Abstract
Aminomethyl derivatives of 1,5-bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one, designed as new cytotoxins, were synthesized and evaluated in terms of their cytotoxic activities. The compounds have low CC50 values in the low micromolar range against HL-60 neoplasms and HSC-2, HSC-3 and HSC-4 carcinoma cells. In general, the average CC50 values of these compounds were higher towards HGF, HPC and HPLF non-malignant cells, which reveals the tumour-selectivity of these aminomethyl derivatives, Mannich bases. Using specific concentrations of compounds 4 and 6 caused cleavage of PARP1 in HSC-2 cells but not HGF cells, which may be a contributing factor to cytotoxicities and the tumour-selectivities.
Introduction
The oldest chemotherapeutic approaches involve using natural products. It is not a surprise that natural compounds or their synthetic analogues have proved to be a rich source of potential anti-cancer therapies. Many natural compounds can be structurally modified to lead new analogues, which may have better pharmacological profileCitation1. Curcumin is a yellow pigment isolated from the rhizome of Curcuma longa (Zingiberaceae). Curcumin [1,7-bis-(4-hydroxy-3-methoxyphenyl)-hepta-1,6-diene-3,5-dione] has been studied fairly extensively. It belongs to the group of diarylheptanoids in various natural products. It has various biological activities such as anti-inflammatoryCitation2, anti-oxidantCitation3, anti-HIVCitation4, chemopreventiveCitation5 and anti-cancerCitation6 activities and it suppresses carcinogenesis of the colon and liver in mice in vivoCitation7.
There are three moieties in the structure of curcumin, which can be modified to produce improved curcumin analogues in terms of bioactivity. These are the aromatic rings, β-diketones and two double bonds (). Synthesis of curcumin analogues considering these modification sites has resulted with more potential anti-cancer candidates in different stages and processes in cancer cell growthCitation8–10. Generally, conjugated enones act as Michael acceptors (Scheme 1) with thiol preferred over amino and hydroxy nucleophilesCitation11. Many monoketone analogues of curcumin are more isolable and stableCitation12,Citation13 than curcumin. A variety of 2,6-bis(arylidene) cyclohexanones, which are mono-ketone analogues, were three to five times more potent than reference anti-cancer compound 5-fluorouracil (5-FU) against various cell linesCitation14. α,β-Unsaturated monoketone curcumin analogues based on piperidone have been found to be anti-neoplastic. These analogues have also exhibited significant cytotoxic activityCitation15.
Figure 1. Chemical structure of curcumin (keto form) [1,7-bis-(4-hydroxy-3-methoxyphenyl)-hepta-1,6-diene-3,5-dione].
![Figure 1. Chemical structure of curcumin (keto form) [1,7-bis-(4-hydroxy-3-methoxyphenyl)-hepta-1,6-diene-3,5-dione].](/cms/asset/f9f8753d-49be-4a15-8666-c9962f564c7c/ienz_a_940934_f0001_b.jpg)
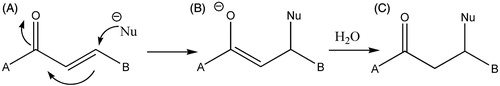
Scheme 1. A Michael acceptor. The α,β-unsaturated ketone (A) undergoes attack at the β-position by the nucleophile (Nu) to generate an enolate intermediate (B). Aqueous quenching gives the product (C), α,β-functionalized ketoneCitation1.
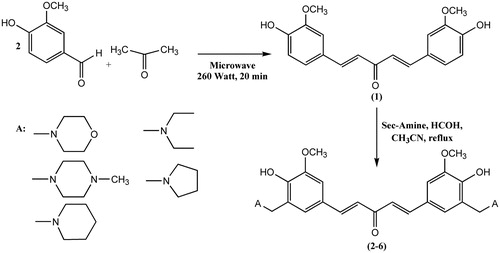
In this study, it was aimed to design new curcumin analogue having 1,5-bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one (1) structure as the main part. This analogue is mono ketone derivative carrying σ,β-unsaturated ketone moiety.
Aminomethyl derivatives, Mannich bases, of σ,β-unsaturated ketones showed remarkably increased cytotoxicity against several cell linesCitation16–18. Starting from the main structure 1, it was planned to synthesize new aminomethyl derivatives, (Mannich bases), to obtain new curcumin analogues, by the expectation to obtain more cytotoxic compounds than compound 1 against several cell lines. It is expected that Mannich bases will produce additional alkylating centre by deamination process leading increased cytotoxicity. Several secondary amines such as morpholine, pyrrolidine, N-methylpiperazine, diethylamine and piperidine (2–6) were used as amine compounds. To test the cytotoxicity of the compounds, several tumour (HL-60, HSC-2, HSC-3, HSC-4) cell lines and non-tumour (HGF, HPC, HPLF) cell lines were planned to use to evaluate their cytotoxicities and to find out their selectivity index (SI), which reflects the selectivity against tumour cells comparing with the non-tumour cells. The higher SI value reflects the most preferable cytotoxic compounds against tumour cells. It was also aimed to investigate the possible mechanism of action of the representative compounds cytotoxicity by testing PARP1 activity. Because many cytotoxins exert their bioactivity by inducing apoptosisCitation19,Citation20, but single-stranded DNA breaks can be repaired by poly(ADP-ribose)polymerase 1 (PARP1)Citation21. Thus, compounds which cleave PARP1 (and hence reduce the extent of repair of DNA damage) to a greater extent in neoplasms than normal cells may be useful agents in cancer chemotherapy.
Results and discussion
1,5-Bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one (1) was synthesized in acidic or basic conditions using different methods of previously reported studiesCitation22,Citation23. But in this study, this starting compound was synthesized by a different method using microwave irradiation. Products were purified by column chromatography and recrystallized with suitable solvents. Chemical structures of the synthesized compounds were confirmed by 1H NMR, 13C NMR, IR and HRMS. Mannich bases (2–6) reported here were reported for the first time by this study. Aminomethyl derivatives of compound 1 were obtained with classical Mannich reaction shown at Scheme 2. To obtain the compounds with a better yield, 1,5-bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one was added into a previously 10-min heated mixture of secondary amine and paraformaldehyde in acetonitrile.
Scheme 2. Synthesis of 1,5-bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-on and its aminomethyl derivatives (Mannich bases) 2–6.
The 1H NMR, 13C NMR, IR, HRMS and UV spectra of compounds 2–6 were in accordance with their chemical structures. The presence of the aminomethyl groups was obvious from the spectral data. For example, in compound 2, the morpholine protons were observed as broad singlet of eight protons at δ 2.61 ppm and broad singlet of the other eight protons at δ 3.75 ppm. The pyrrolidine protons were observed as broad singlet of eight protons at δ 1.86 ppm and broad singlet of the other eight protons at δ 2.56 ppm for compound 3. The piperazine protons were observed as singlet of the six N-methyl protons at δ 2.29 ppm and broad singlet of 16 protons at δ 2.42 ppm for the compound 4. The diethylamine protons were observed as triplet of 12 protons at δ 1.17 ppm and quartet of eight protons at δ 2.71 ppm. The piperidine protons for compound 6 were observed as broad singlet of 12 protons at δ 1.68 ppm and broad singlet of eight protons at δ 2.56 ppm in 1H NMR spectra.
In 13C NMR, chemical shifts of carbons of amine moieties of Mannich bases have been observed at 56.22 and 53.09 ppm for the morpholin, 56.22 and 23.86 ppm for pyrrolidin, 55.04, 52.69 and 46.08 ppm for N-methylpiperazin, 46.63 and 11.31 ppm for diethylamine and 25.98 and 24.06 ppm for piperidin. 13C NMR chemical shifts were in accordance with their chemical structure.
The IR spectra of the Mannich derivatives revealed the C=C–H stretching absorption bands of α,β-unsaturated moiety at 2926–2972 cm−1 and C–N stretching absorption bands of aminomethyl groups in the range of 1265–1291 cm−1. The aromatic C–H and C=C stretching absorption bands were observed at 3359 and 1410–1493 cm−1, respectively. The stretching bands of carbonyl groups were observed at 1574–1589 cm−1.
In HRMS spectra, molecular weights and molecular ion peaks of all of the new synthesized compounds (2–6) were clearly observed by electrospray ionization method at under the positive or negative ionization mode. K bands in UV spectra were observed at between 275 and 398 nm, which resulted in the electronic transition of the styril ketone chromophore π → π*.
Compounds 1–6 and curcumin were evaluated against human HL-60 promyelocytic leukemic cells and human HCS-2, HSC-3 and HSC-4 oral squamous cell carcinomas. In addition, these enones were assayed against non-malignant HGF gingival fibroblasts, HPC pulp cells and HPLF periodontal ligament fibroblasts to observe cytotoxicity of the compound and SI. These data are presented in .
Table 1. Cytotoxic evaluation of the compounds 1–6 and curcumina.
The first question to be addressed is whether the compound 1 and their Mannich bases (2–6) have anti-neoplastic properties. The results portrayed in reveal that CC50 values of the synthesized compounds are 0.55–4.2 micromolar range towards HL-60, HSC-2, HSC-3 and HSC-4 cells. The potencies of 1–6 and curcumin towards the tumour cell lines were compared with two reference compounds, melphalan and 5-FU, which are clinically used anti-cancer agents. All synthesized compounds were exhibited more powerful activity towards HL-60, HSC-2, HSC-3 and HSC-4 cell lines than the reference compound 5-FU except compound 6 against HSC-4. According to the results, all compounds synthesized were found to have 1.8–6.8 times more cytotoxicity towards HSC-2 cell lines than the reference compound Melphalan. Beside this, the compounds synthesized were found 5.0–8.0 and 12.4–22.5 times more cytotoxic towards HSC-3 and HSC-4 cell lines, respectively, compared to melphalan. Curcumin was found to be more potent than melpahalan towards both HSC-3 and HSC-4 cell lines. Curcumin exhibited more cytotoxic activity against HL-60 and HSC-3 cell lines than the reference compound 5-FU.
The average CC50 values of each compound are listed in . Compounds 2 and 5 have the lowest average CC50 figures against all tumour cell lines and seem to be lead molecules. In addition to these compounds, most of the compounds had lower average CC50 values than melphalan towards tumour cell lines. Thus, the conclusion to be drawn is that many of the compounds possess notable anti-neoplastic potency.
The second aspect of these compounds to be considered is whether they are tumour-specific cytotoxins. Thus, the compounds were also evaluated against HGF, HPC and HPLF, which are non-malignant cells, and these data are presented in . Under clinical conditions, tumours are surrounded by different types of normal cells. Hence, SI values were generated which are the quotients of the average CC50 values of the non-malignant cells and the CC50 figure of a compound towards a specific cell line. The results in reveal that average SI values of the compounds synthesized were greater than curcumin. The compounds synthesized seem to be tumour-specific anti-neoplastic agents. An average figure of 4.0 was arbitrarily chosen as evidence of significant average selective toxicity to neoplasms and was displayed by all the compounds in series. The greatest selectivity was noted with compound 5 having an average SI of 5.42. Lead compounds should possess both cytotoxic activity and selective toxicity to tumours. In order to identify such molecules, a potency selectivity expression (PSE) was devised, which is the product of the reciprocal of the average CC50 values towards HL-60, HSC-2-, HSC-3 and HSC-4 cells (a measure of potency) and the average SI figures towards these cell lines (a determination of tumour-selectivity) expressed as a percentage. The PSE data are given in . Compounds 2, 4 and 5 had greater PCE values than the average PSE value (189.8) and serve as lead molecules for analogue development.
CC50 and PSE values of curcumin and its analogue compound 1 were compared. Compound 1 was found 4.3 times more selective against all tumour cell lines than curcumin. According to the CC50 value of compound 1, it was found more effective against all malignant cell lines with a respectable PSE value (138.3) than curcumin. Therefore, based on the results obtained, it can be said that penta-1,4-dien-3-one chemical structure has been useful chemical modification for the selective cytotoxicity. The other issue requiring a response is whether greater cytotoxic potency was found in the Mannich bases of compound 1. Average CC50 values of Mannich bases were evaluated, and it was seen that compounds 4 with PSE value (250.0) and 5 with PSE value (416.9) showed more effective cytotoxicity against whole malign cell lines with high potency SI values than the other Mannich bases (2, 3 and 6) and the starting compound 1. Hence, preparation of Mannich bases of the curcumin analogue 1 was found to be a useful modification for selective cytotoxic activity. The average SI figures reveal that each of the compounds (3–6) in series had a higher SI value than the starting analogue 1. The average SI figure of compounds 3–6 is in the range of 4.1–5.4 while compound 1 has 3.3 as SI value. Hence, the conclusion drawn is that in terms of demonstrating greater toxicity to neoplasms than normal cells, the presence of an aminomethyl moiety on ring is preferable. The three compounds with the highest average SI figures were 4, 5 and 6. Thus, they can be considered as lead molecules. In regard to the PSE figures, the data in reveal that the average PSE value for 2–6 was 231.7. This value was 1.7 times higher than the PSE value of 1 which was 138.3. This observation confirms the earlier conclusions that the presence of aminomethyl group on phenyl ring display greater toxicity to tumourous tissues than non-malignant cells. The log p values of the compounds in series are presented in . They are in the range of 2.07–4.34. No statistically significant correlation was found between selective or non-selective cytotoxicity and lipophilicity.
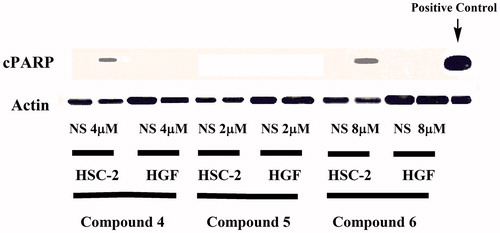
Compounds 4–6, which had the highest average SI values towards the HL-60, HSC-2, HSC-3 and HSC-4 malignant cells, were detected for their levels of cleaved PARP, which is the fragment of human PARP1 produced by caspase cleavage. In general, cleavage of PARP facilitates cellular disassembly and serves as a marker of cells undergoing apoptosis. Concentrations of 2, 4 and 8 µM were used which corresponds to the CC50 values of 4, 5 and 6 towards HSC-2 cells, respectively. The results are displayed in , which reveals that after 24 h some cleavage of PARP occurs only in HSC-2 cells, not in HGF cells for compounds 4 and 6. Hence, a possible factor contributing to the tumour-selectivity of 4 and 6 are the ability to inhibit DNA repair, and enhanced apoptosis preferentially in malignant cells.
Experimental
Materials
All chemicals were purchased from Aldrich Chemical Co. (Munich, Germany). Melting points were determined on a Thomas Hoover Capillary Apparatus (Philadelphia, PA). UV spectra were recorded in CHCl3 by a Thermo Electron Helios (α) (UVA 114903, Cambridge, UK). The IR spectra were measured on a Perkin-Elmer FT-IR spectrometer (Beaconsfield, UK). 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were obtained on Varian spectrometer (Danbury, CT). Mass spectra (HRMS) were recorded on a VG Waters Micromass ZQ by ESI (+) and ESI (−) mode. Microwave syntheses were made by modified household microwave oven. The reactions were monitored using silicagel Thin Layer Chromatography (TLC) plates (Merck, Darmstadt, Germany).
General synthesis of 1,5-bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one (1)
An aqueous solution of sodium hydroxide (10% w/v, 20 ml) was added to a solution of the appropriate aryl aldehyde (0.02 mol) in ethanol (20 ml). Acetone (0.01 mol) was added into the reaction mixture. Reaction mixture was heated with modified household microwave oven at 260 Watt for 20 min. Red viscous residue obtained was diluted with water (30 ml). Then, the mixture was acidified with HCl (10%, pH: 3–5) and the aqueous solution was extracted with EtOAc (3 × 20 ml). The organic layer was dried over anhydrous sodium sulphate. After removal of the solvent in vacuo, the crude products were purified by column chromatography eluting with the mixture of ethyl acetate:n-hexane (1:1). Compound 1 has been previously reported, but synthesis of compound 1 by microwave irradiation is reported here for the first time. 1H-NMR of the compound was in accordance with the chemical structure and melting point of the compound was the same as reported in the literatureCitation22,Citation23.
General synthesis of Mannich bases (2–6)
The solution of compound 1 in acetonitrile (10 ml) was added to a previously heated mixture of paraformaldehyde and suitable secondary amine in acetonitrile (5 ml) at 80 °C for 10 min. The reaction mixture was refluxed until the disappearance of the starting compound 1. The completion of reaction was monitored by TLC for 5–27 h. The reaction solvent was removed in vacuo. Compounds were purified by column chromatography on silica gel (SiO2) for compounds 4–6 and basic Al2O3 (pH 10 ± 0.5) for compound 2. Then, the corresponding fractions related were recrystallized from the suitable solvent.
1,5-Bis-(4-hydroxy-3-methoxy-5-morpholin-4-ylmethyl-phenyl)-penta-1,4-dien-3-one (2)
The crude product was purified by column chromatographyCitation24 on Al2O3 (pH 10 ± 0.5) eluting with CHCl3:MeOH (8:2) and recrystallized from ethanol: yield 53%; m.p. 135–138 °C; (log ɛ) 391 (4.48) nm; IR (KBr) 1976, 1589 (C=O), 1461, 1265, 1083 cm−1; 1H NMR (400 MHz, CDCl3): δ 2.61 (br s, 8H); 3.75 (br s, 12H); 3.93 (s, 6H); 6.91 (s, 2H); 6.92–6.88 (d, 2H, J = 15.75 Hz); 7.25 (s, 2H); 7.65–7.61 (d, 2H, J = 15.75 Hz); 13C NMR (100 MHz, CDCl3): δ 188.69, 150.05, 148.58, 143.25, 126.44, 123.38, 122.82, 121.14, 110.42, 66.93, 61.65, 56.22, 53.09; HRMS m/z 524.2461 (M+), 523.2427 (M − H)+.
1,5-Bis-(4-hydroxy-3-methoxy-5-pyrrolidin-1-ylmethyl-phenyl)-penta-1,4-dien-3-one (3)
The crude product was recrystallized from ethanol: yield 36%; m.p. 130–132 °C; (log ɛ) 396 (4.61) nm; IR (KBr) 1977, 1586 (C=O), 1487, 1268, 1083 cm−1; 1H NMR (400 MHz, CDCl3): δ 1.87–1.85 (br s, 8H); 2.57–2.55 (br s, 8H); 3.87 (s, 4H); 3.93 (s, 6H); 6.90 (s, 2H); 6.94–6.86 (d, 2H, J = 15.76 Hz); 7.07 (s, 2H); 7.68–7.60 (d, 2H, J = 15.75 Hz); 13C NMR (100 MHz, CDCl3): δ 188.82, 150.93, 148.52, 143.44, 128.84, 123.09, 122.71, 122.17, 110.14, 58.60, 56.20, 53.66, 23.86; HRMS m/z 492.2564 (M+), 491.2544 (M − H)+.
1,5-Bis-[4-hydroxy-3-methoxy-5-(4-methyl-piperazin-1-ylmethyl)-phenyl]-penta-1,4-dien-3-one (4)
The crude product was purified by column chromatography on SiO2 eluting with CHCl3/MeOH (1:1) and recrystallized from ethanol; yield 45%; mp 224–226 °C; (log ɛ) 393 (4.67) nm; IR (KBr) 2972, 1977, 1644, 1574 (C=O), 1455, 1291, 1087 cm−1; 1H NMR (400 MHz, CDCl3): δ 2.29 (s, 6H); 2.63–2.42 (br s, 16H); 3.75 (s, 4H); 3.92 (s, 6H); 6.90 (s, 2H); 6.91–6.87 (d, 2H, J = 15.76 Hz); 7.06 (s, 2H); 7.64–7.60 (d, 2HH, J = 15.76 Hz; 13C NMR (100 MHz, CDCl3): δ 188.72, 150.40, 148.57, 143.29, 126.21, 123.26, 122.62, 121.54, 110.39, 61.20, 56.20, 55.04, 52.69, 46.08; HRMS m/z 550.3100 (M+), 549.3069 (M − H)+.
1,5-Bis-(3-diethylaminomethyl-4-hydroxy-5-methoxy-phenyl)-penta-1,4-dien-3-one (5)
The crude product was purified by column chromatography on SiO2 eluting with CHCl3:MeOH (1:1) and recrystallized from cyclohexane: yield 17%; m.p. 80–82 °C; (log ɛ) 398 (4.31) nm; IR (KBr) 3359, 2972, 1976, 1586 (C=O), 1449, 1291, 1080 cm−1; 1H NMR (400 MHz, CDCl3): δ 1.17–1.10 (t, 12H); 2.71–2.61 (q, 8H); 3.82 (s, 4H); 3.85 (s, 6H); 6.90 (s, 2H); 6.94–6.85 (d, 2H, J = 15.75 Hz); 7.05 (s, 2H); 7.67–7.59 (d, 2H, J = 15.93 Hz); 13C NMR (100 MHz, CDCl3): δ 188.84, 151.28, 148.60, 143.44, 125.83, 123.05, 122.62, 121.90, 110.08, 56.81, 56.19, 46.63, 11.31; HRMS m/z 496.2904 (M+), 495.2858 (M − H)+.
1,5-Bis-(4-hydroxy-3-methoxy-5-piperidin-1-ylmethyl-phenyl)-penta-1,4-dien-3-one (6)
The crude product was purified by column chromatography on SiO2 eluting with CHCl3/MeOH (1:1) and recrystallized from acetonitrile; yield 41%; mp 180–182 °C; (log ɛ) 398 nm (4.53); IR (KBr) 2926, 2030, 1589 (C=O), 1410, 1089 cm−1; 1H NMR (400 MHz, CDCl3): δ 1.68–1.49 (br s, 12H); 2.56–2.55 (br s, 8H); 3.72 (s, 4H); 3.93 (s, 6H); 6.89–6.88 (d, 2H); 6.93–6.86 (d, 2H, J = 15.75 Hz); 7.07–7.06 (d, 2H); 7.68–7.60 (d, 2H, J = 15.75 Hz); 13C NMR (100 MHz, CDCl3): δ 188.80, 151.02, 148.56, 143.42, 125.87, 123.05, 122.65, 121.85, 110.08, 62.02, 56.19, 54.08, 25.98, 24.06; HRMS m/z 520.2903 (M+), 519.2853 (M − H)+.
Bioassays
Cytotoxicity evaluation
The compounds in series 1–6 and curcumin were assayed towards HSC-2, HSC-3, HSC-4, HL-60, HGF, HPC and HPLF cells based on a literature procedureCitation25 with some minor modifications. In brief, cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) except the HL-60 cells were cultured in RPMI 1640 medium supplemented with 10% FBS. Varying concentrations of the compound in dimethylsulfoxide were added to the medium and incubated at 37 °C for 48 h. The viable cell numbers were determined by the MTT method except the viability of HL-60 cells was obtained by cell counting with a haemocytometer after staining with 0.15% trypan blue. The CC50 values were determined from dose–response curves.
Immunoblot analysis
Primary antibodies against cPARP were purchased from Cell Signaling Technology (Danvers, MA), and the primary antibody against actin was purchased from Sigma-Aldrich (St. Louis, MO). The horseradish peroxidase-conjugated secondary anti-mouse immunoglobulin G (IgG) and anti-rabbit IgG antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The HSC-2 and HGF cells were cultured in six-well plates for 24 h and then incubated with compounds 4, 5 or 6 for 24 h. The cells were scraped with a rubber policeman and collected in 10× cell lysis buffer (Cell Signaling Technology, Beverly, MA). A solution containing phenylmethanesulfonyl fluoride (1 mM) plus 1× protease inhibitor cocktail (Cell Signaling Technology) was added to each cell lysate. Equal amounts of proteins (50 μg) for each sample were subjected to SDS-polyacrylamide gel electrophoresis, followed by immunoblotting with primary antibodies against cPARP and actin (employed as a loading control) and secondary anti-IgG antibodies, as previously describedCitation26.
Conclusion
In this study, curcumin analogue, compound 1, which has an α,β-unsaturated moiety and their Mannich bases with morpholine, pyrrolidine, N-methylpiperazine, piperidine and diethylamine were designed and their cytotoxic activities against four tumour and three non-malignant cell lines were evaluated. Results obtained revealed that 1,5-bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one and its aminomethyl derivatives (Mannich bases) possessed not only noteworthy potencies towards several neoplasms but demonstrate a greater toxicity to tumours than various non-malignant cells. Generally, all compounds (2–6) in series were found to be more potent than curcumin and its analogue 1, and also greater PSE and SI values were found among compounds 2–6 than compound 1 from which they were derived. Compounds 4 and 6 cleaved PARP1 only in HSC-2 malignant cells, suggesting that possible mechanism of action of the compounds may be inducing apoptosis.
Supplemental Material.pdf
Download PDF (5 MB)Declaration of interest
This research work was supported by Ataturk University Research Fund (Project No: 2013/56), Turkey. The authors have declared no conflicts of interest with the presented data from this article.
References
- Mosley CA, Liotta DC, Snyder JP. Highly active anticancer curcumin analogues. Adv Exp Med Biol 2007;595:77–103
- Ruby AJ, Kuttan G, Babu KD, et al. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett 1995;94:79–83
- Huang SW, Frankel EN. Antioxidant activity of tea catechins in different lipid system. J Agric Food Chem 1997;43:3033–8
- Jordan WC, Drew CR. Curcumin – a natural herb with anti-HIV activity. J Natl Med Assoc 1996;88:333–4
- Kawamori T, Lubet R, Steele VE, et al. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res 1999;59:597–601
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 2003;23:363–98
- Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann New York Acad Sci 2005;1056:206–17
- Chuprajob T, Changtam C, Chokchaisiri R, et al. Synthesis, cytotoxicity against human oral cancer kb cells and structure-activity relationship studies of trienone analogues of curcuminoids. Bioorg Med Chem Lett 2014;24:2839–44
- Brown A, Shi Q, Moore T, et al. Monocarbonyl curcumin analogues: heterocyclic pleiotropic kinase inhibitors that mediate anticancer properties. J Med Chem 2013;56:3456–66
- Manohar S, Khan S, Kandi S, et al. Synthesis, antimalarial activity and cytotoxic potential of new monocarbonyl analogues of curcumin. Bioorg Med Chem Lett 2013;23:112–16
- Mutus B, Wagner JD, Talpas CJ, et al. 1-p-chlorophenyl-4,4-dimethyl-5-ethylamino-1-penten-3-one hydrobromide, a sulfhydryl-specific compund which reacts irreversibly with protein thiols but reversibly with small molecular weight thiols. Anal Biochem 1989;177:237–43
- Ansari MJ, Ahmad S, Kohli K, et al. Stability-indicating HPTLC determination of curcumin in bulk drug and pharmaceutical formulations. J Pharm Biomed Anal 2005;39:132–8
- Wang YJ, Pan MH, Cheng AL, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal 1997;15:1867–76
- Dimmock JR, Padmanilayam MP, Zello GA, et al. Cytotoxic analogues of 2,6-bis (arylidene) cyclohexanones. Eur J Med Chem 2003;38:169–77
- El-Subbagh HI, Abu-Zaid SM, Mahran MA, et al. Synthesis and biological evaluation of certain α,β-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J Med Chem 2000;43:2915–21
- Kucukoglu K, Gul M, Atalay M, et al. Synthesis of some Mannich bases with dimethylamine and their hydrazones and evaluation of their cytotoxicity against Jurkat cells. Arzneimittelforschung 2011;61:366–71
- Kumbhare R, Vijay K, Janaki R, et al. Synthesis and biological evaluation of novel Mannich bases of 2-arylimidazo[2,1-b]benzothiazoles as potential anti-cancer agents. Eur J Med Chem 2011;46:4258–66
- Mete E, Gul H, Canturk P, et al. Biological activity of 1-aryl-3-phenethylamino-1-propanone hydrochlorides and 3-aroyl-4-aryl-1-phenethyl-4-piperidinols on PC-3 cells and DNA topoisomerase I enzyme. Z Naturforsch C 2010;65:647–52
- Gunji H, Kharbanda S, Kufe D. Induction of internucleosomal DNA fragmentation in human myeloid leukemia cells by 1-β-D-arabinofuranosylcytosine. Cancer Res 1991;51:741–3
- Tsurusawa M, Saeki K, Fujimoto T. Differential induction of apoptosis on human lymphoblastic leukemia Nalm-6 and Molt-4 cells by various antitumor drugs. Int J Hematol 1997;66:79–88
- Malanga M, Althaus FR. The role of poly(ADP-ribose) in the DNA damage signaling network. Biochem Cell Biol 2005;83:354–64
- Ge HX, Chen L, Zhang J, et al. Inhibitory effect of curcumin analogs on tissue factor procoagulant activity and their preliminary structure-activity relationships. Med Chem Res 2013;22:3242–6
- Quincoces Suarez JA, Rando DG, Santos R, et al. New antitumoral agents I: in vitro anticancer activity and in vivo acute toxicity of synthetic 1,5-bis(4-hydroxy-3-methoxyphenyl)-1,4-pentadien-3-one and derivatives. Bioorg Med Chem 2010;18:6275–81
- Huang S, Ying H, Hu Y. Synthesis and antitumor activity study of nitrogen-containing curcumin derivatives. Zhongguo Yaowu Huaxue Zazhi 2011;21:88–95
- Motohashi N, Wakabayashi H, Kurihara T, et al. Biological activity of barbados cherry (acerola fruits, fruit of Malpighia emarginata DC) extracts and fractions. Phytother Res 2004;18:212–23
- Umemura N, Zhu J, Mburu YK, et al. Defective NF-κB signaling in metastatic head and neck cancer cells leads to enhanced apoptosis by double-stranded RNA. Cancer Res 2012;72:45–55