Abstract
Objective: This work describes the anti-enzymatic activity of (7-chloroquinolin-4-yl)arylhydrazones against Candida albicans and examines their cytotoxicity.
Material and methods: Ten C. albicans strains [nine isolates and one azole-resistant standard strain (ATCC 62342)] were used to assess the anti-enzymatic activity. Fifteen compounds at sub-antifungal concentrations ranging from 12.5 to 100 µg/ml were assessed after a 30-min exposure. The strains were seeded onto petri dishes with selective agar media for aspartyl proteases (Saps) and phospholipases (PLs). Enzymatic inhibition was measured by the reduction of the precipitation zone (Pz) against untreated strains (positive control). A colorimetric MTT assay was used with 3T3/NIH mouse fibroblasts to evaluate cytotoxicity. Cells were exposed to 15 compounds in concentrations from 6.25 to 100 µg/ml for 24 and 48 h.
Results: Four hydrazones showed enzymatic repression values over 40% to Pl and three over 20% to Saps. The cell viability was over 50% at hydrazone concentrations of 25–100 µg/ml.
Conclusion: These results revealed that select (7-chloroquinolin-4-yl)arylhydrazones may be potential antifungal agents for the control of C. albicans infections.
Introduction
The increase of Candida albicans strains that are resistant to antifungal azoles is one of the major reasons for the investigation of new antimicrobial agentsCitation1. Candida spp. pathogenicity has been related to an imbalance between the host and the opportunist fungusCitation2. In addition, it has been associated with the expression of virulence factors by the yeast, including the production of factors related to adhesion, adherence and biofilm formation as well as exoenzymes.
Recent studies support investigating drugs with specific targets, suggesting a change of focus to the development of new antifungal agents capable of inhibiting virulence factors. Moreover, industry has stimulated the development of new methods to identify and inhibit these virulence factors with lower doses of antimicrobials, aiming to avoid an increase in antifungal resistanceCitation3–5.
The prevalence of exoenzyme production by C. albicans and the effect of several antimicrobials on the enzymatic secretion of these yeasts have been investigatedCitation4,Citation6,Citation7. The most frequent enzymes produced by C. albicans are Secreted Aspartyl Proteases (Saps) and Phospholipases (PLs)Citation5. However, no difference in the production of PLs or Saps was reported between healthy patient isolates and isolates from patients with denture stomatitisCitation4 or between isolates from diabetic patients with candidiasis and non-diabetic patient isolatesCitation6,Citation8.
Saps and PLs have many functions in the virulence of C. albicans, such as the degradation of immunoglobulin and proteins of the extracellular matrix, inhibition of neutrophil phagocytosis, epithelial/mucosal invasion and tissue degradationCitation5,Citation9.
Hydrazones and hydrazides are important in medicinal chemistry because of their versatile biological propertiesCitation10,Citation11. These molecules are easily prepared and have diverse pharmacological potentials, which lead to the synthesis of various novel hydrazone compounds. Numerous studies have centered on hydrazones for the development of better biological molecules with low toxicity profilesCitation12,Citation13.
The biological potential of hydrazone derivatives has been widely described in the literature; considering the variety of biological activities described, the antimicrobial potential seems to be very significantCitation12–14. Biological activity has been described against many strains. However, the strains that hydrazones are most frequently reported to have biological activities against are Staphylococcus aureus, Escherichia coli, Candida albicans, Bacillus subtilis and Pseudomonas aeruginosa. In addition to the antimicrobial activity, hydrazones have also been reported to have anticancer, anti-inflammatory, antiprotozoal, antiplatelet and cardio-protective propertiesCitation14. Many effective compounds, such as iproniazid and isocarboxazid, are synthesized by the reduction of hydrazide-hydrazones and are used in the treatment of tuberculosis (TB) and display an anti-depressant effectCitation12.
The most significant reactivity of hydrazones is the nucleophilicity of the carbon atom. It appears that hard nucleophiles preferentially attack nitrogen, while soft nucleophiles preferentially attack carbon. The functional substituents retain their established reactivity pattern, although they generally become more electrophilicCitation14.
Recently, our research group reported on the in vitro antifungal activity of (7-chloroquinolin-4-yl)hydrazones against strains of Candida spp. and Rhodotorula spp.Citation7 The aim of this study was to evaluate the activity of such hydrazones on the production of PLs and Saps by various strains of C. albicans and their cytotoxicity in 3T3/NIH mouse fibroblasts cultures.
Materials and methods
Chemistry
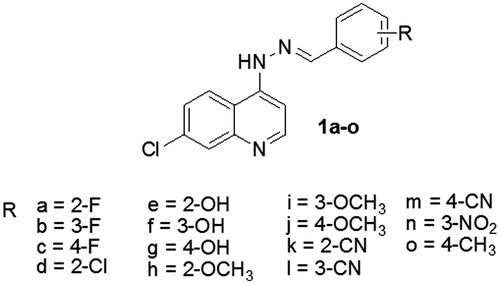
Fifteen hydrazones (1a–1o) with the same basic structure were synthesized from the reaction of 7-chloro-4-hydrazinoquinoline and arenealdehydes refluxed in toluene for 3 hours, according to our previous methodCitation7. The compounds differ from one another by the type (electron-withdrawing or electron-donating) and the position (ortho, meta or para) of the substituent attached to the benzene ring. The general structure of the hydrazones is shown in .
Strain culture conditions
Tests were carried out with 10 different strains of C. albicans, including one azole-resistant strain (ATCC 62342). Candida isolates were obtained from the mouth of patients with denture stomatitis at multiple affected sites and identified as Candida albicans. The strains are part of a fungi bank at the Oral Microbiology Lab at the Federal University of Pelotas, which includes over 100 isolates. For these assays, strains were maintained at −80 °C, defrosted at room temperature and suspended in Sabouraud Dextrose Broth (Acumedia Manufacturers, Inc., Lansing, MI) for overnight incubation. Next, a 20-µl aliquot was seeded in Sabouraud Dextrose Agar with 0.01% chloramphenicol and incubated for 24 h at 37 °C.
Anti-enzymatic assay
For the preparation of inocula, the strains were individually suspended in 5 ml of 1% sterile phosphate-buffered saline (PBS), with turbidity and visual refraction corresponding to 0.5 on the McFarland scale (1–5 × 106 microorganisms/ml).
The chemical compounds () were initially diluted in dimethyl sulfoxide (DMSO) at a concentration of 1 mg/ml and then serially diluted by half until 10 different concentrations were obtained. Yeasts were then exposed to two different concentrations below the minimal inhibitory concentration, as previously describedCitation7. For the exposure, 20 µl aliquots of each concentration were added to 1980 µl of PBS. Immediately, 0.5 ml of the strain suspension was added to the PBS + anti-fungal agent and incubated for 30 min at 37 °C. DMSO exposure was used as a control.
Table 1. Comparison between Pz and inhibition ratio results in percentage for PL production.
After incubation, the solutions were centrifuged at 3000 rpm for 10 min, the supernatant was removed and the pellet was washed in 2 ml of sterile PBS. This process was repeated twice to remove the antifungal agent. The pellet was then suspended in 2 ml of sterile PBS.
For the enzyme production measurement, 20 µl of the final suspension was plated in specific agar medium for PLs (Sabouraud Dextrose Agar 65 g, sodium chloride 57.3 g, calcium chloride 0.55 g and 40 ml of sterile egg yolk) and Saps (Agar–agar 20 g, glucose 20 g, yeast nitrogen base [Difco, Difco Laboratories Detroit, MI. ref. 233520, without amino acids and ammonium sulfate] 1.45 g and bovine serum albumin 2 g).
Results were measured at 48 h for Saps and 96 h for PLs to analyse the enzyme production. Pz was calculated as previously described by Barros et al.Citation5 Colony diameter (cd) and the diameter of the colony plus the precipitation diameter around the colony (cd + p) were measured and Pz was calculated using Equation (1).
(1)
For further analysis, the results of the control Pzc and the test Pzt were compared to calculate the enzymatic inhibition percentage (Ei%) using Equation (2).
(2)
Cell culture
The NIH/3T3 cell strain was obtained from the Rio de Janeiro Cell Bank (PABCAM, Federal University of Rio de Janeiro, RJ, Brazil). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum (FBS) purchased from Vitrocell Embriolife (Campinas, Brazil) and Gibco (Grand Island, NY), respectively. Cells were grown at 37 °C in an atmosphere of 95% humidified air and 5% CO2, as described previouslyCitation15. The experiments were performed with cells in the logarithmic phase of growth.
Cytotoxicity assay
Briefly, cells were seeded at a density of 2 × 104 cells per well in a volume of 100 µl in 96-well plates and grown at 37 °C in a humidified atmosphere of 5% CO2/95% air for 24 h. Cells were incubated with 11 different hydrazone compounds for 24 h at the following concentrations: 6.25, 12.5, 25 and 50 µg/ml. These compounds were previously dissolved in DMSO and added to DMEM supplemented with 10% FBS to the desired concentrations. The final DMSO concentration in the culture medium never exceeded 0.5%, and a control group exposed to an equivalent concentration of DMSO was evaluated. After incubation, 20 µl of MTT (5 mg MTT/ml solution) was added to each well. The plates were incubated for an additional 3 h, and the medium was discarded. About 200 µl of DMSO was added to each well, and the formazan was solubilized on a shaker for 5 min at 100 × g. The viability of the NIH/3T3 cells was determined by measuring the reduction of soluble MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to water-insoluble formazanCitation16. The absorbance of each well was read on a microplate reader (Thermo Plate TP-Reader, Thermo Fisher Scientific, Waltham, MA) at a wavelength of 492 nmCitation15–17.
The rate of cell growth inhibition was determined using Equation (3):
Statistical analysis
Data were typed twice and descriptive and statistical analyses were carried out using the Stata 12.0 software package (StataCorp LP; College Station, TX). To compare all groups, one-way anova was performed followed by Tukey's test. A p value of <0.05 was considered statistically significant.
Results
Anti-enzymatic activity
The compounds with significant results had inhibition values over 40% for PLs and 20% for Saps. The most effective compound was hydrazone 1a, which showed a PL inhibition of 71% at the lowest concentration (12.5 µg/ml) and inhibited Saps by 23% at 25 µg/ml. Compound 1o showed little inhibition of PL (25%); however, 1o inhibited 20% of Saps production at 25 µg/ml. Furthermore, four compounds (1e, 1g, 1j and 1l) also showed important enzymatic suppression of PLs, and one compound (1l) suppressed Saps at 50 µg/ml ( and ).
Table 2. Comparison between Pz and inhibition ratio results in percentage for Spas production.
Cytotoxicity
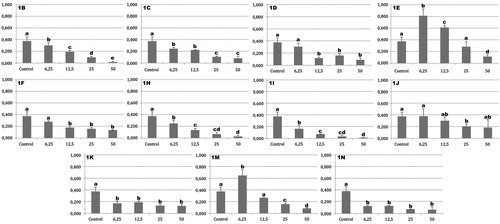
To evaluate whether the hydrazones had cytotoxic effects, the MTT assay was carried out (). The cytotoxic activities of compounds 1a, 1g, 1l and 1o were published in our previous workCitation7. In this study, 1e, 1j and 1m were the least cytotoxic. Interestingly, cell proliferative activity was observed for compounds 1e and 1m at the lowest concentration tested (6.25 µg/ml). Compound 1e also increased the cell count at 12.5 µg/ml. The highest cytotoxicity was presented by 1i, as shown by the following inhibitory ratios: 57.3% (6.25 µg/ml), 81.4% (12.5 µg/ml), 91.1% (25 µg/ml) and 97.3% (50 µg/ml).
Discussion
Although the number of antifungal drugs has increased, the clinical need for novel antifungals remains important in today’s medical practice. Considering the worldwide increase in fungal infections associated with patients with impaired immunity, a new gamma of antifungal drugs has been developed in the last two decades. Unfortunately, the increased use of antifungal drugs caused an expansion of fungal resistance to most commercially available drugsCitation18.
In humans, Candida spp. are the most common fungal pathogens. Moreover, Candida albicans is known as the main fungus associated with human candidiasis, and it is the most virulent species of CandidaCitation18.
Protein and enzyme secretion is considered an essential process in fungal survival, and the characteristics of the secreted proteins define many of the functional capabilities of these microorganisms. The secretion of hydrolytic enzymes by C. albicans, the expression of surface adhesins, morphological mutation between hyphae and yeast, phenotypic switch and biofilm formation are crucial for species perpetuationCitation19.
The methodology used was previously validated by Kadir et al.Citation4 for the inhibition of PLs using chlorhexidine in various concentrations. In this study, the authors observed statistically significant reductions of PLs at concentrations of 0.0012% and 0.002% chlorhexidine against 10 oral isolates of C. albicans. However, their data are presented in Pz results, impairing observations of the amount of enzymatic inhibition and comparisons with percentage valuesCitation4.
The hydrazones are a class of compounds cited as biologically valuable for pharmaceutical applications due to their anti-inflammatoryCitation20, anticancerCitation21, anti-tuberculosisCitation22, anti-HIVCitation23 and anti-Alzheimer's disease (cholinesterase inhibitors) propertiesCitation24. The literature also shows important antifungal properties of hydrazonesCitation25–27. In addition, the quinoline ring is also an important group in medicinal chemistry and has particular utility in antimalarialCitation28, antituberculosis29, antileishmania30 and antimicrobial31 agents.
In this study, the minimal concentrations needed to inhibit the enzymatic activity ranged from 6.25 to 100 µg/ml. It is important to highlight that the basic chain structure was maintained and that only the substituent attached to the heterocyclic ring was changed. These results suggest an effect dependent on the association between the general structure and the substituent.
Although the focus in this study was the inhibition of Candida species growth, it is important to note that compounds 1e and 1m were able to stimulate cell growth. This result indicates that these hydrazones could be used to inhibit fungal growth and to stimulate tissue repair.
The most effective heterocyclic ring substitution for the repression of the PL production appeared to be compound 1a (71% inhibition at 12.5 μg/ml). Unfortunately, this compound also showed cytotoxicity at this concentrationCitation7. This result is important because compound 1a showed the best MIC value against C. albicans strains (25 μg/ml) in our previous studyCitation7. Compound 1a also had the best inhibitory activity against the Sap production. However, hydrazone 1o appears to be the best compound because, at 25 μg/ml, it reduced the production of Saps by 20% and did not display cytotoxicity at 50 μg/ml in 3T3/NIH fibroblast cellsCitation7.
These results do not allow us to determinate a structure–activity relationship for the arylhydrazone moiety. Both electron-donating (1g, 1j and 1o) and electron-withdrawing (1a, 1i and 1l) groups showed PL activity. In addition, the position does not affect the activity. The hydrogen bond-donating property of the R–OH groups might be important to compound–enzyme affinity because only hydrazones with R=2–OH (1e) and R=4–OH (1g) have activity.
Exoenzyme production and secretion in Candida and other fungi have many mechanisms. The main mechanism for Sap production is the chain production between the endoplasmic reticulum and the Golgi complex, a system involving vesicle secretion. PL production is most likely a membrane release mechanismCitation19. These different pathways may underlie the discrepancy in the results between PL inhibition and Sap inhibition in our study.
Conclusion
According to our findings, (7-chloroquinolin-4-yl)arylhydrazones can potently inhibit enzymatic processes in C. albicans and have low cytotoxicity at sub-antifungal concentrations. Based on these results, the quinolin-4-yl hydrazones are promising agents for pharmaceutical use, and the methodology used supports a new target of action in antifungal studies.
Declaration of interest
The authors are thankful to UFPel, Fundação de Amparo a Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq APE Grant No. 462866/2014-9), for the financial support of the research. The authors report no declarations of interest.
References
- Ramage G, Milligan S, Lappin DF, et al. Antifungal, cytotoxic, and immunomodulatory properties of tea tree oil and its derivative components: potential role in management of oral candidosis in cancer patients. Front Microbiol 2012;3:220
- Lund RG, da Silva Nascente P, Etges A, et al. Occurrence, isolation and differentiation of Candida spp. and prevalence of variables associated to chronic atrophic candidiasis. Mycoses 2010;53:232–8
- Taweechaisupapong S, Klanrit P, Singhara S, et al. Inhibitory effect of Streblus asper leaf-extract on adhesion of Candida albicans to denture acrylic. J Ethnopharmacol 2006;106:414–17
- Kadir T, Gümrü B, Uygun-Can B. Phospholipase activity of Candida albicans isolates from patients with denture stomatitis: the influence of chlorhexidine gluconate on phospholipase production. Arch Oral Biol 2007;52:691–6
- Barros LM, Boriollo MF, Alves AC, et al. Genetic diversity and exoenzyme activities of Candida albicans and Candida dubliniensis isolated from the oral cavity of Brazilian periodontal patients. Arch Oral Biol 2008;53:1172–8
- Willis AM, Coulter WA, Fulton CR, et al. The influence of antifungal drugs on virulence properties of Candida albicans in patients with diabetes mellitus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;91:317–21
- Duval AR, Carvalho PH, Soares MC, et al. 7-Chloroquinolin-4-yl arylhydrazone derivatives: synthesis and antifungal activity. ScientificWorldJournal 2011;11:1489–95
- Boriollo MF, Bassi RC, dos Santos Nascimento CM, et al. Distribution and hydrolytic enzyme characteristics of Candida albicans strains isolated from diabetic patients and their non-diabetic consorts. Oral Microbiol Immunol 2009;24:437–50
- Santos PO, Melo JO, Ponzzes CM, et al. Multilocus enzyme electrophoresis analysis and exoenzymatic activity of Candida albicans strains isolated from women with vaginal candidiasis. Mycoses 2012;55:64–72
- Narang R, Narasimhan B, Sharma S. A review on biological activities and chemical synthesis of hydrazide derivatives. Curr Med Chem 2012;19:569–612
- Negim VJ, Sharma AK, Negi JS, Ram V. Biological activities of hydrazone derivatives in the new millennium. Int J Pharma Chem 2012;2:100–9
- Mohammad A. Anti-microbial potentials of hydrazonone derivatives: a promising scaffold. Int J Chem Appl Biol Sci 2014;1:23–37
- Verma G, Marella A, Shaquiquzzaman M, et al. A review exploring biological activities of hydrazonesJ Pharma Bioallied Sci 2014;6:69–80
- Uppal G, Bala S, Kamboj S, Saini M. Therapeutic review exploring antimicrobial potential of hydrazones as promising lead. Der Pharma Chemica 2011;3:250–68
- Nedel F, Campos VF, Alves D, et al. Substituted diaryl diselenides: cytotoxic and apoptotic effect in human colon adenocarcinoma cells. Life Sci 2012;91:345–52
- Henn S, Nedel F, de Carvalho RV, et al. Characterization of an antimicrobial dental resin adhesive containing zinc methacrylate. J Mater Sci Mater Med 2011;22:1797–802
- Nedel F, Begnini K, Carvalho PH, et al. Antiproliferative activity of flower hexane extract obtained from mentha spicata associated with mentha rotundifolia against the MCF7, KB, and IH/3T3 cell lines. J Med Food 2012;15:955–8
- Kathiravan MK, Salake AB, Chothe AS, et al. The biology and chemistry of antifungal agents: a review. Bioorg Med Chem 2012;20:5678–98
- Fonzi WA. The protein secretory pathway of Candida albicans. Mycoses 2009;52:291–303
- Lacerda RB, da Silva LL, de Lima CKF, et al. Discovery of novel orally active antiinflammatory N-phenylpyrazolyl-N-glycinyl-hydrazone derivatives that inhibit TNF-α production. PLoS One 2012;7:e46925
- Tong JQ, Tian FF, Li Q, et al. Probing the adverse temperature dependence in the static fluorescence quenching of BSA induced by a novel anticancer hydrazone. Photochem Photobiol Sci 2012;11:1868–79
- Lessigiarska I, Pajeva I, Prodanova P, et al. Structure-activity relationships of pyrrole hydrazones as new anti-tuberculosis agents. Med Chem 2012;8:462–73
- Ma XD, Yang SQ, Gu SX, et al. Synthesis and anti-HIV activity of aryl-2-[(4-cyanophenyl)amino]-4-pyrimidinone hydrazones as potent non-nucleoside reverse transcriptase inhibitors. ChemMedChem 2011;6:2225–32
- Raza R, Saeed A, Arif M, et al. Synthesis and biological evaluation of 3-thiazolocoumarinyl Schiff-base derivatives as cholinesterase inhibitors. Chem Biol Drug Des 2012;80:605–15
- Aslan HG, Karacan N. Aromatic sulfonyl hydrazides and sulfonyl hydrazones: antimicrobial activity and physical properties. Med Chem Res 2103;22:1330–8
- Altintop MD, Ozdemir A, Turan-Zitouni G, et al. Synthesis and biological evaluation of some hydrazone derivatives as new anticandidal and anticancer agents. Eur J Med Chem 2012;58:299–307
- Syed TS, Santhalakshmi S, Kannappan G. Studies on antimicrobial activity of some hydrazones and its copper complexes. J Pharm Res 2012;3:2759–60
- Kouznetsov VV, Gómes-Barrio A. Recent developments in the design and synthesis of hybrid molecules based on aminoquinoline ring and their antiplasmodial evaluation. Eur J Med Chem 2009;44:3091–113
- Ferreira ML, Goncalves RSB, Cardoso LNF, et al. Synthesis and antitubercular activity of heteroaromatic isonicotinoyl and 7-chloro-4-quinolinyl hydrazone derivatives. Scientific World J 2010;10:1347–55
- Coimbra ES, Antinarelli LMR, da Silva AD, et al. 7-Chloro-4-quinolinyl hydrazones: a promising and potent class of antileishmanial compounds. Chem Biol Drug Des 2013;81:658–65
- Makawana JA, Patel MP, Patel RG. Synthesis and in vitro antimicrobial evaluation of penta-substituted pyridine derivatives bearing the quinoline nucleus. Med Chem Res 2012;21:616–23