Abstract
In the present study, 2-[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]acetohydrazide (1) was used as starting compound for the synthesis of 2-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]acetyl}-4-thiosemicarbazides (2a–c) and 5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-1,3,4-oxadiazole-2-thione (5). The cyclization of compounds 2a–c in the presence of NaOH resulted in the formation of 5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-4-alkyl/aryl-2,4-dihydro-3H-1,2,4-triazole-3-thiones (3a–c). Aminomethylation of compounds 3a–c and 5 with formaldehyde and N-methyl/phenylpiperazine furnished Mannich bases (4a–f and 6a–b). The newly synthesized compounds were well-characterized by IR, 1H NMR, 13C NMR, elemental analysis and mass spectral studies. They were also screened for their lipase and α-glucosidase inhibition. Among the tested compound 2c (IC50 = 2.50 ± 0.50 µM) showed the best anti-lipase activity and compounds 2c (IC50 = 3.41 ± 0.16 µM) and 6a (IC50 = 4.36 ± 0.10 µM) showed the best anti-α-glucosidase activity.
Introduction
Obesity is a major global public health problem, and its prevalence is steadily increasingCitation1. Many serious chronic diseases such as hypertension, high total cholesterol and triglycerides, type II diabetes mellitus, sleep apnea and respiratory problems, cancer, stroke, and coronary heart diseases are attributable to obesityCitation2. Basically, there are two types of anti-obesity agents, classified as inhibitors of lipid (lipase inhibitor) and carbohydrate (α-glucosidase inhibitor) absorptionCitation3.
Lipase is the lipid-digesting enzyme that catalyzes the hydrolysis of ester bonds of triacylglycerols (TAGs) to produce free fatty acids, diacylglycerols, monoglycerols and glycerol. The break down and digestion of dietary TAG in the small intestine of mammals is enabled by pancreatic lipaseCitation4. The resulting free fatty acids are then absorbed by the body and if not utilized, added to the development of obesity. Therefore, if the breakdown and hydrolysis of TAGs can be stopped or minimized, absorption will be lowered and consequently the prevalence of obesity could be reducedCitation5. Hence, an inhibitor of pancreatic lipases could be a useful agent in the fight against obesityCitation6. Orlistat is the only authorized anti-obesity drug in Europe. However, it has some side effects, including fecal incontinence, flatulence and steatorrheaCitation7.
Carbohydrate digestion is catalyzed by the enzyme α-glucosidase which is an exo-acting enzyme located in the brush-border surface membrane of intestinal cellsCitation8. It releases an α-d-glucose from the non-reducing end of the sugar by acting on 1,4-α linkagesCitation9. The α-glucosidase inhibitors in the gut work against α-glucosidase in the gut preventing the release of glucose from disaccharides and oligosaccharides, and therefore reducing postprandial glucose levelsCitation10. It is considered as a significant strategy in the management of diabetes mellitus if the α-glucosidase inhibitors are used. For example, in the treatment of diabetes mellitus patients, some α-glucosidase inhibitors, such as acarbose and voglibose, are extensively used but often causes severe gastrointestinal side effects such as flatulence and diarrheaCitation11–13.
Heterocyclic compounds containing 1,2,4-triazole skeleton are known to possess a wide range of pharmacological activities such as antimicrobial, antitubercular, anti-inflammatory, antiviral, antioxidant, antidepressant, anticonvulsant, antimalarial, anti-diabetic, anti-obesity, enzyme inhibitory and anticancer activitiesCitation14–17. Some present day drugs such as fluconazole, itraconazole, terconazole (antifungal agents), anastrozole, letrozole, vorozole (aromatase inhibitors in the treatment of postmenopausal breast cancer), ribavirin (antiviral agent), etoperidone and nefazodone (antidepressant), rizatriptan (antimigraine agent) and rilmazafon (hypnotic, anxiolytic, used in the case of neurotic insomnia) are examples of potent bioactive molecules possessing 1,2,4-triazole moietiesCitation16,Citation18–21. Similarly, heterocyclic compounds bearing 1,3,4-oxadiazole nucleus are also known to possess a wide spectrum of pharmacological properties, including analgesic, anti-inflammatory, antimicrobial, antifungal, anti-convulsant, anti-diabetic, antihelmintic, hypo-glycemic, antiallergic, enzyme inhibitors, antineoplastic, CNS depressant, antitubercular and anticancerCitation22–26. Furthermore, there are a number of drugs containing 1,3,4-oxadiazole ring such as raltegravir (antiretroviral), nesapidil (antiarrhythemic), furamizole (antibacterial), tiodazosin (antihypertensive) and fenadiazole (hypnotic)Citation27. Other important groups of heterocyclic compounds for medicinal chemistry are the thiosemicarbazides and their corresponding cyclized counterpart 1,2,4-triazole-thions which have been extensively reported in the literature for the synthesis of new biologically active moleculesCitation28.
Mannich base is a β-amino-carbonyl compound, which is formed in the reaction of an amine (primery or secondary), formaldehyde and a reactive hydrogen atomCitation29. Mannich bases have been reported to be useful agent for potential biological activities such as anticancer, antibacterial, antifungal, analgesic, anti-inflammatory, antimalarial and antitubercularCitation30. Many Mannich bases of 1,2,4-triazoles carrying N-methylpiperazine moiety are reported to possess protozoacidal and bactericidal activitiesCitation31. The modern drugs, Prazosin, Lidoflazine and Urapidil, carrying piperazine nucleus are used as cardiovascular agentsCitation31. Furthermore, Mannich bases containing 1,3,4-oxadiazoles ring are also known to posses a number of biological activitiesCitation32.
In view of these findings, the aim of this present study is to obtain 1,2,4-triazole and 1,3,4-oxadiazole derivatives containing 3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazole sekeleton incorporating also Mannich base structures. All newly synthesized compounds were screened for the lipase and α-glucosidase inhibition.
Results and discussion
Chemistry
The reaction sequence employed for synthesis of target compounds is shown in Scheme 1. The starting compound 2-[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]acetohydrazide (1) was prepared according to our previous reportCitation33.
Scheme 1. Reagents and conditions: (i) absolute ethanol, RNCS, reflux; (ii) 2 N NaOH, reflux; (iii) and (v) DMF, HCHO, N-methyl/phenyl piperazine, room temperature; (iv) ethanol, CS2/KOH, relux.
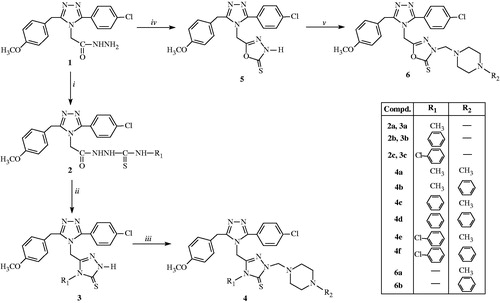
Reaction of compound 1 with various isothiocyanate resulted in the formation of 2-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]acetyl}-4alkyl/arylthiosemicarbazides (2a–c)Citation34. The IR spectra of these compounds showed moderately strong bands around 3150–3360, 1688–1689 and 1177–1182 cm−1, characteristic of the NH, C = O and C = S groups, respectivelyCitation34. The 1H NMR spectra of the compounds displayed three singlets due to N1–H, N2–H and N3–H groups at δ 8.09–9.78, 9.40–9.89 and 10.26–10.53 ppmCitation35. The 13C NMR spectra showed two signals at δ 163.20–166.18 ppm and δ 181.56–182.61 ppm characteristic to C = O and C = S carbons, respectively, which confirm the formation of thiosemicarbazidesCitation34,Citation35. Alkaline cyclization of the compounds (2a–c) using NaOH afforded the corresponding derivatives (3a–c)Citation36. These compounds can exist in two tautomeric forms, 5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-4-alkyl/aryl-2,4-dihydro-3H-1,2,4-triazole-3-thioles and 5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-4-alkyl/aryl-2,4-dihydro-3H-1,2,4-triazole-3-thiones (3a–c). The spectral analysis (IR, 1H NMR and 13C NMR) shows that these compounds exist in latter tautomeric form. According to IR spectroscopic data of compounds (3a–c), the SH vibration band (2550–2600 cm−1) was absent and C = S stretching bands were observed at 1321–1326 cm−1. Moreover, NH stretching bands of 3a–c were observed at 3169, 3236 and 3240 cm−1, respectivelyCitation37,Citation38. In the 1H NMR spectra of these compounds, NH peaks were observed as a singlet at δ 13.75, 14.02 and 14.07 ppm, respectivelyCitation38. The C = S group resonated at δ 168.08–169.15 ppm in the 13C NMR spectra of compounds (3a–c) and so their thionic form was provedCitation39.
Intramolecular cyclization of the acid hydrazide (1) with carbon disulfide in the presence of potassium hydrtoxide in absolute alcohol resulted in 5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-1,3,4-oxadiazole-2(3H)-thione (5)Citation40. The IR spectra of this compound showed NH bands in the region of 3291 cm−1 and C = S absorption band at 1326 cm−1 instead of SH band at around 2550–2600 cm−1 Citation41,Citation42. The N–H protons of 1,3,4-oxadiazole ring (5) were seen at δ 13.83 ppmCitation41,Citation42. The 13C NMR data of 5 which showed a peak at δ 160.12 and 178.55 ppm, due to oxadiazole C-5 and oxadiazole C-2 (C = S)Citation41,Citation42.
Mannich bases 4a–f and 6a–b were synthesized from the compounds 3a–c and 5 by reacting with N-methylpiperazine or N-pheylpiperazine in dimethyl formamide in the presence of formaldehydeCitation33. The IR spectra of these compounds showed moderately strong bands around 1170–1179 and 1325–1339 cm−1, characteristic of the N–CH2–N and C = S groups, respectivelyCitation43. In the 1H NMR spectra, a characteristic signal due to the –N–CH2–N– protons appeared at δ 5.49–5.74 ppm. The signal due to the N–CH3 (for 4a–c and 6a) protons appeared at δ 5.50–5.52 ppmCitation43,Citation44. The signals due to the N–CH2–N and C = S carbons appeared at δ 68.30–70.06 and 168.95–178.27 ppm in the 13C NMR, respectivelyCitation43,Citation44.
Biological evaluation
Anti-lipase activity
All compounds were evaluated with regard to pancreatic lipase activity and 2a, 2c and 6a showed anti-lipase activities at various concentrations (). No significant inhibitory effect was detected for other compounds. Among the tested compounds, 2c showed the best anti-lipase activity. The compound inhibited pancreatic lipase by 100.00 ± 0.49% at concentration of 10 µM (). Orlistat, known pancreatic lipase inhibitor used as anti-obesity drug, showed inhibitory effect by 99.15 ± 0.35% at concentration of 312 nM (IC50 = 0.85 ± 0.042 nM). Compounds 2a and 2c IC50 values were calculated as 10.25 ± 0.40 and 2.50 ± 0.50 µM, respectively. Compund 2c and Orlistat almost completely inhibited lipase activity, whereas 2a and 6a were less effective. The difference was not statistically significant for compound 2c compared to Orlistat. Synthesized compound 2c has a potential to be an alternative for Orlistat.
Table 1. Inhibitory effects of selected synthesized compounds (at final concentration of 10 µM).
α-Glucosidase inhibitory activity
All compounds were evaluated with regard to α-glucosidase activity and 2c, 6a and 6b showed anti-α-glucosidase activity at various concentrations (). These compounds exhibited more inhibitory effect than Acarbose, known α-glucosidase inhibitor was used as anti-diabetic drug (). No significant inhibitory effect was detected for other compounds. Among the tested compounds, 2c and 6a showed the best anti-α-glucosidase activity (p < 0.05). These compounds inhibited α-glucosidase activity by 99.79 ± 1.17% and 99.87 ± 0.38% at a concentration of 100 µM, respectively. Acarbose showed inhibitory effect by 55.4 ± 3.01% at the same concentration (IC50 = 26.12 ± 2.38 µM). Compounds 2c and 6a IC50 values were calculated as 3.41 ± 0.16 and 4.36 ± 0.10 µM, respectively (). Compunds 2c, 6a and 6b almost completely inhibited α-glucosidase activity. The difference was statistically significant for these compounds compared to Acarbose. Synthesized compounds 2c, 6a and 6b have substantial inhibition potential.
Figure 1. Dose-dependent inhibitory effect of compounds 2c, 6a and 6b. Acarbose was used as standard inhibitor. Inhibitory effect of the tested compounds and Acarbose was measured at the range of 0.045–100 µM concentrations. Residual activities of the compounds were expressed as the mean ± S.D., measured in triplicate.
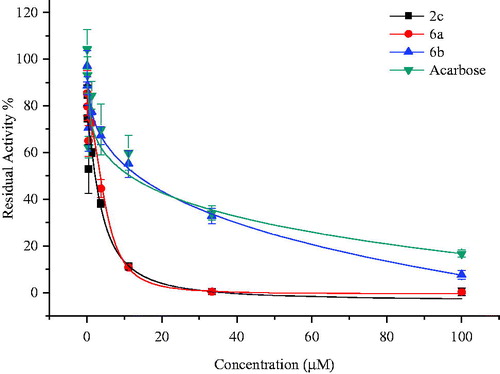
Table 2. Inhibition of α-glucosidase by newly synthesized compounds 2–6.
Statistical analysis
Results are expressed as means ± standard error of the mean for each experiment. Statistical analyses were performed by one-way analysis of variance (ANOVA) followed by Dunnett’s Multiple Comparison Test and p value of less than 0.05 was considered to be statistically significant.
Experimental
Chemistry
Melting points were determined in open capillaries on a Gallenkamp Electrothermal digital melting point apparatus (London, England). IR spectra were recorded in a Perkin-Elmer Frontier FT-IR spectrophotometer (San Jose, CA) using attenuated total reflection (ATR) accessory. 1H and 13C NMR spectra were measured on a BRUKER AVANCE II-400 (400 MHz) (Darmstadt, Germany) using DMSO-d6 as solvent and TMS as internal standard. The elemental analysis was performed on a Costech Elemental Combustion System CHNS-O elemental analyzer (Valencia, CA). All the compounds gave C, H and N analyses within ± 0.4% of theoretical values. The mass spectra were taken on a Quattro LC–MS (70 eV) instrument (Manchester, UK). The progress of the reaction was monitored by thin layer chromatography (TLC) on silica gel plates (silica gel 60 F 2.54 0.2 mm thickness). Compound 1 was synthesized by the methods reported earlierCitation33.
General procedure for the synthesis of 2-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]acetyl}-4-alkyl/arylthiosemicarbazide (2a–c)
2-[3-(4-Chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]acetohydrazide (0.01 mol) was mixed with corresponding isothiocyanate (0.01 mol) in absolute ethanol (50 mL). Then this mixture was refluxed for about 4 h. At the and of the reaction, crude product collapsed. The precipitate formed was filtered off and purified by recrystallization from ethanol.
2-{[3-(4-Chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]acetyl}-4-metylthiosemicarbazide (2a). Yield (3.98 g, 89.44%); m.p. 184–185 °C;IR (ATR, νmax, cm−1): 3228, 3150 (3NH), 1688 (C = O), 1182 (C = S); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 2.90 (d, 3H, CH3, J = 4.0 Hz), 3.73 (s, 3H, OCH3), 4.04 (s, 2H, benzyl CH2), 5.00 (s, 2H, N–CH2), Ar–H: [6.90 (d, 2H,J = 8.4 Hz), 7.23 (d, 2H, J = 8.4 Hz), 7.50 (d, 2H, J = 8.4 Hz), 7.93 (d, 2H, J = 8.4 Hz)], 8.09 (bs, 1H, NH), 9.40 (s, 1H, NH), 10.26 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 30.76 (benzyl CH2), 31.36 (NH–CH3), 49.91 (N–CH2), 55.51 (OCH3), Ar–C: [114.42 (2CH), 127.84 (2CH), 128.36, 129.28 (2CH), 130.32 (2CH), 130.37, 134.11, 158.57], 157.72 (triazole C-3), 159.26 (triazole C-5), 163.20 (C = O), 182.63 (C = S); EI MS m/z (%): 467.35 ([M+Na]+, 58), 434.31 (100), 327.51 (60), 229.27 (10), 163.13 (16), 130.28 (18); Anal. Cald for C20H21ClN6O2S: C, 53.99; H, 4.76; N, 18.89. Found: C, 54.14; H, 4.70 N, 19.00.
2-{[3-(4-Chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]acetyl}-4-phenylthiosemicarbazide (2b). Yield (4.70 g, 92.70%); m.p. 193–194 °C; IR (ATR, νmax, cm−1): 3336, 3252, 3168 (3NH), 1701 (C = O), 1177 (C = S); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 3.72 (s, 3H, OCH3), 4.12 (s, 2H, benzyl CH2), 5.08 (s, 2H, N–CH2), Ar–H: [6.89 (d, 2H, J = 8.4 Hz), 7.19–7.26 (m, 3H), 7.34–7.40 (m, 4H), 7.50 (d, 2H, J = 8.0 Hz), 7.94 (d, 2H, J = 8.4 Hz)], 9.78 (s, 2H, 2NH), 10.51 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 30.71 (benzyl CH2), 50.02 (N–CH2), 55.51 (OCH3), Ar–C: [114.41 (2CH), 128.56 (CH), 127.84 (2CH), 128.39, 128.67 (2CH), 129.27 (2CH), 130.19, 130.35 (2CH), 130.40 (2CH), 134.08, 139.41, 158.57], 157.74 (triazole C-3), 159.27 (triazole C-5), 166.18 (C = O), 181.56 (C = S); LC–MS/MS m/z (%): 529.36 ([M+Na]+, 100), 507.40 ([M]+, 62), 436.38 (20), 394.33 (38), 372.43 (32), 358.29 (61), 300.29 (15), 229.46 (18); Anal. Cald. for C25H23ClN6O2S: C, 59.22; H, 4.57; N, 16.58. Found: C, 59.45; H, 4.53; N, 16.71.
2-{[3-(4-Chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]acetyl}-4-(4-chlorophenyl)thiosemicarbazide (2c). Yield (5.20 g, 95.94%); m.p. 198–199 °C; IR (ATR, νmax, cm−1): 3360, 3240, 3157 (3NH), 1689 (C = O), 1178 (C = S); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 3.72 (s, 3H, OCH3), 4.12 (s, 2H, benzyl CH2), 5.07 (s, 2H, N–CH2), Ar–H: [6.84–6.90 (m, 2H), 7.25 (d, 2H, J = 8.4 Hz), 7.40–7.44 (m, 4H), 7.49–7.51 (m, 2H), 7.92–7.94 (m, 2H)], 9.89 (s, 2H, 2NH), 10.53 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 30.80 (benzyl CH2), 50.02 (N–CH2), 55.51 (OCH3), Ar–C: [114.41 (2CH), 127.83 (2CH), 128.57, 128.60 (2CH), 129.28 (2CH), 130.17, 130.40 (4CH), 134.01, 134.09, 138.42, 158.57], 157.73 (triazole C-3), 159.27 (triazole C-5), 163.24 (C = O), 182.61 (C = S); LC–MS/MS m/z (%): 565.59 ([M+Na]+, 48), 541.50 ([M]+, 62), 415.67 (22), 359.42 (25), 283.46 (39), 229.33 (100), 214.38 (40), 113.45 (26); Anal. Cald. for C25H22Cl2N6O2S: C, 55.46; H, 4.10; N, 15.52. Found: C, 55.63; H, 4.02; N, 15.64.
General procedure for the synthesis of 5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-4-alkyl/aryl-2,4-dihydro-3H-1,2,4-triazole-3-thione (3a–c)
A 10 mL ethanolic solution of corresponding thiosemicarbazide (2a–c) (0.005 mol) was heated in aqueous NaOH solution (2 N, 25 mL) under reflux for 6 h. After completion of reaction, the mixture was cooled and then acidified to pH 3–4 with concentrated HCl. The precipitate formed was filtered, washed with cold water and recrystallized from ethanol–water (3:1) to yield the desired compound. The completion of reaction was monitored by thin-layer chromatography.
5-{[3-(4-Chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-4-methyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (3a). Yield (1.85 g, 86.04%); m.p. 207–208 °C; IR (ATR, νmax, cm−1): 3169 (NH), 1611, 1578 (C = N), 1326 (C = S); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 3.38 (s, 3H, CH3), 3.72 (s, 3H, OCH3), 4.22 (s, 2H, benzyl CH2), 5.67 (s, 2H, N–CH2), Ar–H: [6.86 (d, 2H, J = 8.4 Hz), 7.21 (d, 2H, J = 8.8 Hz), 7.50 (d, 2H, J = 8.4 Hz), 7.94 (d, 2H, J = 8.4 Hz)], 13.75 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 30.59 (benzyl CH2), 30.64 (NH–CH3), 43.58 (N–CH2), 55.52 (OCH3), Ar–C: [114.37 (2CH), 127.86(2CH), 127.94, 128.07 (2CH), 129.33, 129.91 (2CH), 134.30, 158.59], 148.04 (triazole C-5, second ring), 157.40 (triazole C-3), 159.68 (triazole C-5), 168.08 (triazole C-3, second ring); LC–MS/MS m/z (%): 427.48 ([M+1]+, 17), 358.35 (22), 306.48 (15), 219.38 (32), 130.28 (100); Anal. Cald. for C20H19ClN6OS: C, 56.27; H, 4.49; N, 19.69. Found: C, 56.42; H, 4.55; N, 19.73.
5-{[3-(4-Chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (3b). Yield (2.05 g, 80.71%); m.p. 255–256 °C; IR (ATR, νmax, cm−1): 3236 (NH), 1615, 1578 (C = N), 1321 (C = S); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 3.72 (s, 3H, OCH3), 3.81 (s, 2H, benzyl CH2), 5.44 (s, 2H, N–CH2), Ar–H: [6.84 (d, 2H, J = 8.4 Hz), 7.07 (d, 2H, J = 8.4 Hz), 7.28–7.31 (m, 2H), 7.47–7.49 (m, 5H), 7.84 (d, 2H, J = 8.4 Hz)], 14.02 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 30.31 (benzyl CH2), 48.32 (N–CH2), 55.53 (OCH3), Ar–C: [114.40 (2CH), 127.46, 127.80 (2CH), 127.92 (2CH), 128.54 (2CH), 129.24 (2CH), 129.87, 130.18 (2CH), 130.42 (CH), 133.35, 134.22, 158.56], 147.43 (triazole C-5, second ring), 156.99 (triazole C-3), 159.49 (triazole C-5), 169.15 (triazole C-3, second ring); LC–MS/MS m/z (%): 489.38 ([M+1]+, 18), 478.49 (32), 456.46 (13), 329.32 (20), 157.06 (72), 139.11 (100); Anal. Cald. for C25H21ClN6OS: C, 61.41; H, 4.33; N, 17.19. Found: C, 61.63; H, 4.41; N, 17.07.
5-{[3-(4-Chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-4-(4-chlorophenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (3c). Yield (2.35 g, 86.72%); m.p. 247–248 °C; IR (ATR, νmax, cm−1): 3240 (NH), 1615, 1586 (C = N), 1323 (C = S); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 3.73 (s, 3H, OCH3), 3.92 (s, 2H, benzyl CH2), 5.47 (s, 2H, N–CH2), Ar–H: [6.65 (d, 2H, J = 8.4 Hz), 7.07 (d, 2H, J = 8.6 Hz), 7.33 (d, 2H, J = 8.6 Hz), 7.48–7.55 (m, 4H), 7.82 (d, 2H, J = 8.4 Hz)], 14.07 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 29.95 (benzyl CH2), 43.44 (N–CH2), 55.08 (OCH3), Ar–C: [113.95 (2CH), 127.25 (2CH), 127.28, 127.47, 128.84 (2CH), 129.35 (2CH), 129.45 (2CH), 129.88 (2CH), 131.78, 133.77, 134.51, 158.12], 146.92 (triazole C-5, second ring), 156.77 (triazole C-3), 158.92 (triazole C-5), 168.61 (triazole C-3, second ring); LC–MS/MS m/z (%): 523.30 ([M]+, 8), 477.49 (5), 389.46 ((12), 375.36 (13), 277.26 (100), 237.18 (86), 159.97 (28), 117.12 (41), 106.04 (48); Anal. Cald. for C25H20Cl2N6OS: C, 57.37; H, 3.85; N, 16.06. Found: C, 57.48; H, 3.80; N, 15.99.
General procedure for the synthesis of 4a–f and 6a–b
To the solution of corresponding compound 3a–c/5 (0.001 mol) in DMF (10 mL), formaldehyde (37%, 0.155 mL) and 1-methyl/phenylpiperazine (0.001 mol) were added and the mixture was stirred at room temperature for 16–20 h. Petroleum ether (40–60 °C) was added at the end of the reaction and the mixture was then cooled in refrigerator at 1 °C for 12 h. The resulting solid obtained was filtered, dried and crystallized from benzene:petroleum ether (40–60 °C). The completion of reaction was monitored by thin-layer chromatography.
4-Methyl-2-[(4-methylpiperazin-1-yl)methyl]-5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-2,4-dihydro-3H-1,2,4-triazole-3-thione (4a). Yield (0.42 g, 77.78%); m.p. 182–183 °C; IR (ATR, νmax, cm−1): 1610, 1583 (C = N), 1332 (C = S), 1177 (N–CH2–N); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 2.16 (s, 3H, N–CH3), 2.33 (bs, 4H, CH2–N–CH2), 2.61 (bs, 4H, CH2–N–CH2), 3.45 (s, 3H, N–CH3), 3.72 (s, 3H, OCH3), 4.23 (s, 2H, benzyl CH2), 5.67 (s, 2H, N–CH2), 5.74 (s, 2H, N–CH2–N), Ar–H: [6.84 (d, 2H, J = 7.6 Hz), 7.19 (d, 2H, J = 6.8 Hz), 7.51 (d, 2H, J = 7.6 Hz), 7.94 (d, 2H, J = 6.8 Hz)]; 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 30.54 (benzyl CH2), 31.73 (N–CH3), 43.56 (N–CH2), 46.21 (N–CH3), 50.02 (2C, CH2–N–CH2), 54.93 (2C, CH2–N–CH2), 55.49 (OCH3), 69.98 (N–CH2–N), Ar–C: [114.37 (2CH), 127.92 (2CH), 128.06, 129.32 (2CH), 129.89, 130.24 (2CH), 134.29, 158.56], 146.16 (triazole C-5, second ring), 157.37 (triazole C-3), 159.61 (triazole C-5), 168.95 (triazole C-3, second ring); LC–MS/MS m/z (%): 539.00 ([M]+, 14), 449.24 (12), 327.41 (63), 323.26 (100), 130.14 (14), 113.21 (72); Anal. Cald. for C26H31ClN8OS: C, 57.93; H, 5.80; N, 20.79. Found: C, 58.21; H, 5.85; N, 20.63.
4-Methyl-2-[(4-phenylpiperazin-1-yl)methyl]-5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-2,4-dihydro-3H-1,2,4-triazole-3-thione (4b). Yield (0.53 g, 88.33%); m.p. 138–139 °C; IR (ATR, νmax, cm−1): 1601, 1580 (C = N), 1316 (C = S), 1168 (N–CH2–N); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 2.74 (bs, 4H, CH2–N–CH2), 3.06 (bs, 4H, CH2–N–CH2), 3.49 (s, 3H, N–CH3), 3.71 (s, 3H, OCH3), 4.23 (s, 2H, benzyl CH2), 5.01 (s, 2H, N–CH2), 5.74 (s, 2H, N–CH2–N), Ar–H: [6.75–6.88 (m, 4H), 7.18–7.21 (m, 3H), 7.37–7.45 (m, 4H), 7.92 (d, 2H, J = 8.0 Hz)]; 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 30.64 (benzyl CH2), 31.74 (N–CH3), 43.47 (N–CH2), 48.69 (2C, CH2–N–CH2), 50.20 (2C, CH2–N–CH2), 55.47 (OCH3), 68.30 (N–CH2–N), Ar–C: [114.36 (2CH), 116.02 (2CH), 119.37 (CH), 127.30 (2CH), 127.99, 128.78 (2CH), 129.34 (2CH), 129.89, 130.25 (2CH), 134.28, 151.45, 158.56], 146.80 (triazole C-5, second ring), 157.40 (triazole C-3), 159.68 (triazole C-5), 169.05 (triazole C-3, second ring); LC–MS/MS m/z (%): 603.47 ([M+2]+, 18), 601.71 ([M]+, 31), 587.55 (100), 585.60 (84), 571.57 (95), 567.60 (15), 547.59 (15); Anal. Cald. for C31H33ClN8OS: C, 61.94; H, 5.53; N, 18.64. Found: C, 62.17; H, 5.50; N, 18.43.
4-Phenyl-2-[(4-methylpiperazin-1-yl)methyl]-5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-2,4-dihydro-3H-1,2,4-triazole-3-thione (4c). Yield (0.51 g, 85.00%); m.p. 253–254 °C; IR (ATR, νmax, cm−1): 1610, 1585 (C = N), 1325 (C = S), 1178 (N–CH2–N); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 2.16 (s, 3H, N–CH3), 2.32 (bs, 4H, CH2–N–CH2), 2.72 (bs, 4H, CH2–N–CH2), 3.72 (s, 3H, OCH3), 3.84 (s, 2H, benzyl CH2), 5.03 (s, 2H, N–CH2), 5.50 (s, 2H, N–CH2–N), Ar–H: [6.85 (d, 2H, J = 8.4 Hz), 7.07 (d, 2H, J = 8.4 Hz), 7.28–7.32 (m, 2H), 7.47–7.51 (m, 5H), 7.84 (d, 2H, J = 8.4 Hz)]; 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 30.39 (benzyl CH2), 43.71 (N–CH2), 46.18 (N–CH3), 50.07 (2C, CH2–N–CH2), 54.97 (2C, CH2–N–CH2), 55.53 (OCH3), 69.21 (N–CH2–N), Ar–C: [114.38 (2CH), 127.75, 127.81, 127.92 (2CH), 128.29 (2CH), 129.82 (2CH), 129.91 (2CH), 130.15 (2CH), 130.33 (CH), 133.78, 134.22, 158.56], 146.00 (triazole C-5, second ring), 156.99 (triazole C-3), 159.48 (triazole C-5), 169.96 (triazole C-3, second ring); LC–MS/MS m/z (%): 601.46 ([M]+, 100), 589.56 (20), 511.28 (48), 489.45 (22); Anal. Cald. for C31H33ClN8OS: C, 61.94; H, 5.53; N, 18.64. Found: C, 62.17; H, 5.58; N, 18.43.
4-Phenyl-2-[(4-phenylpiperazin-1-yl)methyl]-5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-2,4-dihydro-3H-1,2,4-triazole-3-thione (4d). Yield (0.59 g, 89.39%); m.p. 162–163 °C; IR (ATR, νmax, cm−1): 1600, 1563 (C = N), 1328 (C = S), 1179 (N–CH2–N);1H NMR (DMSO-d6, 400 MHz) δ (ppm): 2.88 (bs, 4H, CH2–N–CH2), 3.13 (bs, 4H, CH2–N–CH2), 3.70 (s, 3H, OCH3), 3.80 (s, 2H, benzyl CH2), 5.15 (s, 2H, N–CH2), 5.49 (s, 2H, N–CH2–N), Ar–H: [6.79–6.80 (m, 1H), 6.84 (d, 2H, J = 8.4 Hz), 6.92 (d, 2H, J = 8.4 Hz), 7.06 (d, 2H, J = 8.4 Hz), 7.19–7.23 (m, 2H), 7.28–7.30 (m, 2H), 7.38 (d, 2H, J = 8.4 Hz), 7.48–7.50 (m, 3H), 7.82 (d, 2H, J = 8.4 Hz)]; 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 30.39 (benzyl CH2), 43.72 (N–CH2), 48.68 (2C, CH2–N–CH2), 48.76 (2C, CH2–N–CH2), 55.61 (OCH3), 69.12 (N–CH2–N), Ar–C: [114.39 (2CH), 116.04 (2CH), 119.44 (CH), 127.73, 127.86 (2CH), 128.28 (2CH), 129.22 (2CH), 129.37 (2CH), 129.83, 129.89 (2CH), 130.14 (2CH), 130.38 (CH), 133.78, 134.20, 151.53, 158.66], 146.07 (triazole C-5, second ring), 156.94 (triazole C-3), 159.46 (triazole C-5), 170.03 (triazole C-3, second ring); LC–MS/MS m/z (%): 685.50 ([M+Na]+, 47), 663.21 ([M]+, 32), 651.29 (27), 585.50 (70), 571.50 (32), 393.47 (28), 323.41 (22), 132.08 (100); Anal. Cald. for C36H35ClN8OS: C, 65.19; H, 5.32; N, 16.89. Found: C, 65.32; H, 5.29; N, 17.00.
4-(4-Chlorophenyl)-2-[(4-methylpiperazin-1-yl)methyl]-5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-2,4-dihydro-3H-1,2,4-triazole-3-thione (4e). Yield (0.55 g, 85.94%); m.p. 247–248 °C; IR (ATR, νmax, cm−1): 1611, 1585 (C = N), 1322 (C = S), 1178 (N–CH2–N); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 2.13 (s, 3H, N–CH3), 2.28 (bs, 4H, CH2–N–CH2), 2.70 (bs, 4H, CH2–N–CH2), 3.71 (s, 3H, OCH3), 3.93 (s, 2H, benzyl CH2), 5.00 (s, 2H, N–CH2), 5.51 (s, 2H, N–CH2–N), Ar–H: [6.84 (d, 2H, J = 8.8 Hz), 7.05 (d, 2H, J = 8.8 Hz), 7.29 (d, 2H, J = 8.8 Hz), 7.46 (d, 2H, J = 8.8 Hz), 7.51 (d, 2H, J = 8.8 Hz), 7.80 (d, 2H, J = 8.4 Hz)]; 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 30.43 (benzyl CH2), 43.76 (N–CH2), 46.23 (N–CH3), 50.06 (2C, CH2–N–CH2), 54.98 (2C, CH2–N–CH2), 55.50 (OCH3), 69.26 (N–CH2–N), Ar–C: [114.36 (2CH), 127.62, 127.89 (2CH), 129.22 (2CH), 129.77, 129.89 (2CH), 130.10 (2CH), 130.28 (2CH), 132.63, 134.20, 135.04, 158.54], 145.87 (triazole C-5, second ring), 156.91 (triazole C-3), 159.29 (triazole C-5), 169.85 (triazole C-3, second ring); LC–MS/MS m/z (%): 637.26 ([M+2]+, 72), 635.25 ([M]+, 100), 623.54 (12), 587.55 (15); Anal. Cald. for C31H32Cl2N8OS: C, 58.58; H, 5.07; N, 17.63. Found: C, 58.77; H, 4.99; N, 17.60.
4-(4-Chlorophenyl)-2-[(4-phenylpiperazin-1-yl)methyl]-5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-2,4-dihydro-3H-1,2,4-triazole-3-thione (4f). Yield (0.65 g, 92.86%); m.p. 162–163 °C; IR (ATR, νmax, cm−1): 1601, 1584 (C = N), 1326 (C = S), 1170 (N–CH2–N); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 2.86 (bs, 4H, CH2–N–CH2), 3.10 (bs, 4H, CH2–N–CH2), 3.71 (s, 3H, OCH3), 3.92 (s, 2H, benzyl CH2), 5.11 (s, 2H, N–CH2), 5.51 (s, 2H, N–CH2–N), Ar–H: [6.75–6.78 (m, 1H), 6.83 (d, 2H, J = 8.4 Hz), 6.89 (d, 2H, J = 8.0 Hz), 7.05 (d, 2H, J = 8.8 Hz), 7.16–7.20 (m, 2H), 7.31 (d, 2H, J = 8.8 Hz) 7.39 (d, 2H, J = 8.4 Hz), 7.52 (d, 2H, J = 8.4 Hz), 7.79 (d, 2H, J = 8.8 Hz)]; 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 30.43 (benzyl CH2), 43.77 (N–CH2), 48.74 (2C, CH2–N–CH2), 50.29 (2C, CH2–N–CH2), 55.48 (OCH3), 69.16 (N–CH2–N), Ar–C: [114.34 (2CH), 116.04 (2CH), 119.44 (CH), 127.60, 127.83 (2CH), 129.22, 129.35 (2CH), 129.75, 129.92 (2CH), 130.10 (2CH), 130.28 (2CH), 132.63, 133.78, 134.18, 135.08, 151.50, 158.53], 145.94 (triazole C-5, second ring), 156.91 (triazole C-3), 159.31 (triazole C-5), 169.94 (triazole C-3, second ring); LC–MS/MS m/z (%): 699.18 ([M+2]+, 64), 697.36 ([M]+, 32), 685.25 (100), 677.59 (15), 635.47 (12), 591.46 (25), 585.40 (26); Anal. Cald. for C36H34Cl2N8OS: C, 61.98; H, 4.91; N, 16.06. Found: C, 62.17; H, 5.00; N, 15.92.
5-{[(3-(4-Chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl)]methyl]}-3-[(4-methylpiperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione (6a). Yield (0.35 g, 66.04%); m.p. 87–88 °C; IR (ATR, νmax, cm−1): 1611, 1586 (C = N), 1339 (C = S), 1177 (N–CH2–N); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 2.18 (s, 3H, N–CH3), 2.70 (bs, 4H, CH2–N–CH2), 2.86 (bs, 4H, CH2–N–CH2), 3.70 (s, 3H, OCH3), 4.19 (s, 2H, benzyl CH2), 5.33 (s, 2H, N–CH2), 5.49 (s, 2H, N–CH2–N), Ar–H: [6.87 (d, 2H, J = 8.8 Hz), 7.23 (d, 2H, J = 8.8 Hz), 7.45 (d, 2H, J = 8.8 Hz), 7.92 (d, 2H, J = 8.8 Hz)]; 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 30.57 (benzyl CH2), 43.63 (N–CH2), 46.21 (N–CH3), 49.91 (2C, CH2–N–CH2), 51.47 (2C, CH2–N–CH2), 55.47 (OCH3), 69.78 (N–CH2–N), Ar–C: [114.32 (2CH), 127.93 (2CH), 128.43, 128.54, 129.23 (2CH), 130.37 (2CH), 134.20, 158.57], 157.13 (triazole C-3), 157.80 (oxadiazole C-5), 159.57 (triazole C-5), 171.15 (oxadiazole C-2); LC–MS/MS m/z (%): 526.49 ([M]+, 8), 524.48 (10), 496.57 (13), 460.22 (38), 458.34 (100), 450.33 (41), 438.51 (18); Anal. Cald. for C25H28ClN7O2S: C, 57.08; H, 5.36; N, 18.64. Found: C, 57.21; H, 5.27; N, 18.52.
5-{[(3-(4-Chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl)]methyl]}-3-[(4-phenylpiperazin-1-yl)methyl]-1,3,4-oxadiazole-2(3H)-thione (6b). Yield (0.48 g, 81.36%); m.p. 118–119 °C; IR (ATR, νmax, cm−1): 1610, 1599 (C = N), 1326 (C = S), 1175 (N–CH2–N); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 2.77 (bs, 4H, CH2–N–CH2), 3.07 (bs, 4H, CH2–N–CH2), 3.70 (s, 3H, OCH3), 4.23 (s, 2H, benzyl CH2), 4.92 (s, 2H, N–CH2), 5.74 (s, 2H, N–CH2–N), Ar–H: [6.74–6.83 (m, 3H), 6.85–6.90 (m, 2H), 7.16–7.22 (m, 4H), 7.48 (d, 2H, J = 8.0 Hz), 7.94 (d, 2H, J = 8.4 Hz)];13C NMR (DMSO-d6, 100 MHz) δ (ppm): 30.42 (benzyl CH2), 43.58 (N–CH2), 48.66 (2C, CH2–N–CH2), 49.91 (2C, CH2–N–CH2), 55.45 (OCH3), 70.06 (N–CH2–N), Ar–C: [114.29 (2CH), 116.09 (2CH), 119.49 (CH), 127.81 (2CH), 127.95, 129.35 (2CH), 129.73, 130.23 (2CH), 130.44 (2CH), 134.40, 151.42, 158.56], 157.64 (triazole C-3), 159.35 (oxadiazole C-5), 159.81 (triazole C-5), 178.27 (oxadiazole C-2); LC–MS/MS m/z (%): 588.37 ([M]+, 11), 578.62 (21), 558.42 (15), 548.41 (43), 546.46 (100); Anal. Calcd. for C30H30ClN7O2S: C, 61.27; H, 5.14; N, 16.67. Found: C, 61.41; H, 5.10; N, 16.53.
Synthesis of 5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-1,3,4-oxadiazole-2(3H)-thione (5)
To solution of the compound 1 (0.01 mol) in ethanol (25 mL) at 0 °C, CS2 (6 mL, 0.01 mol) and a solution of KOH (0.56 g, 0.001 mol) in 50 mL H2O and 50 mL ethanol were added, and the reaction mixture was refluxed for about 6 h until the evolution of H2S gas ceased. Excess solvents were evaporated under reduced pressure to dryness, a solid was obtained. This was dissolved in 200 mL H2O and acidified with conc. HCl to pH ∼5. The precipitate was filtered off, washed with H2O and recrystallized from ethanol to afford the desired compound. Yield (3.12 g, 75.36%); m.p. 180–181 °C; IR (νmax, cm−1): 3294 (NH), 1610, 1586 (C = N), 1323 (C = S); 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 3.71 (s, 3H, OCH3), 4.23 (s, 2H, benzyl CH2), 5.72 (s, 2H, N–CH2), Ar–H: [6.84 (d, 2H, J = 8.2 Hz), 7.19 (d, 2H, J = 8.6 Hz), 7.50 (d, 2H, J = 8.2 Hz), 7.95 (d, 2H, J = 8.6 Hz)], 13.83 (bs, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 30.64 (benzyl CH2), 43.62 (N–CH2), 55.71 (OCH3), Ar–C: [114.62 (2CH), 128.09, 128.21 (2CH), 129.63 (2CH), 129.95, 130.45 (2CH), 134.64, 158.80], 157.92 (triazole C-3), 159.00 (triazole C-5), 160.12 (oxadiazole C-5), 178.55 (oxadiazole C-2); LC–MS/MS m/z (%): 436.31 ([M+Na]+, 100), 412.40 ([M−1]+, 81), 327.31 (36), 174.39 (31), 130.35 (79); Anal. Cald. for C19H16ClN5O2S: C, 55.14; H, 3.90; N, 16.92. Found: C, 55.34; H, 4.07; N, 16.87.
Biological assays
Anti-lipase activity assay
The inhibitory effects of those compounds were evaluated against Porcine Pancreatic Lipase (Applichem, Germany) (15 ng/mL). Lipase activity assay were done according to Kurihara et alCitation45. The lipase activity was measured using 4-methylumbelliferyl oleate (4-MU oleate) as a substrate. Briefly, compounds were mixed with Porcine Pancreatic Lipase (PPL) 1:3 (v/v) and incubated for 30 min. The microtiter plates containing, 50 µL 0.1 mM 4-MU oleate, 25 µL diluted compound-lipase solution, 25 µL dH2O and assay buffer (13 mM Tris–HCl, 150 mM NaCl and 1.3 mM CaCl2, pH 8.0) were incubated at 37 °C for 20 min. After incubation, in order to stop the reaction, 0.1 mL 0.1 M pH 4.2 citrate buffer was added reaction mixture. The amount of 4-methylumbelliferone released by the lipase was measured by using a spectroflourometer (SpectraMax M5, Molecular Devices) at an excitation wavelength of 355 nm and an emission wavelength of 460 nm. The inhibitory activity of those compounds and Orlistat (Xenical, Hoffman, La Roche, Segrate, Italy), an inhibitor control of pancreatic lipase, was measured at various concentration. Residual activities were calculated by comparing to control without inhibitor. The assays were done in triplicate. The IC50 value was determined as the concentration of compound that give 50% inhibition of maximal activity.
α-Glucosidase ınhibition assay
α-Glucosidase inhibition assay was performed spectrophotometricallyCitation46. α-Glucosidase from Saccharomyces cerevisiae (Sigma-Aldrich, St. Louis, MO) was dissolved in phosphate buffer (pH 6.8, 50 mM). Test compounds were dissolved in DMSO. In 96-well microtiter plates, 20 μL of test sample, 20 μL of enzyme (20 mU/mL) and 135 μL of buffer were added and incubated for 15 min at 37 °C. After incubation, 25 μL of p-nitrophenyl-α-d-glucopyranoside (2 mM, Sigma Aldrich) was added and change in absorbance was monitored for 30 min at 400 nm. Test compound was replaced by DMSO (7.5% final) as control. Acarbose (Sigma-Aldrich) was used as a standard inhibitorCitation46.
Conclusion
In this current study, new 2-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]acetyl}-4-alkyl/arylthiosemicarbazides, 5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-4-alkyl/aryl-2,4-dihydro-3H-1,2,4-triazole-3-thiones, 5-{[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]methyl}-1,3,4-oxadiazole-2(3H)-thione were synthesized starting from 2-[3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol-4-yl]acetohydrazide. In addition to these, new N-Mannich bases of 1,2,4-triazole-3-thiones and 1,3,4-oxadiazole-2(3H)-thione were synthesized.
The newly synthesized compounds were tested in vitro for lipase and α-glucosidase inhibitory activity. Among the tested compounds 2a (IC50 = 10.25 ± 0.40 µM), 2c (IC50 = 2.50 ± 0.50 µM)and 6a (IC50 = 42.21 ± 4.54 µM) showed anti-lipase activities. In addition, compounds 2c (IC50 = 3.41 ± 0.16 µM), 6a (IC50 = 4.36 ± 0.10 µM) and 6b (IC50 = 17.68 ± 1.10 µM) showed the high anti-α-glucosidase activity. Compounds 2c and 6a showed the best anti-α-glucosidase activity. These compounds inhibited α-glucosidase activity by 99.79 ± 1.17% and 99.87 ± 0.38% at a concentration of 100 µM, respectively. Based on the obtained results, compounds 2c and 6a can be considered as lead in the search of alternative drugs to Orlistat and Acarbose.
Declaration of interest
The support provided by Karadeniz Technical University, BAP, Turkey (Project No. 10020) is gratefully acknowledged.
References
- Drew B, Dixon A, Dixon J. Obesity management: update on orlistat. Vasc Health Risk Manag 2007;3:817–21
- Van Gaal LF, Mertens IL, de Block CE. Mechanisms linking obesity with cardiovascular disease. Natur 2006;444:875–80
- Tsuji M, Saito N, Inoue S. Inhibitors of absorption as anti-obesity drugs. Nihon Yakurigaku Zasshi 2001;118:340–6
- Gupta R, Gupta N, Rathi P. Bacterial lipases: an overview of production, purification and biochemical properties. Appl Microbiol Biotechnol 2004;64:763–81
- Thomson AB, De Pover A, Keelan M, et al. Inhibition of lipid absorption as an approach to the treatment of obesity. Method Enzymol 1997;286:3–44
- Finer N, James WPT, Kopelman PG, et al. One-year treatment of obesity: a randomized, double-blind placebo-controlled, multicentre study of orlistat, a gastrointestinal lipase inhibitor. Int J Obesity 2000;24:306–13
- McClendon K, Riche D, Uwaifo G. Orlistat: current status in clinical therapeutics. Expert Opin Drug Saf 2009;8:727–44
- Girish TK, Pratape VM, Prasada Rao UJS. Nutrient distribution, phenolic acid composition, antioxidant and α-glucosidase inhibitory potentials of black gram (Vigna mungo L.) and it's milled by-products. Food Res Int 2012;46:370–7
- Ali H, Houghton PJ, Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol 2006;104:449–55
- Casirola DM, Ferraris RP. α-Glucosidase inhibitors prevent diet-induced increases in intestinal sugar transport in diabetic mice. Metabolism 2006;55:832–41
- Stuart AR, Gulve EA, Wang M. Chemistry and biochemistry of type 2 diabetes. Chem Rev 2004;104:1255–82
- Playford RJ, Pither C, Gao R, Middleton SJ. Use of the α-glucosidase inhibitor acarbose in patients with ‘Middleton syndrome’: normal gastric anatomy but with accelerated gastric emptying causing postprandial reactive hypoglycemia and diarrhea. Can J Gastroenterol 2013;27:403–4
- Saito N, Sakai H, Sekihara H, Yajima Y. Effect of an α-glucosidase inhibitör (voglibose), in combination with sulphonylureas, on glycaemic control in type 2 diabetes patients. J Int Med Res 1998;26:219–32
- Patel NB, Khan IH, Rajani SD. Pharmacological evaluation and characterizations of newly synthesized1,2,4-triazoles. Eur J Med Chem 2010;45:4293–99
- Wakale VS, Pattan SR, Tambe V. Therapeutic importance of 1,2,4-triazole: a review. Int J Res Pharm Biomed Sci 2013;4:985–1001
- Kharb R, Sharma PC, Yar MS. Pharmacological significance of triazole scaffold. J Enzyme Inhib Med Chem 2011;26:1–21
- Maddila S, Pagadala R, Jonnalagadda SB. 1,2,4-Triazoles: a review of synthetic approaches and the biologicalactivity. Lett Org Chem 2013;10:693–714
- Banerjee S, Ganguly S, Sen KK. A review on 1,2,4-triazoles. J Adv Pharm Edu Res 2013;3:102–15
- Sztanke K, Tuzimski T, Rzymowska J, et al. Synthesis, determination of the lipophilicity, anticancer and antimicrobial properties of some fused 1,2,4-triazole derivatives. Eur J Med Chem 2008;43:404–19
- Ashok M, Holla BS, Poojary B. Convenient one pot synthesis and antimicrobial evaluation of some new Mannich bases carrying 4-methylthiobenzyl moiety. Eur J Med Chem 2007;42:1095–101
- Holla BS, Kalluraya B, Sridhar KR, et al. Synthesis, structural characterization, crystallographic analysis and antibacterial properties of some nitrofuryl triazolo[3,4-b]-1,3,4-thiadiazines. Eur J Med Chem 1994;29:301–8
- Sahu VKR, Singh AK, Yadav D. Review article on 1,3,4-oxadiazole derivatives and it’s pharmacological activities. Int J Chem Tech Res 2011;3:1362–72
- Pangal A, Shaikh JA. Various pharmacological aspects of 2,5-disubstituted 1,3,4-oxadiazolederivatives: a review. Res J Chem Sci 2013;3:79–89
- Amir M, Shikha K. Synthesis and anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation activities of some new 2-[(2,6-dichloroanilino) phenyl]acetic acid derivatives. Eur J Med Chem 2004;39:535–45
- Namita S, Barthwal JP, Saxena AK, et al. Monoamine oxidase and succinate dehydrogenase inhibitory properties of substituted 1,3,4-oxadiazole-2-thiones. J Heterocycl Chem 1982;19:29–32
- Huiguo L, Dengfeng D, Sridhar A, et al. Design, synthesis and characterization of novel 1,2-benzisothiazol-3(2H)-one and 1,3,4-oxadiazole hybrid derivatives: potent inhibitors of Dengue and West Nile virus NS2B/NS3 proteases. Bioorg Med Chem 2013;21:102–13
- Oliveira CS, Lira BF, Falcao-Silva VS, et al. Synthesis, molecular properties prediction, and anti-staphylococcal activity of N-acylhydrazones and new 1,3,4-oxadiazole derivatives. Molecules 2012;17:5095–107
- Shaker RM. The chemistry of mercapto- and thione- substituted 1,2,4-triazoles and their utility in heterocyclic synthesis. ARKIVOC 2006;ix:59–112
- Dimmock JR, Kumar P. Anticancer and cytotoxic properties of Mannich bases. Curr Med Chem 1997;4:1–22
- Subramaniapillai SG. Mannich reaction: a versatile and convenient approach to bioactive skeletons. J Chem Sci 2013;125:467–82
- Karthikeyan MS, Prasad DJ, Poojary B, et al. Synthesis and biological activity of Schiff and Mannich bases bearing 2,4-dichloro-5-fluorophenyl moiety. Bioorg Med Chem 2006;14:7482–89
- Shahzad SA, Yar M, Bajda M, et al. Synthesis and biological evaluation of novel oxadiazole derivatives: a new class of thymidine phosphorylase inhibitors as potential anti-tumor agents. Bioorg Med Chem 2014;22:1008–15
- Bekircan O, Mentese E, Ulker S, Kucuk C. Synthesis of some new 1,2,4-triazole derivatives starting from 3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1,2,4-triazol with anti-lipase and anti-urease activities. Arch Pharm Chem Life Sci 2014;347:387–97
- Demirbas A, Sahin D, Demirbas N, Karaoglu SA. Synthesis of some new 1,3,4-thiadiazol-2-yl-methyl-1,2,4-triazole derivatives and investigation of their antimicrobial activities. Eur J Med Chem 2009;44:2896–903
- Plech T, Wujec M, Siwek A, et al. Synthesis and antimicrobial activity of thiosemicarbazides, s-triazoles and their Mannich bases bearing 3-chlorophenyl moiety. Eur J Med Chem 2011;46:241–8
- Terzioglu N, Capan G, Gürsoy A, et al. Synthesis, structure, and antifungal evaluation of some novel 1,2,4-triazolylmercaptoacetylthiosemicarbazide and 1,2,4-triazolylmercaptomethyl-1,3,4-thiadiazole analogs. J Enzyme Inhib Med Chem 2010;25:126–31
- Rollas S. Synthesis and spectrometric analysis of some 1,2,4-triazoline-5-thiones. J Fac Pharm Istanbul 1981;17:155–63
- Gulerman NN, Dogan HN, Rollas S, et al. Synthesis and structure elucidation of some new thioether derivatives of 1,2,4-triazoline-3 thiones and their antimicrobial activities. Il Farmaco 2001;56:953–8
- Bekircan O, Kucuk M, Kahveci B, Bektas H. Synthesis and anticancer evaluation of some new 4-amino-3-(p-methoxybenzyl)-4,5-dihydro-1,2,4-triazole-5-one derivatives. Z Naturforsch 2008;63b:1305–14
- Sahin G, Palaska E, Ekizoglu M, Ozalp M. Synthesis and antimicrobial activity of some 1,3,4-oxadiazole derivatives. Il Farmaco 2002;57:539–42
- Ramaprasad GC, Kalluraya B, Sunil Kumar B, Hunnur RK. Synthesis and biological property of some novel 1,3,4-oxadiazoles. Eur J Med Chem 2010;45:4587–93
- Joshi SD, Vagdevi HM, Vaidya VP, Gadaginamath GS. Synthesis of new 4-pyrrol-1-yl benzoic acid hydrazide analogs and some derived oxadiazole, triazole and pyrrole ring systems: a novel class of potential antibacterial and antitubercular agents. Eur J Med Chem 2008;43:1989–96
- Almajan GL, Barbuceanu SF, Almajan ER, et al. Synthesis, characterization and antibacterial activity of sometriazole Mannich bases carrying diphenylsulfone moieties. Eur J Med Chem 2009;44:3083–9
- Koparır M, Orek C, Parlak AE, et al. Synthesis and biological activities of some novel aminomethylderivatives of 4-substituted-5-(2-thienyl)-2,4-dihydro-3H-1,2,4-triazole-3-thiones. Eur J Med Chem 2013;63:340–6
- Kurihara H, Asami S, Shibata H, et al. Hypolipemic effect of cyclocarya paliurus (Batal) Iljinskaja in lipid-loaded mice. Biol Pharm Bull 2003;26:383–5
- Choudhary MI, Adhikari A, Rasheed S, et al. Cyclopeptide alkaloids of Ziziphus oxyphylla Edgw. as novel inhibitors of α-glucosidase enzyme and protein glycation. Phytochem Lett 2011;4:404–6
