α-dihydroxy methyl caryophyllan oxide (50) showed an average inhibitory activity against the enzyme, when compared with substrate caryophyllene oxide (40) ()Citation75,Citation78,Citation79.
Microbial enzymatic transformation of an anti-inflammatory sesquiterpene (−) caryophyllene oxide by a number of bacteria, afforded a known metabolite 4β,5α-dihydroxycaryophyll-8 (13)-ene (42) which was earlier reported as a synthetic derivative of 5α-hydroxycaryophyll-8(13)-ene-3,4-epoxideCitation76 ().
Microbial enzymatic transformation of triterpenes
Microbial transformation of triterpenoids has provided new derivatives that are potentially useful for pharmacological studies. Biotransformation of pentacyclic triterpenes, such as ursaneCitation80–82 oleananeCitation83–86 have been reported to have anti-cancer activityCitation87.
Pentacyclic transformed triterpenes of the lupane typeCitation88–93, such as lupeol, betulin and betulinic acid, have been reported to have interesting bioactivities including antiviral, in particular, against the human immunodeficiency virusCitation93–95, herpes simplex virusCitation96 and Epstein–Barr virusCitation97, anti-inflammatoryCitation98 and antitumour against human melanoma and other types of human malignanciesCitation99,Citation100.
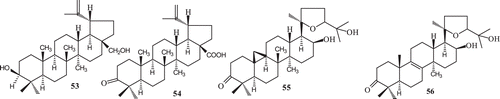
Examples of transformation of triterpenes include the microbial enzymatic transformation of two lupene-type triterpenes, betulin (53) and betulonic acid (54) by the fungus Chaetomium longirostre and microbial enzymatic transformation of a mixture of argentatin A (55) and incanilin (56) by Gibberella suabinetti and Septomyxa affinisCitation60,Citation101,Citation102 ().
Microbial transformation of 18β−glycyrrhetinic acid
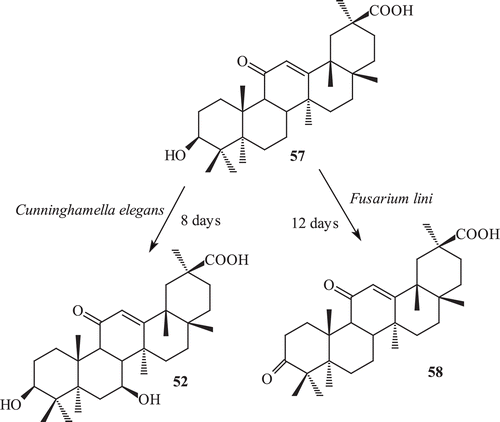
18β glycyrrhetinic acid (57) has been used for the treatment of pulmonary diseases and inflammatory disorders. 18β glycyrrhetinic acid is well known for the anti-inflammatory activityCitation103,Citation104. It was subjected to fermentation with C. elegans,Citation105 which afforded a hydroxylated metabolite, 3β,7β-dihydroxy-11-oxo-olean-12-en-30-oic acid (52) (). 3β,7β-Dihydroxy-11-oxo-olean-12-en-30-oic acid was earlier reported as a metabolite of glycyrrhetinic acid with C. lunata.
Scheme 18. The incubation of 57 with Fusarium lini afforded a oxidative metabolite 3,11-dioxo-olean-12-en-30-oic acid (58) while with Cunninghamela elegans afforded a metabolite 3β, 7β-dihydroxy-11-oxo-olean-12-en-30-oic acid (52).
C. lunata, a well-known fungus used for 11-hydroxylation of steroidsCitation84 also performed triterpenoid hydroxylations. The major product of the microbial transformation of glycyrrhetinic acid, also known as 18-glycyrrhetinic acid, by C. lunata ATCC 13432 was identified as 7-hydroxyglycyrrhetinic acidCitation106.
Epimeres of glycyrrhetinic acid, such as liquiritic acid, were partially converted by the same strain as well as by the fungi Cunninghamella sp. ATCC 3229 and Mucor griseo cyanus ATCC 1207-a to their 7-hydroxy, 15-hydroxy, and/or 7-15-dihydroxy derivatives. The fungal strains Mucor polymorphosporus AS 3.3443 and Mucor spinosus AS 3.3450 also produced 7-hydroxy glycyrrhetinic acid as a major metabolite from glycyrrhetinic acid.
The incubation of 18β-glycyrrhetinic acid (57) with F. lini afforded a oxidative metabolite 3,11-dioxo-olean-12-en-30-oic acid (58) from Maytenus undataCitation107, which was earlier reported as a semisynthetic derivative of 18β-glycyrrhetic acidCitation108 (). 3,11-Dioxo-olean-12-en-30-oic acid was previously reported as a microbial metabolite of glycyrrhetinic acidCitation109.
In mammalian cells, lipoxygenases catalyze the biosynthesis of a variety of bioregulatory compounds, such as hydroxyeicosatetraenoic acids, leukotrienes, lipoxins and hepoxylinesCitation110. It has been found that these lipoxygenase products play a major role in a variety of disorders, such as bronchial asthma, inflammation and tumour angiogenesisCitation89,Citation111, raising the exciting possibility of using nonsteroidal anti-inflammatory drugs and Lox inhibitors as anti-angiogenesis agents for cancer treatment.
Compounds 18β-glycyrrhetinic acid and metabolites 52–58 were screened against the enzyme lipoxygenase. 3,11-Dioxo-olean-12-en-30-oic acid 58 showed a strong lipoxygenase inhibitory activity against the lipoxygenase, (IC50 =144.2 µM) more than the parent 18β-glycyrrhetinic acid (57) (IC50 = 225.1), whereas 3β, 7β-dihydroxy-11-oxo-olean-12-en-30-oic acid (52) showed only a low inhibitory activity against the enzyme with an IC50 value of 300 µM.
In another biotransformation, betulonic acid was produced from betulinic acid using two Bacillus megaterium strainsCitation90,Citation112,Citation113. Arthrobotrys converted betulonic acid into 3-oxo-7β-hydroxylup-20(29)-en-28-oic acid, 3-oxo-7β,15α-dihydroxylup-20(29)-en-28-oic acid and 3-oxo-7β,30-dihydroxy-lup-20(29)-en-28-oic acid. Colletotrichum converted betulinic acid into 3-oxo-15α-hydroxylup-20(29)-en-28-oic acid whereas, betulonic acid was converted into the same product and 3-oxo-7β,15α-dihydroxy-lup-20(29)-en-28-oic acid. Chaetophoma converted betulonic acid (4) into 3-oxo-25-hydroxy-lup-20(29)-en-28-oic acid and both Chaetophoma and Dematium converted betulinic acid into betulonic acidCitation11.
Biotransformation of cycloartenol, 24-methylenecycloartenol and cycloartenone
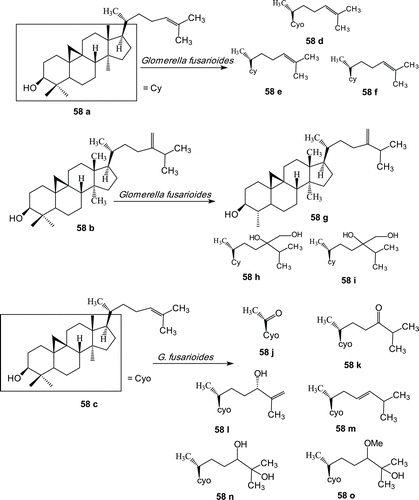
Most of these biotransformations have been performed onto tetracyclic triterpenoids. Biotransformation of three cycloartane-type triterpenes, cycloartenol (58a), 24-methylene cycloartenol (58b) and cycloartenone (58c), by the fungus Glomerella fusarioides was studied. Cycloartenol was converted into cycloartenone (58d), cycloart-25-ene-3β,24-diol (58e) and cycloartane-3β,24,25-triol (58f). 24-Methylene cycloartanol (58b) was metabolized to cycloeucalenol (58g) and two new compounds, 24-methylcycloartane-3β, 24,24-triol (58h) and 24-methoxy-24-methylcycloartane-3β,24-diol (58i). Cycloartenone was converted into two new metabolites, 4α,4β,14α-trimethyl-9β,19-cyclopregnane-3,20-dione (58j) and 25-hydroxy-24-methoxy cycloartan-3-one (58o), and four known compounds, viz., cycloartane-3,24-dione (58k), 24-hydroxycycloart-25-en-3-one (58l), (23E)-25-hydroxycycloart-23-en-3-one (58m) and 24,25-dihydroxycycloartan-3-one (58n) ()Citation114.
Scheme 19. Structures of cycloartenol (58a), 24-methylene cycloartenol (58b), cycloartenol (58c) and metabolites (58d–58o).
Of 49 microbial strains screened for their capabilities to transform ginsenoside Rb1, R. stolonifer and C. lunata produced four key metabolites, 3-O-[β-D-glucopyranosyl-(1,2)-β-D-glucopyranosyl]-20-O-[β-D-glucopyranosyl]-3β,12β,20(S) trihydroxydammar-24-ene, 3-O-[β-D-glucopyranosyl-(1,2)-β-Dglucopyranosyl]-20-O-[β-D-glucopyranosyl]-3β,12β,20(S)-trihydroxydammar-24-ol, 3-O-[β-D-glucopyranosyl-(1,2)-β-D-glucopyranosyl]-3β,12β,20(S)-trihydroxydammar-24-ene, and 3-O-β-D-glucopyranosyl-3β,12β,20(S)-trihydroxydammar-24-eneCitation115. Transformation of G-Rb1, G-Rb3 and G-Rc by F. sacchari was respectively experimented. Kinetic evolutions of G-Rb1, G-Rb3 and G-Rc and their metabolites during the cell incubation were monitored by HPLC analysis. High-performance liquid chromatography was used for monitoring the transformation kinetics of bioactive compounds during F. sacchari metabolismCitation116. Cucurbitacin E 2-O-P-~-glucopyranoside was transformed by Cuwularia lunata NRRL 2178 into cucurbitacin E 21 and three new metabolites. The novel metabolites were identified as (24R)-hydroxy-23,24-dihydrocucurbitacinE and (24S)-hydroxy-23,24-dihydrocucurbitacinE 14 and 51, and the 3-acetyloxy-3-methylbutyl ester of (23–27)-penta-norcucurbitacin I 22-oic acidCitation117. More than seventy strains of aerobic bacteria showing glucosidase activity were isolated from a ginseng field, using a newly designed Esculin-R2A agar. Of these microorganisms, twelve strains could convert the major ginsenoside, Rb1, to the pharmaceutically active minor ginsenoside Rd. Three strains, Burkholderia pyrrocinia GP16, B. megaterium GP27 and Sphingomonas echinoides GP50, were phylogenetically studied, and observed to be most potent at converting ginsenoside Rb1 almost completely within 48 hCitation118.
Enzymatic transformation of β-amyrin acetate by Rhodobacter sphaeroides
The microbial transformation of β-amyrin acetate occurred in the culture of Rhodobacter sphaeroides producing three metabolites, β-amyrin,(3β)-olean-12-ene-3,23-diol and erythrodiol. Initially, it was found that β-amyrin acetate could be deacetylated and hydroxylated by microbial methodsCitation119.
Enzymatic transformation of oleanolic acid by F. lini
The fermentation of oleanolic acid (2) with F. lini afforded two oxidative metabolites, 2α,3β-dihydroxyolean-12-en-28-oic acid and 2α,3β,11β-trihydroxyolean-12-en-28-oic acid. 2α,3β,11β-trihydroxyolean-12-en-28-oic acid was found to be a new compound. These metabolites exhibited a potent inhibition of α-glucosidase enzymeCitation80. In another biotransformation, oleanolic acid was produced by the microbe Nocardia sp.NRRL. 5646 to selectively furnish their corresponding 28-methyl estersCitation80.
Microbial enzymatic transformation of diterpenes
Methyl ent-17-hydroxy-16β-kauran-19-oate was fed to a 2-day-old culture of the fungus R. stolonifer, fermenting at room temperature (25°C). Two novel compounds methyl ent-9α,17-dihydroxy-16β-kauran-19-oate and methyl ent-7α,17-dihydroxy-16β-kauran-19-oate were isolated from this experiment.
Many compounds of therapeutic and industrial interest were obtained by microbial transformation. Examples are the biotransformation of aphidicolin (59)Citation120 () and methyl ent-15-oxokaur-16-en-19-oate (60)Citation121 by various micro organisms 122.
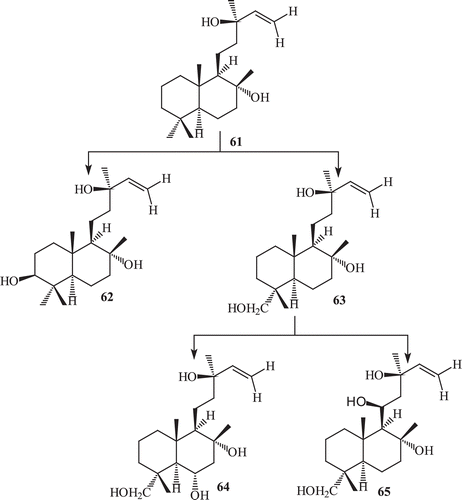
Sclareol (61), a labdane diterpene ditertiary alcohol, was first isolated from Salvia sclarea L. (Labiatae) in 1931Citation123 and also from Astrogalus brachystochysCitation124. Sclareol have a potent antibacterial activity. Commercially, sclareol, is used as a fixative and in perfumery, as a flavoring agent in the tobacco industry, and as a eynthon for the preparation of a series of ambra odourants in perfumeryCitation125–127.
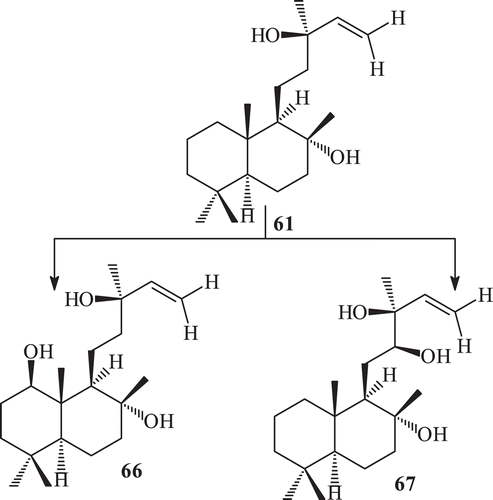
The metabolism of sclareol by R. stolonifer (ATCC 10404) yielded four metabolites 3β-hydroxy sclareol (62) 18α-hydroxy sclareol (63), 6α,18α-dihydroxy sclareol (64), 11S,18α-dihydroxy sclareol (65) (). Incubation of sclareol with R. stolonifer afforded in high yield a mixture of triols with 18-hydroxy-sclareol as the main component. The fermentation of sclareol with F. lini yielded two metabolites, 66–67 ().
Sclareol is reported to have an antibacterial activity against different pathogenic strains. The metabolites 62–67 produced from sclareol were screened for antibacterial activity against three pathogenic strains. 1β-Hydroxy sclareol (66) was found to be more active than sclareol, whereas compound 6α,18α-dihydroxy sclareol (64) and 11S,18α-dihydroxy sclareol (67) were found to be as active as sclareol against Bacillus subtilisCitation128.
Incubation of the diterpene 2β-hydroxy-ent-13-epi-manoyl oxide with Gibberella fujikuroi afforded in good yield 2β,6β-dihydroxy-ent-13-epi-manoyl oxide, 2β,12β-dihydroxy-ent-13-epi-manoyl oxide and 2β,20-dihydroxy-ent-13-epi-manoyl oxide, confirming that although ent-13-epi-manoyl oxide is a final metabolite of a biosynthetic branch in this fungus, more polar derivatives of this compound can be transformed by this micro-organismCitation129.
Microbial enzymatic transformation of monoterpene
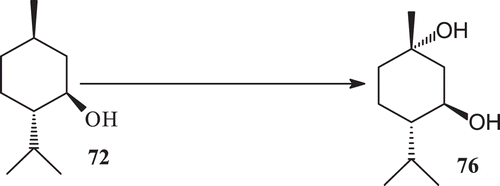
Hydroxylation is found to be the most common reaction in monoterpene, such as in 1,4-cineole (68)Citation130, geraniol (70) and nerol (71)Citation131 afforded mainly hydroxylated derivatives with various microbes. Scientists have investigated the microbial transformation of a monoterpene (1R,3R,4S)-(−)-menthol (72), by using various fungal strains.
In search of potent antibacterial compounds for commercial products as dental root canal sealers, antiseptics, food preservatives and feed supplementsCitation132,Citation133, a monoterpene (1R, 3R, 4S)-(−)-menthol (72), has been investigatedCitation134.
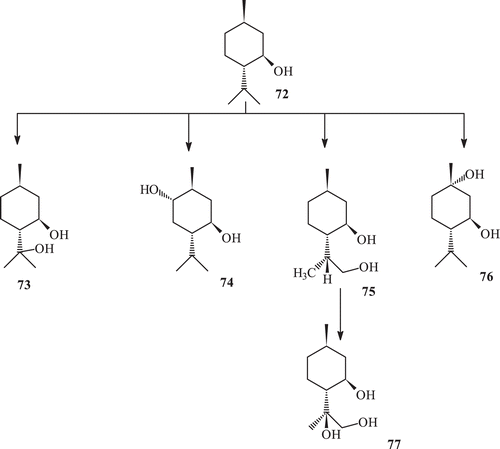
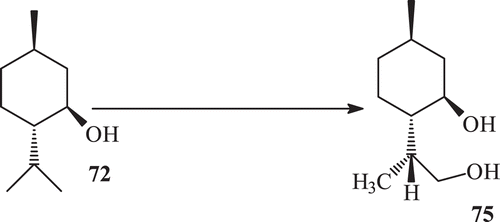
Microbial enzymatic transformation of (−) menthol with Macrophomina phaseolina yielded the metabolites 73–77 (). Microbial enzymatic transformation of (1R, 3R,4S) (−) menthol with R. stolonifer yielded the metabolite (8R)-9-hydroxy menthol (75) (). Microbial enzymatic transformation of (1R, 3R, 4S)–(−), menthol (72) with C. elegans, while incubation of (−) menthol (64) with C. elegans yielded the metabolite 76 ().
Compound (1R,3R,4S)-(−)-menthol is known to possess antibacterial activity against food born pathogens such as Salmonella typhimurium, Staphylococcus aureus and Vibrio parahoemolyticusCitation135. (1R, 3R, 4S)-(−)-menthol was incubated with three fungal cultures to obtain five known metabolites 8-hydroxy menthol, 6α-hydroxy menthol, (8R)-9-hydroxy menthol, 1α-hydroxy menthol and 8R-9-hydroxy menthol (73–77) (). Metabolite 8-hydroxy menthol (73) has been reported as transformation product of (1R, 3R, 4S)-(−)-menthol by various Aspergillus speciesCitation136. Evaluation of (1R, 3R, 4S)-(−)-menthol and 73–77 for anti-bacterial activity against the gram positive bacteria had potent activities.
6α-Hydroxy menthol (74) reported earlier from the microbial enzymatic transformation of l-menthol by Rhizoctonia solani AG-11`-1A and 1BCitation137. (8R)-9-Hydroxy menthol, which was reported earlier as a synthetic derivative of (−)-(1R, 3R, 4S, 8R)-9-Phenyl thio mentholCitation138.
1α-Hydroxy menthol (76) reported earlier from the microbial transformation of l-menthol (64) by Rhizoctonia solani AG-1-1A and 1BCitation137. 8R,9-dihydroxy menthol (77) which was reported as synthetic derivative of (−)-isopulegolCitation139. Metabolites (73–77) are the strong candidates as menthol against Escherichia coli. Metabolites 1α-hydroxy menthol and 8R-9-hydroxy menthol 76–77 are the strong candidates as menthol against Pseudomonas aeruginosa. Metabolite 1α-hydroxy menthol is the strong candidate as menthol against Salmonella typhi. Compound 78 showed significant inhibitory potential against the BuCh inhibition with an IC50 value of 91.9 µM, whereas compound 78 showed only low activity against AchE with an IC50 value of 219.0 µM140. This class of compounds also possess antibiotic and cytotoxic properties and can be good candidates for chemotherapy.
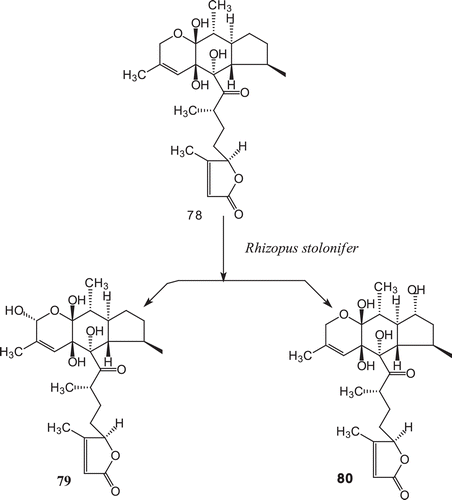
Microbial enzymatic transformation of sesterterpene leucosceptrine
The microbial transformation of a novel sesterterpene, leucosceptrine (78), the first member of class leucosesterterpenes, by R. stolonifer afforded two hydroxylated metabolites, 1α-hydroxyleucosceptrine (79), and 8α-hydroxyleucosceptrine (80)143 ().
Conclusion
Enzyme plays a pivotal role in modifying the naturally occurring substance. Attempts are being made in our lab to enhance the activity of existing bioactive compounds and drug design. The hydrolytic and reductive capabilities of microorganisms have been known and are currently used in preparative and industrial reactions. Various classes of bioactive organic compounds have been subjected to enzymatic transformation in an effort to obtain more active and less toxic substances, or to elucidate their metabolic pathways.
Many drugs produced by chemical synthesis were racemates. However, their beneficial effects often reside in only one enantiomer. This need has given an immense stimulus to the use of enzymes in chiral synthesis. In recent years the synthesis of chiral molecules by enzymatic methods has led to the widespread use of the hydrolytic properties of enzymes such as esterases and lipases. Consequently, predictive models of these transformations were also developed.
In some cases, it is extremely difficult to provide sufficient amounts of active substances from isolation method and fungi due to their limited levels of biosynthesis. The limited quantity might be due to the rare occurrence of the organisms. Although fermentation of microorganisms is a possible way, it does not apply to metabolites produced strictly by macroorganisms. Use of biotransformation and biotechnological methods may help to obtain potent candidates for the treatment of diseases.
In sesquiterpenes, pyrethrosin, plectranthone and costunolide diverse microorganisms have oxidized hydroxyl group afforded mainly their hydroxylated derivatives with various fungal strains. In ambrox biotransformation with F. lini afforded many new mono, di- and tri-hydroxylated metabolites, 1α-hydroxy ambrox, 1α,11α-dihydroxyambrox, 1α,6α-dihydroxy ambrox, 1α,6α-11α−trihydroxy ambrox due to enantio selective α-hydroxylation at C-1, C-6 and C-11. In monoterpenes, the metabolite 1β-hydroxy sclareol produced from sclareol was screened for antibacterial activity, was found to be more active than sclareol, whereas compound 6α,18α-dihydroxy sclareol and 11S,18α-dihydroxy sclareol were found to be as active as sclareol against B. subtilis. In 3β-hydroxylated tetracyclic or pentacyclic triterpenes, diverse microorganisms have oxidized this hydroxyl group. Many triterpenes have been converted to 3,4-seco-type compounds, through a Baeyer-Villiger-type oxidation of these derivatives, followed by hydrolysis of the resulting seven-membered ring lactones.
Mycobacterium sp. (NRRL B-3805) has produced a series of complex chemical changes in tetracyclic terpenoids, such as cycloartenol, lanosterol and 24-methylenecycloartanol, including the selective cleavage of the side chain of these triterpenoids, to produce C19 steroids. Different microorganisms have been used to investigate the biotransformation pathways of ginsenosides, and also to convert major ginsenosides into more active minor saponins. These microorganisms have produced regioselective deglycosylation reactions to produce specific ginsenosides using an appropriate combination of ginsenoside substrates and specific microorganism.
Among the metabolites observed, approximately 80% were more potent than the parent compound and planned research can produce more potent terpenoids by using enzymatic transformation methods. Specifically, highly oxygenated terpenes are potential candidates responsible for antioxidant, antibiotic and anti-cancer properties can also be obtained by these biotransformed products. It is highly valuable to construct the optimal transformation system through process-optimization strategy. For the preparation of terpenoids, the reaction parameters have to be optimized with regard to high yields and biocatalysts stability, signifying low production costs. Further studies need to focus on the enzymatic evidences for terpenoid production.
Declaration of interest
The authors report no conflicts of interest.
References
- Nighat S, Ather A. Oleanolic acid and related derivatives as medicinally important compounds. J Enzym Inhib Med Chem 2008;23:739–756.
- Nighat S. Clinically useful anticancer, antitumor and antiwrinkle agent, ursolic acid and related derivatives as medicinally important natural product. J Enzym Inhib Med Chem 2011;26:616–642.
- Carla de Carvalho CCR, Manuela M, Da Fonseca R. Biotransformation of terpenes. Biotechnol Adv 2006;24:134–142.
- Setzer WN, Setzer MC. Plant-derived triterpenoids as potential antineoplastic agents. Mini-Rev Med Chem 2003;3:540–556.
- Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N et al. Plants and human health in the twenty-first century. Trends Biotechnol 2002;20:522–531.
- Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer 2007;7:357–369.
- Laszczyk MN. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med 2009;75:1549–1560.
- Petronelli A, Pannitteri G, Testa U. Triterpenoids as new promising anticancer drugs. Anticancer Drugs 2009;20:880–892.
- Wang X, Zhang F, Yang L, Mei Y, Long H, Zhang X, et al. Ursolic acid inhibits, proliferation and induces apoptosis of cancer cells in vitro and in vivo. J Biomed Biotechnol 2011.
- Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX. Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat Prod Rep 2005;22:386–399.
- Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res 2003;63:1270–1279.
- Sun J, Blaskovich MA, Jove R, Livingston SK, Coppola D, Sebti SM. Cucurbitacin Q: a selective STAT3 activation inhibitor with potent antitumor activity. Oncogene 2005;24:3236–3245.
- Rodriguez N, Vasquez Y, Hussein AA, Coley PD, Solis PN, Gupta MP. Cytotoxic cucurbitacin constituents from Sloanea zuliaensis. J Nat Prod 2003;66:1515–1516.
- Jayaprakasam B, Seeram NP, Nair MG. Anticancer and antiinflammatory activities of cucurbitacins from Cucurbita andreana. Cancer Lett 2003;189:11–16.
- Olmedo D, Rodríguez N, Vásquez Y, Solís PN, López-Pérez JL, Feliciano AS et al. A new coumarin from the fruits of Coutarea hexandra. Nat Prod Res 2007;21:625–631.
- Yang L, Wu S, Zhang Q, Liu F, Wu P. 23,24-Dihydrocucurbitacin B induces G2/M cell-cycle arrest and mitochondria-dependent apoptosis in human breast cancer cells (Bcap37). Cancer Lett 2007;256:267–278.
- Wakimoto N, Yin D, O’Kelly J, Haritunians T, Karlan B, Said J et al. Cucurbitacin B has a potent antiproliferative effect on breast cancer cells in vitro and in vivo. Cancer Sci 2008;99:1793–1797.
- Kongtun S, Jiratchariyakul W, Kummalue T, Tan-ariya P, Kunnachak S, Frahm AW. Cytotoxic properties of root extract and fruit juice of Trichosanthes cucumerina. Planta Med 2009;75:839–842.
- Ramalhete C, Mansoor TA, Mulhovo S, Molnár J, Ferreira MJ. Cucurbitane-type triterpenoids from the African plant Momordica balsamina. J Nat Prod 2009;72:2009–2013.
- Phongmaykin J, Kumamoto T, Ishikawa T, Suttisri R, Saifah E. A new sesquiterpene and other terpenoid constituents of Chisocheton penduliflorus. Arch Pharm Res 2008;31:21–27.
- Tsai ZT, Liaw SL. (1985). The use and the effect of ganoderma. Taichung: San Yun Press.
- Yeh CT, Rao YK, Yao CJ, Yeh CF, Li CH, Chuang SE, et al. Cytotoxic triterpenes from Antrodia camphorate and their mode of action in HT-29 human colon cancer cells. Cancer Lett 2009;285:73–79.
- Setzer WN, Holland MT, Bozeman CA, Rozmus GF, Setzer MC, Moriarity DM et al. Isolation and frontier molecular orbital investigation of bioactive quinone-methide triterpenoids from the bark of Salacia petenensis. Planta Med 2001;67:65–69.
- Chang FR, Hayashi K, Chen IH, Liaw CC, Bastow KF, Nakanishi Y et al. Antitumor agents. 228. five new agarofurans, Reissantins A-E, and cytotoxic principles from Reissantia buchananii. J Nat Prod 2003;66:1416–1420.
- Wu CC, Chan ML, Chen WY, Tsai CY, Chang FR, Wu YC. Pristimerin induces caspase-dependent apoptosis in MDA-MB-231 cells via direct effects on mitochondria. Mol Cancer Ther 2005;4:1277–1285.
- Idris AI, Libouban H, Nyangoga H, Landao-Bassonga E, Chappard D, Ralston SH. Pharmacologic inhibitors of IkappaB kinase suppress growth and migration of mammary carcinosarcoma cells in vitro and prevent osteolytic bone metastasis in vivo. Mol Cancer Ther 2009;8:2339–2347.
- Sung B, Park B, Yadav VR, Aggarwal BB. Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the downregulation of cell survival proteins and upregulation of death receptors. J Biol Chem 2010;285:11498–11507.
- Zeng L, Gu ZM, Chang CJ, Wood KV, McLaughlin JL. Meliavolkenin, a new bioactive triterpenoid from Melia volkensii (Meliaceae). Bioorg Med Chem 1995;3:383–390.
- Rogers LL, Zeng L, Kozlowski JF, Shimada H, Alali FQ, Johnson HA et al. New bioactive triterpenoids from Melia volkensii. J Nat Prod 1998;61:64–70.
- Setzer WN, Setzer MC, Bates RB, Jackes BR. Biologically active triterpenoids of Syncarpia glomulifera bark extract from Paluma, north Queensland, Australia. Planta Med 2000;66:176–177.
- Amico V, Barresi V, Condorelli D, Spatafora C, Tringali C. Antiproliferative terpenoids from almond hulls (Prunus dulcis): identification and structure-activity relationships. J Agric Food Chem 2006;54:810–814.
- Rzeski W, Stepulak A, Szymanski M, Sifringer M, Kaczor J, Wejksza K et al. Betulinic acid decreases expression of bcl-2 and cyclin D1, inhibits proliferation, migration and induces apoptosis in cancer cells. Naunyn Schmiedebergs Arch Pharmacol 2006;374:11–20.
- Basu S, Ma R, Boyle PJ, Mikulla B, Bradley M, Smith B et al. Apoptosis of human carcinoma cells in the presence of potential anti-cancer drugs: III. Treatment of Colo-205 and SKBR3 cells with: cis -platin, Tamoxifen, Melphalan, Betulinic acid, L-PDMP, L-PPMP, and GD3 ganglioside. Glycoconj J 2004;20:563–577.
- Kessler JH, Mullauer FB, de Roo GM, Medema JP. Broad in vitro efficacy of plant-derived betulinic acid against cell lines derived from the most prevalent human cancer types. Cancer Lett 2007;251:132–145.
- Rzeski W, Stepulak A, Szymanski M, Juszczak M, Grabarska A, Sifringer M et al. Betulin elicits anti-cancer effects in tumour primary cultures and cell lines in vitro. Basic Clin Pharmacol Toxicol 2009;105:425–432.
- Lambertini E, Lampronti I, Penolazzi L, Khan MT, Ather A, Giorgi G et al. Expression of estrogen receptor alpha gene in breast cancer cells treated with transcription factor decoy is modulated by Bangladeshi natural plant extracts. Oncol Res 2005;15:69–79.
- Sticher O. (1977). In new natural products and plant drugs with pharmacological, biological or therapeutical activity. Wagner H, Wolff P, eds. Berlin: Springer, 1–22.
- Barrero AF, Oltra JE, Raslan DS, Saude DA. Microbial transformation of sesquiterpene lactones by the fungi cunninghamella echinulata and rhizopus oryzae. J Nat Prod 1999;62:726–729.
- EI Syed KA, Hamann MT, Wadling CA, Jansen C, Lee SK, Dunstan CA, et al. Microbial transformation of podocarpic acid and evaluation of transformation products for antioxidant activity. J Org Chem 1998;63:7449–7455.
- Khalid A, Syed EI, Mayer AMS, Kelly M, Hamann MT. The biocatalytic transformation of furan to amide in the bioactive marine natural product palinurin. J Org Chem 1999;64:9258–9260.
- Purohit SS. (2004). Biotechnology fundamentals and applications. 3rd Edition. Jodhpur, India: Shyam Printing press, 392–393.
- Boyle AW, Phelps CD, Young LY. Isolation from estuarine sediments of a Desulfovibrio strain which can grow on lactate coupled to the reductive dehalogenation of 2,4, 6-tribromophenol. Appl Environ Microbiol 1999;65:1133–1140.
- Roberts SMN, Turner J, Willetts AJ, Turner MK. (1995). Introduction to biocatalysis using enzymes and micro organisms. Cambridge, UK: Cambridge University Press.
- Holland HL. (1992). Organic synthesis with oxidative enzymes. New York: VCH Publishers, 6.
- Alexander LS, Goff HM. Chemicals, cancer and cytochrome P-4. J Chem Edu 1982;59:179.
- Wang JC, Staba JE. Peppermint and spearmint tissue culture. ii. dual-carboy culture of spearmint tissues. J Pharm Sci 1963;52:1058.
- Becker H. Untersuchungen Zur Frange der Building Fluchtiger stoffwechsel produkte in calluskutturen. Biochem Physiol Pflanz 1970;161:425.
- Suga T, Hirata T, Yamamoto Y. Biotransformation of exogenous substrates by plant cell cultures. Agric Biol Chem 1980;44:325.
- Bohm H. In Proceedings of the 5th International Congress on plant tissue and cell culture, 1982, 325.
- Furuya T, Thorpe TA, ed. (1978). Frontiers of plant Tissue Culture. Calgary: University of Calgary, 191.
- Charlwood VB, Hegatry KP, Charlwood AK, Morris, P. (1986). Scragg HA, Stafford A, Fowler WM, eds. Secondary Metabolism in Plant Cell Cultures. London: Cambridge University press, 15.
- Lippin G, Tampion J, Sride J, Morris P, Scragg HA, Stafford A, Fowler WM, eds. (1986). Secondary Metabolism in Plant Cell Cultures. London: Cambridge University press, 113.
- Suga T, Hirata T. Biotransformation of exogenous substrates by plant cell cultures. Phytochemistry 1990;29:2393.
- Hamada H, Miyamoto Y, Nakajima N, Furuya T. Highly selective transformation by plant catalysts. J Mol Catal B Enzyme 1998;5:187.
- Schmitt E, Dekant W, Stopper H. Assaying the estrogenicity of phytoestrogens in cells of different estrogen sensitive tissues. Toxicol In Vitro 2001;15:433–439.
- Decroos K, Vanhemmens S, Cattoir S, Boon N, Verstraete W. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch Microbiol 2005;183:45–55.
- Garcia-Granados A, Martinez A, Parra A, Rivas F. Manoyl-oxide biotransformations with filamentous fungi. Curr Org Chem 2007;11:679–692.
- Arantes SF, Hanson JR. The biotransformation of sesquiterpenoids by Mucor plumbeus. Curr Org Chem 2007;11:657–663.
- Demyttenaere JCR. (2001). Biotransformation of terpenoids by microorganisms. studies in natural products chemistry. Atta-ur-Rahman, ed. Amsterdam: Elsevier Science BV, 25, 125–178.
- Parra A, Rivas F, Garcia-Granados A, Martinez A. Microbial transformation of triterpenoids. Mini-Rev Org Chem 2009:6:307–320.
- Williams TR. (1979). Detoxication mechanism. London: Cunningham and Sons.
- Orabi KY. Microbial transformation of the eudesmane sesquiterpene plectranthone. J Nat Prod 2000;63:1709–1711.
- Maatooq GT. Microbial metabolism of partheniol by Mucor circinelloides. Phytochemistry 2002;59:39–44.
- Hanson JR. (1995). An introduction to biotransformation in organic chemistry. Oxford: Oxford University press.
- Atta-ur-Rahman, Muhammed IC, Syed GM. Novel hydroxylated enantiomers of (-) 3A,6,6,9A-tetramethylper hydronaphtho [2,1-B] furan as perfuming agents derived from a fungal fermentation process. United states patent application number; US 2006/0223883 A 1, 5 Oct. 2006.
- Choudhary MI, Musharraf SG, Sami A, Atta-ur-Rahman. Microbial transformation of (-)-ambrox and sclareolide. Helv Chim Acta 2004;87:2685–2694.
- Hashimoto T, Noma Y, Asakawa Y. Biotransformation of terpenoids from the crude drugs and animal origion by micro organisms. Heterocycles 2001;54:529.
- Nasib A, Musharraf SG, Hussain S, Khan S, Anjum S, Ali S, et al. Biotransformation of (-)-ambrox by cell suspension cultures of Actinidia deliciosa. J Nat Prod 2006;69:957–959.
- Rodriguez E, Towers GHN, Mitchell JC. Biological activities of sesquiterpene lactones. Phytochemistry 1976;15:1573–1580.
- Tahara SZ, Farooq A. Oxidative metabolism of ambrox and sclareolide by Botrytis cinerea. Z Naturforsch 2000;55c:341–346.
- Muhammed IC, Syed GM, Khan MTH, Abdelrahman D, Pervaz, M, Shaheen F, et al. Microbial transformation of (+)-isolongifolen-4-one. Helv Chim Acta 2003;86:3450–3460.
- Aleu J, Hanson JR, Galan RH, Collado IG. Biotransformation of the fungistatic sesquiterpenoid patchoulol by botrytis cinerea. J Nat Prod 1999;62:437–440.
- Muhammed IC, Syed GM, Nawas S, Shazia A, Pervez M, Fun H-K, et al. Microbial transformation of (-)-isolongifolol and butyrylcholinesterase inhibitory activity of transformed products. Bioorg Med Chem 2005;13:1939–1944.
- Shimizu M, Shogawa H, Matsuzawa T, Yonezawa S, Hayashi T, Arisawa M et al. Anti-inflammatory constituents of topically applied crude drugs. IV. Constituents and anti-inflammatory effect of Paraguayan crude drug “alhucema” (Lavandula latifolia Vill.). Chem Pharm Bull 1990;38:2283–2284.
- Choudhary MI, Siddiqui ZA, Nawaz SA, Atta-ur-Rahman. Microbial transformation and butyrylcholinesterase inhibitory activity of (-)-caryophyllene oxide and its derivatives. J Nat Prod 2006;69:1429–1434.
- Collado GI, Hanson RJ, Hitchcock BP, Sanchez-Macias JA. Stereochemistry of epoxidation of some caryophyllenols. J Org Chem 1997;62:1965.
- Yang D, Michel L, Chaumont JP, Millet-Clerc J. Use of caryophyllene oxide as an antifungal agent in an in vitro experimental model of onychomycosis. Mycopathologia 1999;148:79–82.
- Duran R, Corrales E, Hernandez-Galan R, Collado IG. Biotransformation of caryophyllene oxide by botrytis cinerea. J Nat Prod 1999;62:41–44.
- Muhammed IC, Siddique ZA, Saifullah SK, Musharraf SG, Atta-ur- Rahman. Biotransformation of caryophyllene oxide by cell suspension culture of Catharanthus roseus”. Z Naturforsch 2005;61b:197–200.
- Zhang J, Cheng ZH, Yu BY, Cordellb GA, Qiu SQ. Novel biotransformation of pentacyclic triterpenoid acids by Nocardia sp. NRRL 5646. Tetrahedron Lett 2005;46:2337–2340.
- Ibrahim A, Khalifa SI, Khafagi I, Youssef DT, Khan S, Mesbah M et al. Microbial metabolism of biologically active secondary metabolites from Nerium oleander L. Chem Pharm Bull 2008;56:1253–1258.
- Oleander L. Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Chem Pharm Bull 2008;56:1253–1258.
- Chi H, Ji G-E. Transformation of ginsenosides Rb1and Re from panax ginseng by food micro organisms. Biotechnol Lett 2005;27:765–771.
- Xin X, Liu Y, Ye M, Guo H, Guo D. Microbial transformation of glycyrrhetinic acid by Mucor polymorphosporus. Planta Med 2006;72:156–161.
- Shirane N, Hashimoto Y, Ueda K, Takenaka H, Katoh K. Ring-A cleavage of 3-oxo-olean-12-en-28-oic acid by the fungus Chaetomium longirostre. Phytochemistry 1996;43:99–104.
- Cheng ZH, Yu BY, Cordell GA, Qiu SX. Biotransformation of quinovic acid glycosides by microbes: direct conversion of the ursane to the oleanane triterpene skeleton by Nocardia sp. NRRL 5646. Org Lett 2004;6:3163–3165.
- Chen QH, Liu J, Zhang HF, He GQ, Fu ML. The betulinic acid production from betulin through biotransformation by fungi. Enzyme Microb Tech 2009;45:175–180.
- Qian LW, Zhang J, Liu JH, Yu BY. Direct microbial-catalyzed asymmetric [alpha]-hydroxylation of betulonic acid by Nocardia sp., NRRL 5646. Tetrahedron Lett 2009;50:2193–2195.
- Kouzi SA, Chatterjee P, Pezzuto JM, Hamann MT. Microbial transformations of the antimelanoma agent betulinic acid. J Nat Prod 2000;63:1653–1657.
- Chatterjee P, Kouzi SA, Pezzuto JM, Hamann MT. Biotransformation of the antimelanoma agent betulinic acid by Bacillus megaterium ATCC 13368. Appl Environ Microbiol 2000;66:3850–3855.
- Akihisa T, Takamine Y, Yoshizumi K, Tokuda H, Kimura Y, Ukiya M et al. Microbial transformations of two lupane-type triterpenes and anti-tumor-promoting effects of the transformation products. J Nat Prod 2002;65:278–282.
- Muhammed IC, Siddique ZA, Khan SS, Musharraf SG, Atta-ur- Rahman. Biotransformation of (–)-caryophyllene oxide by cell suspension culture of Catharanthus roseus. Z Naturforsch 2006;59b:197–200.
- Barroso-González J, El Jaber-Vazdekis N, García-Expósito L, Machado JD, Zárate R, Ravelo AG et al. The lupane-type triterpene 30-oxo-calenduladiol is a CCR5 antagonist with anti-HIV-1 and anti-chemotactic activities. J Biol Chem 2009;284:16609–16620.
- Qian K, Yu D, Chen CH, Huang L, Morris-Natschke SL, Nitz TJ et al. Anti-AIDS agents. 78. Design, synthesis, metabolic stability assessment, and antiviral evaluation of novel betulinic acid derivatives as potent anti-human immunodeficiency virus (HIV) agents. J Med Chem 2009;52:3248–3258.
- Badria FA, Abu-Karam M, Mikhaeil BR, Maatooq GT, Amer MM. Anti-herpes activity of isolated compounds from frankincense. Biosci Biotechnol Res Asia 2003;1:1–10.
- Nakagawa-Goto K, Yamada K, Taniguchi M, Tokuda H, Lee KH. Cancer preventive agents 9. Betulinic acid derivatives as potent cancer chemopreventive agents. Bioorg Med Chem Lett 2009;19:3378–3381.
- Nguemfo EL, Dimo T, Dongmo AB, Azebaze AG, Alaoui K, Asongalem AE et al. Anti-oxidative and anti-inflammatory activities of some isolated constituents from the stem bark of Allanblackia monticola Staner L.C (Guttiferae). Inflammopharmacology 2009;17:37–41.
- Fulda S, Kroemer G. Targeting mitochondrial apoptosis by betulinic acid in human cancers. Drug Discov Today 2009;14:885–890.
- Saleem M, Murtaza I, Tarapore RS, Suh Y, Adhami VM, Johnson JJ et al. Lupeol inhibits proliferation of human prostate cancer cells by targeting beta-catenin signaling. Carcinogenesis 2009;30:808–817.
- Galal TM, Joseph JH. Microbial transformation of a mixture of argentatin A and incanilin. Z Naturforsch C 2002;57:489.
- Mufflera K, Leipolda D, Schellera MC, Haasb C, Steingroewerb J. Biotransformation of triterpenes. Process Biochemistry 2011;46:1–15.
- Tolstikov AG, Baltina AL, Shultz EE, Pokrovskyi AG. Glycyrrhizic acid. Russian J Bioorg Chem 1997;23:625.
- Tolstikov AG, Baltina AL, Serduyk GN. Glycyrrhetic acid. J Pharm Chem 1998;32:5.
- Muhammed IC, Siddiqui ZA, Nawaz SA, Atta-ur-Rahman. Microbial transformation of 18beta-glycyrrhetinic acid by Cunninghamella elegans and Fusarium lini, and lipoxygenase inhibitory activity of transformed products. Nat Prod Res 2009;23:507–513.
- Collins DO, Reese PB. Biotransformation of cedrol by Curvularia lunata ATCC 12017. Phytochemistry 2001;56:417–421.
- Baran JS, Langford DD, Liang C, Pitzele BS. Synthesis and biological activities of substituted glycyrrhetic acids. J Med Chem 1974;17:184–191.
- Lands MEM. Mechanisms of actions of anti inflammatory drugs. Adv Drug Res 1985;14:147.
- Yamada Y, Nakamura A, Yamamoto K, Kikuzaki H. Transformation of glycyrrhizic acid by Aspergillus spp. Biosci. Biotech Biochem 1994;58:436.
- Muhammad I, EI Sayed AK, Mossa SJ, Al-Said SM, EI-Feraly FS, Clark AM, et al. Bioactive 12-oleanene triterpene and secotriterpene acids from Maytenus undata. J Nat Prod 2000;63:605.
- Steinhilber D. 5-Lipoxygenase: a target for antiinflammatory drugs revisited. Curr Med Chem 1999;6:71–85.
- Nie D, Honn KV. Cyclooxygenase, lipoxygenase and tumor angiogenesis. Cell Mol Life Sci 2002;59:799–807.
- Muhammed S, Nighat S, Mustafa K. Biotransformation of a bioactive secondary metabolite from Alstonia scholaris. J Biocatal Biotransfor (Accepted, August 2012)
- Akihisa TKW, Yoneima R, Suzuki T, Kimura Y. Biotransformation of cycloartane-type triterpenes by the fungus Glomerella fusarioides. J Nat Prod 2006;69:604–607.
- Dong A, Ye M, Guo H, Zheng J, Guo D. Microbial transformation of ginsenoside Rb1 by Rhizopus stolonifer and Curvularia lunata. Biotechnol Lett 2003;25:339–344.
- Han Y, Sun B, Jiang B, Hu X, Spranger MI, Zhang Y et al. Microbial transformation of ginsenosides Rb1, Rb3 and Rc by Fusarium sacchari. J Appl Microbiol 2010;109:792–798.
- Maatooq G, El-Sharkawy S, Afifi MS, Rosazza JPN. Microbial transformation of cucurbitacin E 2-O-Î2-d-glucopyranoside. J Nat Prod 1995;58:165–171.
- Kim MK, Lee JW, Lee KY, Yang DC. Microbial conversion of major ginsenoside rb(1) to pharmaceutically active minor ginsenoside rd. J Microbiol 2005;43:456–462.
- Yang GE, Zhang Z, Bai H, Gong J, Wang Y, Li B, et al. Biotransformation of β-amyrin acetate by Rhodobacter sphaeroides. J Biosci Bioeng 2008;105:558–561.
- Atta-ur-Rehman, Nighat S, Muhammed IC. Microbial transformation of oleanolic acid by Fusarium lini and α-glucosidase inhibitory activity of its transformed products. Nat Product Research 2008;22:489–494.
- Boaventura DAM, Oliveria Hanson BARJ, Hitchcock BP, Takahashi AJ. The biotransformation of methyl ent-15-oxokaur-16-en-19-oate by Rhizopus stolonifer and Mucor plumbeus. Phytochemistry 1995;40:1667.
- Ruzicka L, Janot MM. Kenntnis des Sclareol, Zur Höhere Terpenverbindungen L. Zur Kenntnis des Sclareols. Helv Chim Acta 1931;14:645.
- Ipsen J, Fuska J, Foskova, A, Rossaza JP. Microbial transformations of natural antitumor agents. 21. Conversions of aphidicolin. J Org Chem 1982;47:3278.
- Jassbi AR, Zamanizadehnajari S, Azar PA, Tahara S. Antibacterial diterpenoids from Astragalus brachystachys. Z Naturforsch, C, J Biosci 2002;57:1016–1021.
- Ulubelen A, Miski M, Johansson C, Lee E, Mabry JT, Matlin AS. Terpenoids from Salvia palaestina. Phytochemistry 1985;24:1386.
- Decorzant R, Vial C, Naf F, Whitesides MG. A short synthesis of ambrox from sclareol. Tetrahedron 1987;43:1871.
- Ohloff G. (1982). In Fragrance Chemistry, The Science of the SEBSE of Smell. Theimer ET, ed. New York: Academic press, 535–573.
- Díez D, Sanchez JM, Rodilla JM, Rocha PM, Mendes RS, Paulino C et al. Microbial hydroxylation of sclareol by Rhizopus stolonifer. Molecules 2005;10:1005–1009.
- Braulio M, Fragaa P, Gonzá l, Melchor G, Herná N, Sergio S. The biotransformation of the diterpene 2-hydroxy-ent-13-epimanoyl oxide by Gibberella fujikuroi. Phytochemistry 2003;62:67–70.
- Rosazza JP, Steffens JJ, Sariaslani FS, Goswami A, Beale JM, Reeg S et al. Microbial hydroxylation of 1,4-cineole. Appl Environ Microbiol 1987;53:2482–2486.
- Demyttenaere RCJ, Pooter De LH. Biotransformation of geraniol and nerol by spores of penicillium italica. Phytochemistry 1996;41:1079.
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev 1999;12:564–582.
- National N. (1994). Spices, Herbs and Edible Fungi. In: Charalambous G, ed. Amsterdam, The Netherlands: Elsevier Science BV, 251.
- Bruneton J. (1995). Pharmacognosy, Phytochemistry, Medicinal plants. New York, USA: Lavoisier c/o Springer- Verlag, 431.
- Karapinar M, Aktug SE. Inhibition of food borne pathogens by thymol, eugenol, menthol and anethol. . Int J. Food Microbiol 1987;4:161–166.
- Asakawa Y, Takahashi H, Toyota M, Noma Y. Biotransformation of monoterpenenoids, (–)- and (+)-menthols, terpinolene and carvotanacetone by Aspergillus species. Phytochemistry 1991;30:3981.
- Miyazawa M, Kawazoe H, Hyakumachi M. Biopreparation of (1S,3R,4S,6S)-6-hydroxymenthol and (–)-(1S,3R,4S)-1-hydroxymenthol from 1-menthol by Rhizoctonia solani AG-1-IA and IB. Nat Prod Res 2003;17:307–309.
- Petrovic G, Saicic NR, Ckovic Z. Regioselective free radical phenylsulfenation of nonactivated δ-carbon atom by the photolysis of alkyl benzenesulfenates. Tetrahedron 2003;59:187–191.
- Yoshifumi Y, Yoko, Y. Synthesis and absolute configuration at C(8) of ‘p-menthane-3,8,9-triol’ derived from (–)-isopulegol. Helv Chim Acta 2004;87:2602–2607.
- Choudhary MI, Ranjit R, Atta-Ur-Rahman, Devkota KP, Musharraf SG, Shrestha TM. Hydroxylation of the sesterterpene leucosceptrine by the fungus Rhizopus stolonifer. Phytochemistry 2006;67:439–443.
- Bastos DZ, Pimentel IC, de Jesus DA, de Oliveira BH. Biotransformation of betulinic and betulonic acids by fungi. Phytochemistry 2007;68:834–839.
- Sathyamoorthy N, Wang TT. Differential effects of dietary phyto-oestrogens daidzein and equol on human breast cancer MCF-7 cells. Eur J Cancer 1997;33:2384–2389.
- Ueno T, Uchiyama S, Kikuchi N. The role of intestinal bacteria on biological effects of soy isoflavones in humans. J Nutr 2002;132:594S.
- Choudhary MI, Ranjit R, Atta-ur-Rahman, Devkota KP, Musharraf SG, Shrestha TM. Hydroxylation of the sesterterpene leucosceptrine by the fungus Rhizopus stolonifer. Phytochemistry 2006;67:439–443.