Abstract
Some new benzenesulfonylthiourea derivatives substituted with phthalazones (2a–q) were synthesized by refluxing the appropriate 4-aryl-1-oxophthalazin-2(1H)yl benzenesulfonamides with isothiocyanate in dry acetone over anhydrous K2CO3. All the synthesized compounds were characterized on the basis of IR, 1H NMR, MS data and elemental analysis. These synthesized compounds (2a–q) at the dose of 20 mg/kg were tested for antihyperglycemic activity in the glucose-fed hyperglycemic normal rat model and among these compounds 2f and 2m showed modest antihyperglycemic activity.
Introduction
Non-Insulin Dependent Diabetes Mellitus (NIDDM) is a multifactorial metabolic disease characterized by abnormalities at multiple organ sites. These defects include insulin resistance and insulin deficiencyCitation1,Citation2. The former is primarily represented by decreased insulin-stimulated glucose uptake in skeletal muscle, augmented endogenous glucose production (predominately in the liver) and enhanced lipolytic activity in fatCitation3. The latter is an apparent progressive process with both functional defects in islet cell function and, eventually, apparent loss of β-cell massCitation4,Citation5. These defects are intimately linked with derangements in one system exacerbating those in the othersCitation6. NIDDM is the most common form of diabetes constituting nearly 90% of the diabetic population in any country.
Despite recent advances both in chemistry and molecular pharmacology of antidiabetic drugs, diabetes still remains as a life-threatening disease, which tends to spread all over the world. The clinical profile of diabetic subjects is often worsened by the presence of several long-term complications, namely neuropathy, nephropathy, retinopathy and cataract.
Over the last 40 years, oral therapy for type 2 Diabetes Mellitus (DM) has focused on sulfonylureas (SU) and biguanidesCitation7. SU drugs improve glucose levels by stimulating insulin secretion by the pancreatic β-cellsCitation8. The SU were discovered accidentally; some sulfonamides were demonstrated to induce hypoglycaemia in experimental animals. This observation led to the development of a group of substituted arylsulfonylureas differing at the para-position of the benzene ring at one nitrogen residue of the urea moiety. Carbutamide (1-butyl-3-sulfonylurea) became the first drug in the diabetic therapy later withdrawn due to its adverse effect on bone marrow. However, this compound initiated the synthesis of many thousands of analogs. Two generations have been differentiated among them, according to their substitution patterns. In contrast to the first generation (tolbutamide, acetohexamide, tolazamide, chlorpropamide), the aliphatic side chain is substituted with a cyclohexyl ring in the second generation (glibenclamide, glipizide, glimepiride) of these oral hypoglycemic agents.
Side effects of SUs include weight gainCitation9–11 and hypoglycemiaCitation10,Citation12. Hypoglycemia risk becomes a more important issue as patients’ overall glucose control approaches the normal range. During the past several years, the cardiology community has become disquieted because of the potential effect of SUs on myocardial ischemic preconditioningCitation13. The actual importance of this issue in clinical practice remains unclear, but it has likely been exaggerated.
Obesity and weight gain are also contributors to development of diabetes and Coronary Heart Disease (CHD). The risk of diabetes for obese adults can be more than 90-fold the risk for slender adults. Small increase in physical activity and sustained weight losses of 5% of initial body weight can reduce risk for developing diabetes by 58%. Thus, an emphasis on weight management may be the most important therapeutic taskCitation14. Obesity, the principle cause of type 2 diabetes, remains an important target for possible drug therapy. It is assumed that weight loss agents will likely play an increasingly important role in the future therapy of obese type 2 diabetic patientsCitation15.
Chronic diabetes is accompanied by complications, such as neuropathy, nephropathy, cataracts and retinopathy, which are practically not controlled by insulin. These complications are considerately caused by an accumulation of sorbitol, which is produced from glucose by Aldose Reductase (AR) in polyol pathway. AR converts glucose to sorbitol only at high glucose levels in plasma and tissue in diabetes. The difficulty in obtaining normalization of blood glucose values has underlined the importance of the search for new and effective Aldose Reductase Inhibitors (ARIs) to control the consequences of elevated glucose levels and thereby delaying the onset and retarding the progression of diabetic complications, such as neuropathy, nephropathy, retinopathy and cataract. A large number of AR inhibitors have been prepared synthetically and some of them are used therapeuticallyCitation16. Recently compounds containing pyridazine nucleus have been reported as AR inhibitorsCitation17–19. Zopolrestat is a phthalazinone derivative that has been in clinical trials; it inhibits AR and has potential use in the prevention of retinopathy, neuropathy and cataract formation in diabetesCitation20.
The levels of serum lipids are usually elevated in DM and such an elevation represents a risk factor for CHDCitation21. The abnormal high level of serum lipids is mainly due to the uninhibited actions of lipolytic hormones on the fat depots mainly due to the action of insulin, since under normal circumstances, insulin activates the enzyme lipoprotein lipase, which hydrolyses triglycerides. However, in diabetic state, lipoprotein lipase is not activated due to insulin deficiency resulting in hypertriglyceridaemiaCitation21. In addition, insulin has an inhibitory action on 3-hydroxy-3-methyl glutaryl Co A (HMG- Co A) reductase, a key rate-limiting enzyme responsible for the metabolism of cholesterol-rich LDL particlesCitation22. Recently two compounds derived from phthalazones have been reported as HMG- Co A reductase inhibitorsCitation23. Azelastine, a phthalazone derivative, has shown the best plasma glucose lowering activity as well as vasorelaxant activityCitation24.
Since the phthalazone nucleus have been reported to have lipid lowering and AR inhibitory activity, it was considered to synthesize SU substituted with 4-aryl-1-oxophthalazin-2(1H)yl-ones ().
Figure 1. Structure of biologically active agent of benzenesulfonylthiourea’s as blood glucose lowering and rationally designed template for targeted compound.
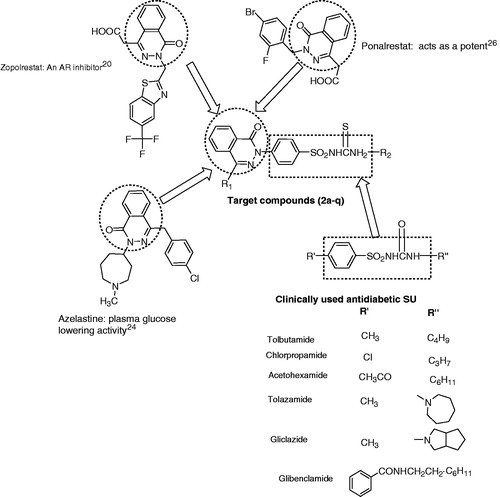
In the present study, 17 new target compounds (2a–q) were synthesized by condensing appropriate 4-aryl-1-oxophthalazin-2(1H)yl benzenesulfonamides with isothiocyanate () and these compounds were assessed for oral antihyperglycemic effects in glucose-fed hyperglycemic normal rats.
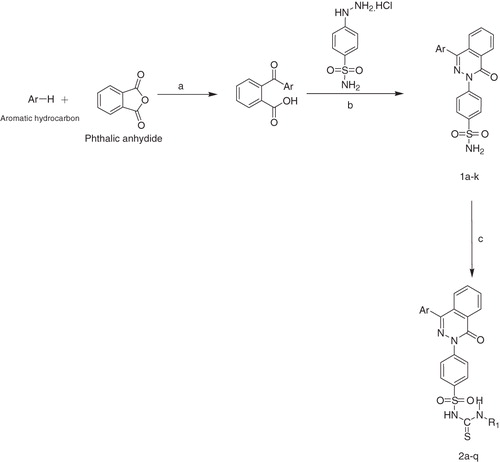
Scheme 1. Reagents and conditions: (a) anhydrous AlCl3, room temperature; (b) absolute alcohol, reflux; (c) isothiocyanate, K2CO3, dry acetone, reflux 24–72 h. 1a, 2a, 2f, 2j, 2o: Ar = Phenyl; 1b, 2b, 2g, 2k, 2p: Ar = 4-Methylphenyl 2a–2e:R1 = –CH2C6H5; 1c, 2c, 2h, 2l: Ar = 4-Chlorophenyl; 1d, 2d, 2i, 2m, 2q: Ar = 4-Chloro-3-methylphenyl; 1e, 2e, 2n, 2k: Ar = 2-Chloro-5-methyl; 2f–2i: R1 = –C3H7; 2j–2n: R1 = –C4H9; 2o–2q: R1 = –C6H11.
Experimental
Melting points were determined by open capillary tubes and are uncorrected. Infrared (IR) spectra were recorded (in KBr) on a BIO-RAD FTS-135 spectrophotometer (Waltham, MA) and νmax values are given in cm−1. 1H NMR spectra were recorded on a Bruker Spectrospin DPX 300-MHz/400-MHz spectrometer (Fällanden, Switzerland) using deuterated DMSO or CDCl3 as solvent and tetramethyl silane (TMS) as an internal standard. Chemical shifts are given in δ (ppm) scale and coupling constants (J values) are expressed in Hertz. Mass spectra (MS) were scanned by using ESI Bruker Esquire 3000 (Billerica, MA). The m/z values of the more intense peaks are mentioned. Purity of the compounds was checked on TLC plates (silica gel G), which were visualized by exposing to iodine vapors. Elemental analysis was carried out on a CHNS Elementar (Vario EL III, Hanau, Germany).
General procedure for the synthesis of sulfonylthioureas (2a–q)
A solution of appropriate 4-arylphthalazone (1 mmol) in dry acetone was refluxed over anhydrous K2CO3 for 1–1.5 h. At this temperature, a solution of isothiocyanate (1.2 mmol) in dry acetone of 5 mL was added in a drop-wise manner. Refluxing was continued for 24–72 h. On completion of the reaction, acetone was removed by distillation method under reduced pressure and the residue was suspended in water. The suspension was acidified with acetic acid. It was filtered, washed and dried residue was crystallized by ethyl alcohol.
N-(benzylcarbamothioyl)-4-(1-oxo-4-phenylphthalazin-2(1H)-yl)benzenesulfonamide (2a)
Yield = 25%, m.p. 231–232 °C, Rf = 0.30 (toluene:ethyl acetate:formic acid, 7.5: 2: 0.5). IR υmax (KBr, in cm−1): 3368 and 2892 (NH of thiouriedo group), 1570 (C=S of thiourea), 1664 (cyclic carbonyl), 1592 (C=N), 1340 and 1134 cm−1 (SO2N). 1H NMR (300 MHz, DMSO, δ): 4.52 (2H, d, J = 6.3 Hz, −CH2 of benzyl unit), 7.16–7.29 (5H, m, benzyl unit protons), 7.58–7.98 (13H, m, H-4′, H-3′, H-5′, H-2′, H-6′, H-6, H-7, H-8/H-5, NHCSNHCH2, H-3′′, H-5′′, H-2′′, H-6′′), 8.46–8.47 (1H, m, H-5/H-8). ESI-MS (m/z): 526 [M+], 527 [M+ + 1]. Anal. Calcd. for C28H22N4O3S2; C = 63.86, H = 4.21, N = 10.64, S = 12.18. Found: C = 63.88, H = 4.18, N = 10.60, S = 12.16.
N-(benzylcarbamothioyl)-4-(1-oxo-4-p-tolylphthalazin-2(1H)-yl)benzenesulfonamide (2b)
Yield = 72%, m.p. 218–219 °C, Rf = 0.46 (toluene:ethyl acetate:formic acid, 7.5:2:0.5). IR υmax (KBr, in cm−1): 3358 and 2853 (NH of thiouriedo group), 1503 (C=S of thioureido group), 1656 (cyclic C=O), 1591 (C=N), 1372 and 1137 cm−1 (SO2N). 1H NMR (400 MHz, CDCl3, δ): 2.45 (3H, s, CH3), 4.52 (2H, s, −CH2 of benzyl unit), 7.13–7.19 (5H, m, benzyl unit protons), 7.25 (2H, d, J = 6.8 Hz, H-3′, H-5′), 7.45 (2H, d, J = 7.2 Hz, H-2′, H-6′), 7.63–7.89 (8H, m, H-6, H-7, H-8/H-5, H-3′′, H-5′′, H-2′′, H-6′′ and NHCSNHCH2), 8.59 (1H, s, H-5/H-8). ESI-MS (m/z): 541[M+ + 1], 563 [M+ + Na]. Anal. Calcd. for C29H24N4O3S2; C = 64.42, H = 4.47, N = 10.36, S = 11.86. Found: C = 64.44, H = 4.44, N = 10.37, S = 11.85.
N-(benzylcarbamothioyl)-4-(4-(4-chlorophenyl)-1-oxophthalazin-2(1H)-yl)benzenesulfonamide (2c)
Yield = 40%, m.p. 204–206 °C, Rf = 0.66, (toluene:ethyl acetate:formic acid, 7.5:2.0:0.5). IR υmax (KBr, in cm−1): 3342 and 2862 (NH of thioureido group), 1531 (C=S of thiourea), 1662 (cyclic carbonyl), 1584 (C=N), 1382 and 1106 (SO2N). 1H NMR (400 MHz, DMSO, δ): 4.64 (2H, d, J = 5.1 Hz, –CH2 of benzyl unit), 7.07–7.21 (5H, m, benzyl unit protons), 7.50 (2H, d, J = 8.4 Hz, H-3′, H-5′), 7.61 (2H, d, J = 8.0 Hz, H-2′, H-6′), 7.69–7.72 (1H, m, H-8/H-5), 7.79–7.87 (2H, m, H-6, H-7), 7.89 (2H, d, J = 8.8 Hz, H-3′′, H-5′′), 7.96 (2H, d, J = 8.0 Hz, H-2′′, H-6′′), 8.45 (1H, ortho–meta coupled dd, J = 3.2 Hz, J = 5.6 Hz, H-5/H-8), 8.58–8.59 (1H, m, NHCSNHCH2 protons). ESI-MS (m/z): 562[M+ + 2]. Anal. Calcd. for C28H21ClN4O3S2; C = 59.94, H = 3.77, N = 9.99, S = 11.43. Found: C = 60.00, H = 3.75, N = 10.00, S = 11.43.
The characterization data of compounds (2d–q) can be obtained online as supplementary data.
Effect of synthesized compounds on oral glucose tolerance in normal rats
Albino rats (either sex) of Wistar strain weighing 150–200 g were fasted overnight and divided into groups of six animals each. Animals of group I received only vehicle (10 ml/kg) orally to serve as control, while animals of group II were given gliclazide 20 mg/kg orally, suspended in the vehicle (10 ml/kg). The test compounds in the dose of 20 mg/kg suspended in the vehicle (10 ml/kg) were administered orally to respective groups. All the animals were given glucose (3 g/kg, p.o.) 30 min after dosing. Blood samples were collected from retro-orbital plexus just prior to and 60 min after the glucose loading and blood glucose levels were measured with an autoanalyzer by using AccuCheck Advantage II glucose kit (Roche, West Sussex, UK).
Results and discussion
Synthesis of compounds
The synthetic route used to synthesize title compounds (2a–q) is outlined in . The 4-aryl-phthalazone-substituted benzenesulfonamide derivatives (1a–k) synthesized through reported methodCitation25 were converted to SU by refluxing with appriopriate isothiocyanates in dry acetone containing K2CO3. Purity of the compounds was checked on TLC plates (Merck, Darmstadt, Germany) (silica gel G) which were visualized by exposing to iodine vapors.
Structures of the synthesized compounds (2a–q) were established on the basis of elemental analysis and various spectroscopic methods, viz. IR, 1H NMR and MS. Elemental analysis (C, H, N and S) data were within ±0.4% of the theoretical values. In the IR spectra of 2a–q three bands characteristic of SU moiety out of which one band for thioureido group at (1503–1570 cm−1) and two bands for NH at (3320–3371 cm−1 and 2850–2960 cm−1) were observed. Apart from these a band in the region 1578–1594 cm−1 corresponding to C=N stretching, a band at 1647–1712 cm−1 for cyclic carbonyl group (C=O) and two bands at 1340–1387 and 1080–1183 cm−1 for >SO2NH group were also observed. The structure was further established by proton NMR spectral data. The H-5/H-8 proton of phthalazone ring appeared in the NMR spectra at δ 8.41–8.62.
Antihyperglycemic activity
In the present study, oral antihyperglcemic effects of 17 compounds (2a–q) were assessed in glucose-fed hyperglycemic normal rats. The marketed SU drug gliclazide was selected as positive control. Quantitative glucose tolerance of each animal was calculated by the area under curve (AUC) method by using the Prism software (Irvine, CA). Comparing AUC of experimental and control groups determined the percentage of antihyperglycemic activity. Samples showing significant inhibition (p < 0.05) were considered as active samples. Statistical comparison was made by Dunnett’s test.
The results showed that two compounds namely 2f and 2m were found to be in abundance. The compounds 2f and 2m significantly inhibited the rise in postprandial hyperglycemia to the tune of 16.0% (p < 0.05) and 17.53% (p < 0.01), respectively. The standard drug Gliclazide caused nearly 35% (p < 0.01) inhibition. With regard to the SAR, it seems impossible to extract an obvious structure–activity relationship from the data shown in .
Table 1. Antihyperglycemic activity of SU derivatives (2a–q) and the standard drug gliclazide in glucose-fed (3g/kg) hyperglycemic normal rats.
Supplementary material
The characterization data of compounds (2d--q) can be obtained online as supplementary data. Supplementary data associated with this article can be found, in the online version.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
One of the authors, Shafiya Yaseen is thankful to DST for INSPIRE fellowship.
Supplementary Material
Download PDF (96.4 KB)Acknowledgements
Thanks are due to IIM Jammu for providing Mass spectra.
References
- Ferrannini E. Insulin resistance versus insulin deficiency in non-insulin-dependent diabetes mellitus: problems and prospects. Endocr Rev 1998;19:477–90
- Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus: prospective studies of Pima Indians. Engl J Med 1993;329:1988–92
- Sulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 2000;106:171–6
- Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003;46:3–19
- LeRoith D. β-cell dysfunction and insulin resistance in type 2 diabetes: role of metabolic and genetic abnormalities. Am J Med 2002;113:3–11
- Robertson RP, Harmon J, Tran PO. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004;53:5119–24
- Kecskemeti V, Bagi Z, Posa I, et al. New trends in the development of oral antidiabetic drugs. Curr Med Chem 2002;9:53–71
- Muller G, Hartz D, Punter J, et al. Differential interaction of glimperide and glibenclamide with β-cell sulfonylurea receptor. Biochim Biochim Acta 1994;1191:267--77
- Schade DS, Jovanovic L, Schneider J. A placebo-controlled, randomized study of glimepiride in patients with type 2 diabetes mellitus for whom diet therapy is unsuccessful. J Clin Pharmacol 1998;38:636–41
- Zimmerman BR. Sulfonylureas. Endocrinol Metab Clin North Am 1997;26:511–21
- Simonson DC, Ferrannini E, Bevilacqua S, et al. Mechanism of improvement in glucose metabolism after chronic glyburide therapy. Diabetes 1984;33:838–45
- Kilo C, Meenan A, Bloomgaren Z. Glyburide versus glipizide in the treatment of patients with noninsulin-dependent diabetes mellitus. Clin Ther 1992;14:801–12
- Klepzig H, Kober G, Matter C, et al. Sulfonylureas and ischaemic preconditioning. A double-blind, placebo-controlled evaluation of glimepiride and glibenclamide. Eur Heart J 1999;20:403–5
- Anderson JW, Kendall CWC, Jenkins DJA. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Nut 2003;22:331–9
- Kimmel B, Inzucchi SE. Oral agents for type 2 diabetes: an update. Clin Diab 2005;23:64–76
- Kawanishi K, Ueda H, Moriyasu M. Aldose reductase inhibitors from the nature. Curr Med Chem 2003;10:1353–74
- Costantino L, Rastelli G, Vescovini K, et al. Synthesis, activity, and molecular modeling of a new series of tricyclic pyridazinones as selective aldose reductase inhibitors. J Med Chem 1996;36:4396–405
- Costantino L, Rastelli G, Cignarella G, Barlocco D. Synthesis and aldose reductase inhibitory activity of a new series of benzo[H]cinnolinone derivatives. Farmaco 2000;55:544–52
- Courdert P, Duroux E, Bastide P, Couquelet J. Synthesis and evaluation of the aldose reductase inhibitory activity of new diaryl pyridazine-3-ones. J Pharm Belg 1991;46:375–80
- Napoletano M, Norcini G, Pellacini F, et al. Phthalazine PDE4 inhibitors. Part 2: the synthesis and biological evaluation of 6-methoxy-1,4-disubstituted derivatives. Bioorg Med Chem Lett 2001;11:33–7
- Pushparaj PN, Low HK, Manikandan J, et al. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J Ethnopharmacol 2007;111:430–4
- Murali B, Upadhyaya UM, Goyal RK. Effect of chronic treatment with Enicostemma littorale in non-insulin dependent diabetic (NIDDM) rats. J Ethnopharmacol 2002;81:199–204
- Ghaffar NF, Mohamed MA, Ghanem HM, Zaki HM. Synthesis and biochemical evaluation of some substituted phthalazines. J Am Sci 2011;7:771–81
- Olmo ED, Barboza B, Ybarra MI, et al. Vasorelaxant activity of phthalazinones and related compounds. Bioorg Med Chem Lett 2006;16:2786–90
- Yaseen S. Synthesis of blood glucose lowering heterocyclic compounds [PhD thesis]. New Delhi: Jamia Hamdard (To be submitted)
- Laudadio C, Sima AA. Progression rates of diabetic neuropathy in placebo patients in an 18-month clinical trial. J Diabetes Complications 1998;12:121–7

