Abstract
Sulfamerazine and sulfaguanidine are clenched with p-nitrobenzoyl chloride and the products obtained are reduced to NaxS in ethanol–water. Novel sulfonamides (6a–g and 9a–g) were synthesized by the reaction of these reduced products (4 and 8) with various sulfonyl chlorides (5a–g). The structures of these compounds were characterized using spectroscopic analysis (IR, 1H-NMR, 13C-NMR and HRMS) technique. Antimicrobial activity of sulfonamides (3, 4, 7, 8, 6a–g and 9a–g) was evaluated by the agar diffusion method. These compounds showed antimicrobial activity against tested microorganism strains (Gram-positive bacteria, clinic isolate and yeast and mold). Compounds 9d, 9e, 9a, 6d and 6e showed particularly antimicrobial activity against tested Gram-positive (Bacillus cereus and B. subtilis) and Gram-negative (Enterobacter aerogenes) bacteria.
Introduction
Sulfonamide compounds, included in “privileged structures” in medical chemistry, indicate many advantageous pharmacokinetic properties including metabolic resistance mechanismsCitation1. Sulfonamides have many advantages like being cheap and durable besides being broad-spectrum, bacteriostatic action and easily implementable medicinesCitation2. Sulfonamide compounds, which are used both for human health and in veterinary applications, have been clinically used as: antimicrobialCitation3, diureticCitation4, antiobesityCitation5,Citation6, anticancerCitation7 and antiglaucomaCitation8–14.
Sulfamerazine is a sulfonamide drug that inhibits bacterial synthesis of dihydrofolic acid by competing with para-amino benzoic acid for binding to dihydropteroate synthesizes. Thus, this molecule can be used to treat anti-bacterial, anti-bronchitis, anti-prostatitis and urinary tract infections.
Sulfaguanidine is an antimicrobial substance used to treat intestinal infections. Moreover, it is also reported that sulfaguanidine prevents infections that cannot be determined by natural and clinical studiesCitation15,Citation16.
Structures of sulfonamide compounds containing newly synthesized sulfamerazine/sulfaguanidine were analyzed using spectroscopic analysis methods (IR, 1H-NMR, 13C-NMR and HRMS, respectively).
These newly synthesized sulfonamide compounds (6a–g and 9a–g) were evaluated for their antimicrobial activity against Shigella sonnei, Salmonella 21.3, Bacillus subtilis, B. cereus, Staphylococcus aureus, Escherichia coli, Staphylococcus epidermidis, Pseudomonas aeruginosa, Candida albicans and Saccharomyces cerevisiae. These compounds were active on both Gram-positive (Bacillus subtilis, B. cereus, Staphylococcus aureus and Staphylococcus epidermidis) and Gram-negative (Shigella flexneri, Salmonella spp., Escherichia coli and Pseudomonas aeruginosa) bacteria.
Materials and methods
Materials
The chemicals used in the synthesis of sulfonamide derivatives were obtained from Merck and Aldrich Chemical Company. All chemicals and solvents used for the synthesis were of spectroscopic reagent grade. Melting points were measured on a Bibby Scientific Stuart Digital, Advanced and SMP30. Fourier Transform Infrared (FTIR) spectra were recorded on Bruker Optics, ALPHA FT-IR spectrometer. The 1H-NMR with Bruker DPX-300 and 13C-NMR spectra was obtained in DMSO-d6 as solvents with tetramethylsilane as the internal reference. HRMS spectra were detected on an Agilent Technologies 6530 Accurate-Mass Q-TOF LC/MS at the advanced technology research center of Dumlupinar University (ILTEM).
General procedure for preparation of N-(4-(N-(4-methylpyrimidin-2-yl) sulfamoyl) phenyl)-4-nitrobenzamide compound (3)
Sulfamerazine [4-amino-N-(4-methylpyrimidin-2-yl)benzene sulfonamide; 2.164 g, 10.1 mmol], p-nitrobenzoyl chloride (1.856 g, 10 mmol), in dry 5 mL pyridine were stirred for 5 h at room temperature. The completion of the reaction is monitored by TLC. Then the solvent was removed in vacuous and washed with H2O. The raw product was purified by recrystallization from ethanol (75%).
General procedure for preparation of 4-amino-N-(4-(N-(4-methylpyrimidin-2-yl) sulfamoyl) phenyl) benzamide compound (4)
The compounds (3) were dissolved in 10 ml ethanol. Then, this solution of sodium poly-sulfur was added drop wise to a stirred and warm solution of compound 3 (1 mmol) in 50 ml ethanol–water. The progress of the reaction was monitored by TLC. Once the reaction is completed, the mixture was cooled to room temperature and solid filtered off and washed with H2O. The sulfonamide product was purified and recrystallized from the ethanol (80%).
4-Amino-N-(4-(N-(4-methylpyrimidin-2-yl)sulfamoyl)phenyl)benzamide (4)
As yellow crystals (1, 906 g, 74%), m.p. 248–251 °C (ethanol). IR (cm−1): 3449 w (–NH), 3394 w (–NH2), 3036 w (Ar–H), 1664 s (C = O), 1531 s (C = C), 1181 s (SO2); 1H NMR (300 MHz, DMSO-d6) δ (ppm): 2.30 (s, 3H, –CH3), 5.60–6.10 (br, 2H, –NH2), 6.60 (d, 2H, J = 13.40 Hz, Ar–H), 6.90 (d, 1H, J = 5.13 Hz, Ar–H), 7.72 (d, 2H, J = 8.66 Hz, Ar–H), 7.95 (s, 4H, Ar–H), 8.33 (d, 1H, J = 5.11 Hz, Ar–H), 10.10 (s, 1H, –NH), 11.40–11.80 (br, 1H, –NH); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 23.74, 112.44, 113.02, 115.37, 119.43, 120.82, 129.25, 130.09, 134.11, 144.24, 152.98, 153.40, 157.08, 166.07; HRMS (QTOF-ESI): m/z [M + H]− calcd. for C18H16N5O3S: 382.0974; found [M − H]− : 382.0978.
General procedure for preparation of sulfonamide derivatives (6a–g, 9a--g)
A mixture of the 4-amino-N-(4-(N-(4-methyl pyrimidin-2-yl) sulfamoyl) phenyl) benzamide (0.5 mmol) and the sulphonyl chlorides 5a–g (0.5 mmol) in dry pyridine (5 mL) was stirred for 5 h at room temperature. After the solvent was removed in vacuum, the crude product was purified by recrystallization from ethanol (30–86%).
4-(Ethylsulfonamido)-N-(4-(N-(4-methylpyrimidin-2-yl)sulfamoyl)phenyl)benzamide (6a)
As white crystals (0.071 g, 30%), m.p. 268–270 °C (ethanol). IR (cm−1): 3381 w (–NH), 3035 w (Ar–H), 2942 w (–CH3), 1669 s (C = O), 1536 s (C = C), 1146 s (SO2); 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.20 (t, 3H, J = 7.31 Hz, –CH3), 2.40 (s, 3H, –CH3), 3.20 (q, 2H, J = 7.32 Hz, –CH2), 6.91 (d, 1H, J = 5.14 Hz, Ar–H), 7.32 (d, 2H, J = 8.78 Hz, Ar–H), 7.92–7.99 (m, 5H, Ar–H), 8.33 (d, 1H, J = 5.11 Hz, Ar–H), 10.25 (s, 1H, –NH), 10.45 (s, 1H, –NH), 11.30–11.70 (br, 1H, –NH); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 8.48, 23.72, 46.06, 113.41, 115.31, 118.13, 119.45, 119.79, 129.26, 129.84, 130.08, 134.95, 142.42, 143.57, 157.03, 158.04, 165.77; HRMS (QTOF-ESI): m/z [M + Na]+ calcd. for C20H21N5NaO5S2: 498.0882; found [M − H]+ : 498.0888.
N-(4-(N-(4-Methylpyrimidin-2-yl)sulfamoyl)phenyl)-4-(phenylsulfonamido)benzamide (6b)
As white crystals (0.192 g, 73%), m.p. 287–288 °C (ethanol). IR (cm−1): 3395 w (–NH), 3046 w (Ar–H), 2980 w (–CH3), 1606 s (C = O), 1504 s (C = C), 1156 s (SO2); 1H NMR (300 MHz, DMSO-d6) δ (ppm): 2.25 (s, 3H, –CH3), 6.90 (d, 1H, J = 5.07 Hz, Ar–H), 7.23 (d, 2H, J = 8.67 Hz, Ar–H), 7.54–7.66 (m, 3H, Ar–H), 7.80–7.96 (m, 8H, Ar–H), 8.31 (d, 1H, J = 5.09 Hz, Ar–H), 10.40 (s, 1H, –NH), 10.80 (s, 1H, –NH), 11.40–11.70 (br, 1H, –NH); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 23.71, 115.30, 118.70, 119.73, 126.42, 127.15, 129.31, 129.69, 129.84, 129.90, 133.68, 134.96, 139.73, 141.57, 143.50, 157.01, 157.93, 165.72; HRMS (QTOF-ESI): m/z [M − H]− calcd. for C24H20N5O5S2: 522.0906; found [M − H]−: 522.0914.
4-(4-Methylphenylsulfonamido)-N-(4-(N-(4-methylpyrimidin-2-yl)sulfamoyl)phenyl) benzamide (6c)
As gray crystals (0.204 g, 76%), m.p. 287–293 °C (ethanol). IR (cm–1): 3358 w (–NH), 3042 w (Ar–H), 2927 w (–CH3), 1641 s (C = O), 1605 s (C = C), 1157 s (SO2); 1H NMR (300 MHz, DMSO-d6) δ (ppm): 2.32 (s, 6H, –CH3), 6.90 (d, 1H, J = 5.02 Hz, Ar–H), 7.21 (d, 2H, J = 8.74 Hz, Ar–H), 7.37 (d, 2H, J = 8.08 Hz, Ar–H), 7.70–7.96 (m, 9H, Ar-H), 8.32 (d, 1H, J = 5.08 Hz, Ar–H), 10.38 (s, 1H, –NH), 10.70 (s, 1H, –NH), 11.30–11.70 (br, 1H, –NH); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 21.42, 23.72, 118.55, 119.71, 127.23, 129.32, 129.67, 129.71, 130.31, 134.94, 136.89, 141.71, 143.53, 144.10, 157.03, 165.72; HRMS (QTOF-ESI): m/z [M − H]− calcd. for C25H22N5O5S2: 536.1062; found [M − H]−: 536.1059.
4-(4-Bromophenylsulfonamido)-N-(4-(N-(4-methylpyrimidin-2-yl)sulfamoyl)phenyl) benzamide (6 d)
As white crystals (0.229 g, 76%), m.p. 288–289 °C (ethanol). IR (cm–1): 3392 w (–NH), 3045 w (Ar–H), 2871 w (–CH3), 1654 s (C = O), 1572 s (C = C), 1156 s (SO2); 1H NMR (300 MHz, DMSO-d6) δ (ppm): 2.30 (s, 3H, –CH3), 6.90 (d, 1H, J = 5.14 Hz, Ar–H), 7.22 (d, 2H, J = 8.68 Hz, Ar–H), 7.73–7.96 (m, 10H, Ar–H), 8.32 (d, 1H, J = 5.10 Hz, Ar–H), 10.40 (s, 1H, –NH), 10.85 (s, 1H, –NH), 11.40–11.70 (br, 1H, –NH); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 23.71, 115.30, 119.02, 119.75, 127.61, 129.18, 129.33, 129.76, 130.17, 133.02, 135.00, 138.97, 141.22, 143.49, 157.02, 158.00, 165.68, 168.71; HRMS (QTOF-ESI): m/z [M − H]− calcd. for C24H19BrN5O5S2: 600.0011; found [M − H]−: 600.0002.
4-(4-Methoxyphenylsulfonamido)-N-(4-(N-(4-methylpyrimidin-2-yl)sulfamoyl)phenyl) benzamide (6e)
As white crystals (0.192 g, 69%), m.p. 260–262 °C (ethanol). IR (cm−1): 3391 w (–NH), 3042 w (Ar–H), 1666 s (C = O), 1591 s (C = C), 1155 s (SO2); 1H NMR (300 MHz, DMSO-d6) δ (ppm): 2.30 (s, 3H, –CH3), 3.80 (s, 3H, –OCH3), 6.91 (d, 1H, J = 5.02 Hz, Ar–H), 7.09 (d, 2H, J = 8.98 Hz, Ar–H), 7.20 (d, 2H, J = 19.80 Hz, Ar–H), 7.75–7.96 (m, 8H, Ar–H), 8.32 (d, 1H, J = 5.05 Hz, Ar–H), 10.40 (s, 1H, –NH), 10.65 (s, 1H, –NH), 11.40–11.60 (br, 1H, –NH); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 23.72, 56.13, 115.00, 115.32, 118.48, 119.72, 129.31, 129.44, 129.59, 129.66, 131.30, 134.94, 141.82, 143.53, 157.03, 163.10, 165.72, 168.75; HRMS (QTOF-ESI): m/z [M − H]− calcd. for C25H22N5O6S2: 552.1011; found [M − H]−: 552.1002.
N-(4 -(N-(4-Methylpyrimidin-2-yl)sulfamoyl)phenyl)-4-(2,4,6-trimethylphenylsulfonamido) benzamide (6f)
As white crystals (0.162 g, 57%), m.p. 237–238 °C (ethanol). IR (cm − 1): 3375 w (–NH), 3030 w (Ar–H), 2932 w (–CH), 1662 s (C = O), 1502 s (C = C), 1147 s (SO2); 1H NMR (300 MHz, DMSO-d6) δ (ppm): 2.20 (s, 3H, –CH3), 2.30 (s, 3H, –CH3), 2.60 (s, 6H, –CH3), 6.89–7.08 (m, 5H, Ar–H), 7.79–7.95 (m, 6H, Ar–H), 8.30 (d, 1H, J = 5.06 Hz, Ar–H), 10.35 (s, 1H, –NH), 10.75 (s, 1H, –NH), 11.40–11.70 (br, 1H, –NH); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 20.83, 23.71, 36.24, 115.00, 115.32, 118.48, 119.72, 129.31, 129.44, 129.59, 129.66, 131.30, 134.94, 141.82, 143.53, 157.03, 163.10, 165.72, 168.75; HRMS (QTOF-ESI): m/z [M – H]− calcd. for C27H26N5O5S2: 564.1375; found [M − H]−: 564.1387.
N-(4-(N-(4-Methylpyrimidin-2-yl)sulfamoyl)phenyl)-4-(naphthalene-2-sulfonamido) benzamide (6 g)
As white crystals (0.246 g, 86%), m.p. 224–228 °C (ethanol). IR (cm−1): 3390 w (–NH), 3044 w (Ar–H), 1666 s (C = O), 1591 s (C = C), 1130 s (SO2); 1H NMR (300 MHz, DMSO-d6) δ (ppm): 2.30 (s, 3H, –CH3), 6.89 (d, 1H, J = 5.12 Hz, Ar–H), 7.26 (d, 2H, J = 8.77 Hz, Ar–H), 7.55–8.01 (m, 11H, Ar–H), 8.13–8.20 (m, 2H, Ar–H), 8.30 (d, 1H, J = 5.10 Hz, Ar–H), 8.55 (s, 1H, Ar–H), 10.35 (s, 1H, –NH), 10.90 (s, 1H, –NH), 11.30–11.70 (br, 1H, –NH); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 23.70, 115.29, 118.64, 119.70, 122.32, 128.27, 128.30, 128.74, 129.31, 129.63, 129.69, 129.76, 119.82, 129.90, 130.17, 131.98, 134.80, 134.94, 136.66, 141.55, 143.50, 157.02, 165.69, 168.66; HRMS (QTOF-ESI): m/z [M − H]− calcd. for C28H22N5O5S2: 572.1062; found [M − H]−: 572.1073.
N-(4-(N-(Diamino methylene)sulfamoyl)phenyl)-4-(ethylsulfonamido)benzamide (9a)
As black crystals (0.112 g, 72%), m.p. 233–235 °C (ethanol). IR (cm−1): 3427 w (–NH), 3352 w (–NH2), 3193 w (Ar–H), 1654 s (C = O), 1606 s (C = C), 1136 s (SO2); 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.20 (t, 3H, J = 7.33 Hz, –CH3), 3.19 (q, 2H, J = 7.32 Hz, –CH2), 6.70–6.90 (br, 4H, 2 × –NH2), 7.33 (d, 2H, J = 8.74 Hz, Ar–H), 7.74 (d, 2H, J = 8.78 Hz, Ar–H), 7.91 (d, 2H, J = 8.77 Hz, Ar–H), 7.99 (d, 2H, J = 8.68 Hz, Ar–H), 10.30 (s, 1H, –NH), 10.50 (s, 1H, –NH); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 8.47, 46.06, 118.15, 120.13, 126.79, 129.33, 129.87, 139.47, 142.24, 142.39, 158.69, 165.59; HRMS (QTOF-ESI): m/z [M − H]− calcd. for C16H18N5O5S2: 424.0749; found [M − H]−: 424.0751.
N-(4-(N-(Diamino methylene)sulfamoyl)phenyl)-4-(phenyl sulfonamido)benzamide (9b)
As white crystals (0.2318 g, 81%), m.p. 250–252 °C (ethanol). IR (cm−1): 3423 w (–NH), 3339 w (–NH2), 3110 w (Ar–H), 2905 w (C–H), 1650 s (C = O), 1529 s (C = C), 1152 s (SO2); 1H NMR (300 MHz, DMSO-d6) δ (ppm): 6.50–6.80 (br, 4H, 2 × –NH2), 7.23 (d, 2H, J = 8.73 Hz, Ar–H), 7.55–7.67 (m, 3H, Ar–H), 7.70 (d, 2H, J = 8.80 Hz, Ar–H), 7.81–7.86 (m, 6H, Ar–H), 10.30 (s, 1H, –NH), 10.80 (s, 1H, –NH); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 118.74, 119.97, 126.84, 127.16, 129.63, 129.90, 130.01, 133.67, 139.56, 139.74, 141.46, 142.06, 158.50, 165.55; HRMS (QTOF-ESI): m/z [M − H]− calcd. for C20H18N5O5S2: 472.0749; found [M − H]−: 472.0753.
N-(4-(N-(Diaminomethylene)sulfamoyl)phenyl)-4-(4-methylphenylsulfonamido)benzamide (9c)
As white crystals (0.235 g, 80%), m.p. 280–282 °C (ethanol). IR (cm–1): 3511 w (–NH), 3436 and 3343 w (–NH2), 1653 s (C = O), 1588 s (C = C), 1293 and 1154 s (SO2); 1H NMR (300 MHz, DMSO-d6) δ (ppm): 2.30 (s, 3H, –CH3), 6.40–6.70 (br, 4H, 2 × –NH2), 7.22 (d, 2H, J = 8.68 Hz, Ar–H), 7.37 (d, 2H, J = 8.26 Hz, Ar–H), 7.69–7.74 (m, 4H, Ar–H), 7.83 (dxd, 4H, J1 = 1.68 Hz, J2 = 10.47 Hz, Ar–H), 10.30 (s, 1H, –NH), 10.70 (s, 1H, –NH); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 21.42, 118.60, 119.99, 126.85, 127.23, 129.61, 129.86, 130.30, 136.88, 139.54, 141.61, 142.08, 144.10, 158.51, 165.58; HRMS (QTOF-ESI): m/z [M − H]− calcd. for C21H20N5O5S2: 486.0906; found [M − H]−: 486.0907.
4-(4-Bromophenylsulfonamido)-N-(4-(N-(diaminomethylene)sulfamoyl)phenyl)benzamide (9 d)
As white crystals (0.252 g, 83%), m.p. 268–270 °C (ethanol). IR (cm − 1): 3462 w (–NH), 3361 and 3339 w (–NH2), 3102 w (Ar–H), 2906 w (C–H), 1645 s (C = O), 1590 s (C = C), 1303 and 1151 s (SO2); 1H NMR (300 MHz, DMSO-d6) δ (ppm): 6.50–6.80 (br, 4H, 2 × –NH2), 7.23 (d, 2H, J = 8.75 Hz, Ar–H), 7.70–7.87 (m, 10H, Ar–H), 10.35 (s, 1H, –NH), 10.85 (s, 1H, –NH); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 118.57, 119.50, 126.35, 127.11, 128.68, 129.20, 129.83, 132.53, 138.47, 139.09, 140.62, 141.55, 158.01, 165.02; HRMS (QTOF-ESI): m/z [M − H]− calcd. for C20H17BrN5O5S2: 549.9854; found [M − H]−: 549.9856.
N-(4-(N-(Diaminomethylene)sulfamoyl)phenyl)-4-(4-methoxyphenylsulfonamido)benzamide (9e)
As white crystals (0.270 g, 81%), m.p. 262–265 °C (ethanol). IR (cm−1): 3411 w (–NH), 3374 and 3325 w (–NH2), 1651 s (C = O), 1590 s (C = C), 1247 and 1149 s (SO2); 1H NMR (300 MHz, DMSO-d6) δ (ppm): 3.80 (s, 3H, –CH3), 6.50–6.90 (br, 4H, 2 × –NH2), 7.08 (d, 2H, J = 9.88 Hz, Ar–H), 7.21 (d, 2H, J = 8.70 Hz, Ar–H), 7.69–7.84 (m, 8H, Ar–H), 10.30 (s, 1H, –NH), 10.70 (s, 1H, –NH); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 56.13, 115.00, 118.53, 119.99, 126.85, 129.45, 129.60, 129.76, 131.30, 139.55, 141.72, 142.09, 158.52, 163.09, 165.58; HRMS (QTOF-ESI): m/z [M − H]− calcd. for C21H20N5O6S2: 502.0855; found [M − H]−: 502.0855.
N-(4-(N-(Diaminomethylene)sulfamoyl)phenyl)-4-(2,4,6-trimethyl phenylsulfonamido) benzamide (9f)
As white crystals (0.251 g, 81%), m.p. 270–271 °C (ethanol). IR (cm−1): 3355 w (–NH2), 3185 w (Ar–H), 2964 w (C–H), 1679 s (C = O), 1542 s (C = C), 1267 and 1149 s (SO2); 1H NMR (300 MHz, DMSO-d6) δ (ppm): 1.22 (s, 3H, –CH3), 2.65 (s, 6H, –CH3), 6.50–6.80 (br, 4H, 2 × –NH2), 7.04–7.09 (m, 4H, Ar–H), 7.70 (d, 2H, J = 8.83 Hz, Ar–H), 7.82 (dxd, 4H, J1 = 2.86 Hz, J2 = 11.59 Hz, Ar–H), 10.30 (s, 1H, –NH), 10.70 (s, 1H, –NH); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 20.83, 22.85, 117.33, 119.91, 126.85, 129.22, 129.65, 132.39, 133.93, 139.20, 139.50, 141.60, 142.12, 142.90, 158.51, 165.54; HRMS (QTOF-ESI): m/z [M − H]− calcd. for C23H24N5O5S2: 514.1219; found [M − H]−: 514.1214.
N-(4-(N-(Diaminomethylene)sulfamoyl)phenyl)-4-(naphthalene-2-sulfonamido)benzamide (9 g)
As brown crystals (0.260 g, 82%), m.p. 290–292 °C (ethanol). IR (cm − 1): 3419 w (–NH), 3336 w (–NH2), 1653 s (C = O), 1589 s (C = C), 1259 and 1154 s (SO2); 1H NMR (300 MHz, DMSO-d6) δ (ppm): 6.50–6.90 (br, 4H, 2 × –NH2), 7.28 (d, 2H, J = 8.74 Hz, Ar–H), 7.64–7.74 (m, 4H, Ar–H), 7.79–7.85 (m, 5H, Ar–H), 8.01 (d, 1H, J = 7.51 Hz, Ar–H), 8.10–8.24 (m, 2H, Ar–H), 8.57 (d, 1H, J = 1.35 Hz, Ar–H), 10.29 (s, 1H, –NH), 10.90 (s, 1H, –NH); 13C NMR (75 MHz, DMSO-d6) δ (ppm): 117.95, 118.96, 119.97, 120.31, 122.33, 123.20, 126.85, 127.21, 128.74, 129.76, 129.98, 130.16, 131.98, 134.80, 136.67, 139.93, 141.45, 142.08, 158.50, 165.55; HRMS (QTOF-ESI): m/z [M − H]− calcd. for C24H20N5O5S2: 522.0906; found [M − H]−: 522.0910.
Antimicrobial activity test
The synthesized sulfonamide compounds were tested for their antimicrobial (antibacterial and antifungal) activities by disc-diffusion methodCitation17,Citation18. A total of 22 microbial species including 16 bacteria, 4 yeasts and 2 molds were used as test organisms in this study. Seven of these microorganisms are clinical isolated bacteria. Legionella pneumophila, being one of these bacteria, was isolated from environmental (water).
To detect antimicrobial activity agar diffusion test with standard test organisms were performed using Staphylococcus aureus ATCC 25923, Bacillus cereus ATCC 7064, Bacillus pumilus, Staphylococcus epidermidis ATCC 12228, Enterococcus fecalis ATCC 29112, B. subtilis NRRL B-200, Methicillin-resistant Staphylococcus aureus (MRSA) and Staphylococcus spp. (clinic isolates) and Gram-negative (Escherichia coli ATCC 25922, Enterobacter aerogenes ATCC 13048, Aeromonas hydrophilia NRRL 406); Vancomycin-resistant enterococci (VRE), Moraxella catarrhalis, Salmonella spp. S. flexneri (clinic isolates), Legionella pneumohila (isolated in our lab.) environmental isolate, five yeasts Candida albicans (ATCC 10231), Rhodotorula rubra DSM 70403, Saccharomyces cerevisa (wild), S. boulardii (wild) and C. tropicalis (clinic isolate) and two mold species (Aspergillus niger ATCC 10949 and A. fumigatus NRRL 163). In the disc-diffusion method, steril paper disc (Ø 6 mm) impregnated with 10 µL of the test compounds (dissolved DMSO compound at concentrations of 100 µg) was used. Test bacteria were transferred to tubes containing 4–5 mL of Nutrient Broth. The test cultures were incubated at 37 °C until they were visibly turbid. The density of these cultures was adjusted to 0.5 Mc Farland (at 625 nm, 0.08–0.1 absorbance) with sterile saline. A suspension containing approximately 108 CFU/ml for bacteria, 107 CFU/mL for yeasts and 105 CFU/mL for mound was spread on the plates of NA. The entire surface of the NA plates was inoculated by streaking with a sterile swab dipped into adjusted suspension. Legionella pneumophila strain was isolated from water. The strain was stored at −70 °C in sterile skimmed milk until use. Subculture of frozen bacteria was grown on Buffered Charcoal Yeast Extract agar (BCYE). The density of this culture was adjusted to 0.5 Mc Farland (at 625 nm, 0.08–0.1 absorbance) with sterile saline. This suspension contains approximately 108 CFU/ml for bacteria. Then, the paper discs impregnated with the tested solutions of the sulfonamide compounds was placed on the surface of the media inoculated with the microorganisms. After pre-incubation for 1 h at 4 °C, the plates were incubated at 37 °C for 24 h for bacterial strains, 48 h for yeast and for 72 h for fungi at room temperature. L. pneumophila was incubated at 37 °C in humidified air for 72 h. Each assay in this experiment was repeated thrice. Erythromycin (15 µg/disc) for bacteria and Nystatin (100 U/disc) for yeast and fungi were used as positive controls.
Results and discussion
Chemistry
General synthesis method of novel sulfonamide derivatives containing sulfamerazine/sulfaguanidine (6a–g and 9a–g) is shown in Scheme 1.
Scheme 1. The synthesis of novel sulfonamide derivatives containing sulfamerazine and sulfaguanidine.
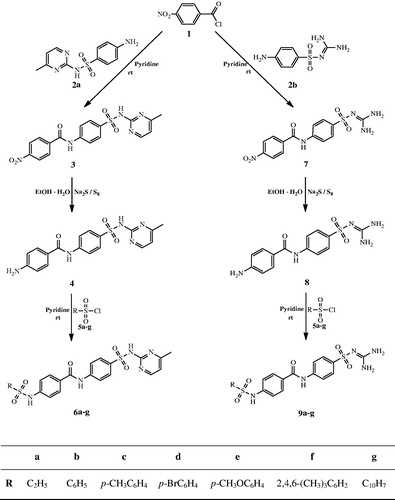
In the first step, N-(4-(N-(4-methyl pyrimidin-2-yl) sulfamoyl) phenyl)-4-nitrobenzamide (3) and N-(4-(N-(diamino methylene) sulfamoyl) phenyl)-4-nitrobenzamide (7) compounds are obtained in high yields by interacting p-nitrobenzoyl chloride (1), sulfamerazine (2a) and sulfaguanidine (2b) in the presence of pyridine at room temperature, respectively.
In the second step, synthesized starting compounds (3, 7) are reduced in the presence of sodium poly-sulfur in ethanol–water mixture (1:1). As a result of reduction, 4-amino-N-(4-(N-(4-methyl pyrimidin-2-yl) sulfamoyl) phenyl) benzamide (4) and 4-amino-N-(4-(N-(diamino methylene) sulfamoyl) phenyl) benzamide (8) compounds are obtained.
In the final step, new sulfonamide derivatives containing sulfamerazine/sulfaguanidine (6a–g and 9a–g) were obtained in pyridine at room temperature by the reaction of various sulfonyl chlorides (5a–g) with the compounds 4 and 8, which are obtained as a result of reduction reaction (Scheme 1).
Infrared (IR) spectra of all sulfonamide compounds are taken into consideration 4, 7, 8 and 9a–g of molecular vibration bands specific to –NH2 were observed in the broad peaks between 3325 and 3436 cm−1. Characteristic peaks of –NH stretching vibrations of all molecules were observed at the intervals of 3355–3511 cm−1. Aromatic C–H stretching bands are observed between 3193 and 3035 cm−1 and the aliphatic C–H stretching bands were observed between 2980 and 2871 cm−1. Stretching vibration of the carbonyl group contained in the structures of the synthesized molecule was observed at 1679–1641 cm−1 and the stretching vibrations of the C = C bonds in the aromatic structure were observed between 1606 and 1504 cm−1. Symmetric and asymmetric vibration bands of SO2, existing in the structure of the novel sulfonamide compounds, were determined to give severe vibrations in the range of 1193–1136 cm−1.
The 1H-NMR spectra of compounds 4, 6a–g, 9c and 9f showed singlet peaks that belong to protons of the methyl groups between 2.22 and 2.85 ppm. When the spectrums of compounds 6a and 9a are taken into consideration, the ethyl group –CH3 protons were observed in the triplet peaks at 1.20 ppm, –CH2 protons were observed in quartet peaks at 3.20 ppm. Protons of –OCH3 group 6e and 9e compounds were observed in singlet peak at 3.80 ppm. NH2 protons of compound 4 were observed as broad peak at 5.90 ppm. Aromatic CH protons of all molecules were observed in the range of 6.60–8.54 ppm. –NH protons of carboxamide and sulfonamide groups were observed between 10 and 12 ppm for all compounds (4, 6a–g, 9a–g).
The signals observed in 13C-NMR (APT) spectrums of all the novel sulfonamide molecules are determined to be in line with the recommended molecule structures. Also, when their high resolution mass spectra (HRMS) are examined, the observed molecule ion peaks are in compliance with the recommended structures.
Antimicrobial activity
The results of the antimicrobial activity of the 4, 6a–g and 9a–g by the agar diffusion method is presented in . The comparison of the obtained result with the antibacterial and antifungal agents used in the study is presented in as well. Stock solutions of the sulfonamide compounds were prepared by dissolving with DMSO and loaded on disc (100 µg). All sulfonamide compounds showed antimicrobial effective to against at least one microorganism. Compound 9d showed highest antimicrobial activity against tested Gram-positive bacteria B. subtilis (14 mm) at 100 µg/disc test concentration. Compounds 9e and 6d good showed antimicrobial activity against E. aerogenes (13 mm) and B. cereus (12 mm), respectively. 6e and 9a showed antibacterial activity against B. cereus and E. aerogenes with diameters of zone 10 mm at 100 µg/disc concentration. The B. cereus in Gram-positive spore-forming bacteria can cause gastrointestinal diseases and severe eye infections in humansCitation19. Only 9d showed weak antibacterial activity against L. pneumophila. Legionella are Gram-negative bacteria that are ubiquitous in both natural aquatic and moist soil environment and in artificial aquatic habitats. Human infection with Legionella has two distinct forms: Legionnaires’ disease, which is a severe form of infection which includes pneumonia; and Pontiac fever, a milder flu like illness without pneumoniaCitation20.
Table 1. Antimicrobial activities of sulfonamide compounds (4, 6a–g and 9a–g) (discs Ø 6 mm).
9d and 9f showed antifungal activity against C. tropicalis (8 mm) and S. boulardii (8 mm) at 100 µg/disc concentration. Candida tropicalis is a diploid ascomycetes yeast commonly found on the skin and in digestive tracts of healthy human hosts worldwide. Infections caused by C. tropicalis are reported in 4–24% of patients with candidemiaCitation21,Citation22. In general, sulfonamides (4, 6a–g and 9a–g) show the low antifungal activity against the yeast and mold. These compounds show no high activity against the tested microorganisms. It was thought that the reason for this event was the inability of sulfonamide molecules to pass through the bacterial cell membrane due to their huge molecular structuresCitation23. IR, 1H-NMR, 13C-NMR and HRMS spectrums of all compounds are given in Supplementary information.
Conclusion
Novel sulfonamide compounds containing sulfamerazine/sulfaguanidine were synthesized and their structures were characterized. In particular, 6d, 6e, 9a, 9d and 9e showed antimicrobial activity against tested bacteria.
Supplementary material available online Supplementary material
IENZ_1079183_Supplemental_file.pdf
Download PDF (5.1 MB)Declaration of interest
This research was financed by Dumlupınar University Research Fund (Grant No. 2012-38).
References
- Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors. Curr Med Chem 2001;1:61–97
- Richard HJ. The mode of action of sulfonamıdes. Bacteriol Rev 1943;7:175–262
- Kaya M, Demir E, Bekci H. Synthesis, characterization and antimicrobial activity of novel xanthene sulfonamide and carboxamide derivatives. J Enzyme Inhib Med Chem 2013;28:885–93
- Carta F, Supuran CT. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013). Expert Opin Ther Pat 2013;23:681–91
- Arechederra RL, Waheed A, Sly WS, et al. Effect of sulfonamides as carbonic anhydrase VA and VB inhibitors on mitochondrial metabolic energy conversion. Bioorg Med Chem 2013;21:1544–8
- Scozzafava A, Supuran CT, Carta F. Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Expert Opin Ther Pat 2013;23:725–35
- Monti SM, Supuran CT, Simone GD. Anticancer carbonic anhydrase inhibitors: a patent review (2008–2013). Expert Opin Ther Pat 2013;23:737–49
- Zolnowska B, Slawinski J, Pogorzelska A, et al. Carbonic anhydrase inhibitors. Synthesis, and molecular structure of novel series N-substituted N′-(2-arylmethylthio-4-chloro-5-methylbenzenesulfonyl)guanidines and their inhibition of human cytosolic isozymes I and II and the transmembrane tumor-associated isozymes IX and XII. Eur J Med Chem 2014;71:135–47
- Slawinski J, Brzozowski Z, Zolnowska B, et al. Synthesis of a new series of N4-substituted 4-(2-aminoethyl)benzenesulfonamides and their inhibitory effect on human carbonic anhydrase cytosolic isozymes I and II and transmembrane tumor-associated isozymes IX and XII. Eur J Med Chem 2014;84:59–67
- Slawinski J, Pogorzelska A, Zolnowska B, et al. Carbonic anhydrase inhibitors. Synthesis of a novel series of 5-substituted 2,4-dichlorobenzenesulfonamides and their inhibition of human cytosolic isozymes I and II and the transmembrane tumor-associated isozymes IX and XII. Eur J Med Chem 2014;82:47–55
- Ghorab MM, Ceruso M, Alsaid MS, et al. Novel sulfonamides bearing pyrrole and pyrrolopyrimidine moieties as carbonic anhydrase inhibitors: synthesis, cytotoxic activity and molecular modeling. Eur J Med Chem 2014;87:186–96
- Kaya M, Basar E, Çakir E, et al. Synthesis and characterization of novel dioxoacridine sulfonamide derivatives as new carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:509–14
- Ulus R, Yeşildağ İ, Tanç M, et al. Synthesis and characterization of novel dioxoacridine sulfonamide derivatives as new carbonic anhydrase inhibitors. Bioorg Med Chem 2013;21:5799–805
- Yeşildağ İ, Ulus R, Basar E, et al. Facile, highly efficient, and clean one-pot synthesis of acridine sulfonamide derivatives at room temperature and their inhibition of human carbonic anhydrase isoenzymes. Monatsh Chem 2014;145:1027–34
- Sayın F. Hayvanlarda coccidiosisin tedavisi ve bu hususta bilinmesi gereken bazı özellikler. Bornova Vet Araşt Ens Derg 1968;9:118–35
- Mimioğlu M, Göksu K, Sayın F. Veteriner ve Tıbbi Protozcoloji II. Ankara: A.Ü. Basımevi; 1969
- National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility test. 4th ed. Vol. 10. Approved Standard NCCLS Document, Villanova; 1990:9–15
- National Committee for Clinical Laboratory Standard. 2nd ed, vol. 10. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard NCCLS Document, Villanova; 1999:12–15
- Ramarao N, Sanchis V. The pore-forming haemolysins of Bacillus cereus. Toxins 2013;5:1119–39
- Anbumani SS, Gururajkumar A, Chaudhury A. Isolation of Legionella pneumophila from clinical & environmental sources in a tertiary care hospital. Indian J Med Res 2010;131:761–4
- Odds FC. Ecology of Candida and epidemiology of candidosis. Candida and candidosis: a review and bibliography. 2nd ed. London: Bailliere Tindall; 1988:68–82
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007;20:133–63
- Özkan ŞÇ, Yilmaz A, Arslan E, et al. Novel copper (II) complexes of p-tert-butylcalix[4]arenediamide derivatives: synthesis, antimicrobial and DNA cleavage activities. Supramol Chem 2015;27:255–67
