Abstract
Three salts of 5-amino-2-sulfonamide-1,3,4-thiadiazole (Hats) were prepared and characterized by physico-chemical methods. The p-toluensulfonate, the methylsulfonate, and the chlorhydrate monohydrate salts of Hats were evaluated as carbonic anhydrase (CA, EC 4.2.1.1) inhibitors (CAIs) and as anticonvulsants and diuretics, since many CAIs are clinically used as pharmacological agents. The three Hats salts exhibited diuretic and anticonvulsant activities with little neurotoxicity. The human (h) isoforms hCA I, II, IV, VII, IX, and XII were inhibited in their micromolar range by these salts, whereas pathogenic beta and gamma CAs showed similar, weak inhibitory profiles.
Introduction
Sulfonamide inhibitors of the metalloenzyme carbonic anhydrase (CA, EC 4.2.1.1), such as acetazolamide, have been used clinically as diuretics, antiglaucoma, or anticonvulsant agents for a long periodCitation1–9, whereas more recent drug design studies have evidenced other CA inhibitors (CAIs) belonging to the sulfonamide/sulfamate/sulfamide classes as molecules of interest for developing novel therapies for obesityCitation10,Citation11 and cancerCitation12–15, based on the selective inhibition of CA isozymes involved in such pathologies, among which 15 such isoforms described so far in humansCitation1,Citation16.
Epilepsy is one of the most common serious neurologicaldisorders characterized by recurrent seizures. Since several decades, acetazolamide is used as an anticonvulsant agent in the treatment ofepilepsyCitation1,Citation10,Citation11. Despite the development of a rapid tolerance consisting in diminished therapeutic efficacy after the initial response of the patients, acetazolamide is still used in combination therapy with other antiepileptic drugs or inrefractory epilepsiesCitation1.
We have obtained and determined the crystal structures of sulfonamides incorporating the 1,3,4-thiadiazole ringCitation17–23. The results obtained by our group with these sulfonamides indicated a notable increase in the CA inhibitory properties of metal complexes of sulfonamides such as acetazolamide or its deacetylated precursor, 5-amino-1,3,4-thiadiazole-2-sulfonamide (Hats)Citation23. Furthermore, continuing our studies on unsubstituted heterocyclic sulfonamides and their metal complexesCitation17–23, we started to study the role of new salts of Hats with regard to their biological activity. In this work, we report new salts of Hats with p-toluenesulfonic acid, methylsulfonic acid, and the hydrochloride monohydrate salts. We evaluated the CA inhibition and anticonvulsant/diuretic activities of these three sulfonamide salts.
Experimental
Salt formation
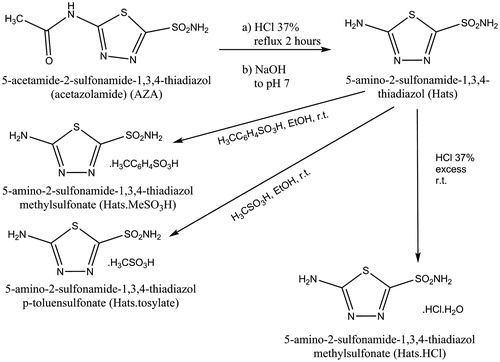
The synthesis of Hats was performed in two steps (). In the first step, the deacetylation of acetazolamide (AZA) to give Hats was performed following the procedure reported in the literatureCitation17. This product was characterized by its FTIR spectrum resulting identical to that reported by Pedregosa et al.Citation17,Citation18. In the second step, 100 mg of Hats (0.55 mmol) were suspended at room temperature in 50 mL of absolute ethanol (99.5%, Merck, Darmstadt, Germany) with permanent stirring. Then, 1.2 g of p-toluenesulfonic acid (Aldrich, Seelze, Germany; 6.31 mmol); 0.45 mL of methylsulfonic acid (Aldrich, Seelze, Germany; 6.7 mmol); 0.45 mL of HCl (37%, Merck, Darmstadt, Germany; 5.5 mmol) were added maintaining the stirring during 2 h at room temperature. White powders were obtained for each salt, 5-amino-1,3,4-thiadiazole-2-sulfonamide p-toluenesulfonate (Hats.tosylate), 5-amino-1,3,4-thiadiazole-2-sulfonamide methylsulfonate (Hats.MeSO3H), and 5-amino-1,3,4-thiadiazole-2-sulfonamide hydrochloride monohydrate (Hats.HCl). Colorless prismatic single crystals of Hats.tosylate, suitable for DRX studies, were obtained after 2 weeks at 15 °C avoiding light exposure.
Methods
X-ray data for Hats.tosylate were collected with an Enraf–Nonius FR590 CCD area detector (Labx, Schwerzenbach, Switzerland) using CollectCitation24 and HKL Denzo–ScalepackCitation25 software with graphite monochromated Mo-Kα (k = 0.71073 Å) at 293Citation2 K. The structure was solved by direct methods using SHELXS-97Citation26 and all the non-hydrogen atoms were refined anisotropically by full-matrix least-squares on F2 using SHELXL-97Citation26. The H atoms attached to N were found in a difference Fourier map, further idealized (N–H:0.86 Å), and finally allowed to ride. All calculations, including , were performed using WinGXCitation27 and Ortep-3 for windowsCitation28. The crystal structure of Hats tosylate has been deposited in the CSD with reference number CCDC 730598. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. The compound crystallizes forming colorless triclinic prisms with the space group P1.
Elemental analysis (C, H, N, and S) was carried out with a Carlo Erba EA 1108 microanalyser (Triad Scientific, Manasquan, NJ) belonging to the Instituto de QuímicaFísica de Materiales, MedioAmbiente y Energía (INQUIMAE), Universidad de Buenos Aires, Argentine; sulfanilamide was used as the standard.
Fourier transformed infrared (FT-IR) spectra were recorded on a Nicolet Protégé 460 spectrometer (Conquer Scientific, San Diego, CA) provided with a CsI beam splitter in the 4000–250 cm−1 range with 32 scans and spectral resolution of 4 cm−1, using the KBr pellet technique.
Thermogravimetric (TGA) and differential thermal analysis (DTA) curves were obtained with a Shimadzu TGA-51 Thermal Analyzer (Shimadzu Corporation, Kyoto, Japan) and DTA-50 Thermal Analyzer, using platinum pans, flowing air at 50 ml min−1 and at a heating rate of 10 °C min−1 from room temperature to 1000 °C.
To confirm these, melts or crystalline phase changes were used a Leitz Westlar heating microscope at this stage. The equipment used for mass spectra was a VG AUTOSPEC. The spectral data were obtained through of Electronic Impact (EI) at 70 eV and Fast Atom Bombardment (FAB). The values listed for each of the compounds are expressed in unit of m/z.
The reaction for obtaining Hats.tosylate occurred with a yield of 95%. Elemental analysis for : Found (calculated): C: 31.21% (30.95%); H: 2.29% (2.58%); N: 15.78% (16.05%); S: 28.53% (27.50%).
All animals used in experiments were handled in accordance with international standard for the use and care of laboratory animals. The corresponding protocols were approved by the Universidad Nacional de San Luis, following the provisions of the ANMATCitation29 6344/96.
The diuretic effect was evaluated using male and female adult Wistar rats weighing 220 g. The administration of solutions was performed by oral gavage in a volume of 5 mL/100 g body weight following the method proposed by Lipschitz et al.Citation30. All animals were deprived of water 12 h before the experiment having free access to food. In addition to the urine volume, sodium excretion and urinary potassium were recordedCitation31 and the concentrations of these ions were quantified in a flame spectrometer Metrolab model 315 (Shimadzu Corporation, Kyoto, Japan).
The anticonvulsant activity of drugs was tested in mice Rockland (weighing 25–30 g, administering the sulfonamides intraperitoneally), against seizures induced by intraperitoneal administration of nikethamide or picrotoxin as control drugs. Through the “rota-rod test”Citation32, the neurological deficit was evaluated, which is evidenced by the ability of the mouse to stay in balance (on a cylinder rotating at 15 rpm) in three trials of 1 min each. Animals were distributed into three lots: negative control (saline), positive control (phenobarbital), and experimental compounds (Hats.tosylate, Hats.MeSO3H, and Hats.HCl). The compounds were administered intraperitoneally. The mice are placed on the rota-rod bar and put into operation the apparatus. Animals that do not fall off the bar in 5 min will be chosen for the test. Thirty minutes after intraperitoneal administration, the mice are placed on the moving bar and the number of falls in 5 min was registered. Then the analysis of the recorded data was achieved.
The “chimney” testCitation33 is another in vivo test used to evaluate the mouse motor coordination impairments. It is based on the time that the rodent needs to traverse a tube of glass or clear acrylic 20 cm long in a span of 10 s. In this assay, the degree of muscular relaxation in mice is evaluated. Animals were distributed into three lots: negative control (saline), positive control (phenobarbital), and experimental (sulfonamides). The rota-rod test was used to evaluate the activity of drugs that interfere with motor coordination (Test of Dunham, 1957), while the “chimney” test was used to estimate locomotor activityCitation33. Neurotoxicity experiments were carried out with adults Rockland albino mice weighing 25–30 g. The doses of sulfonamides studied were 20, 50, and 90 mg/kg of mouse.
An Applied Photophysics stopped-flow instrument was used for assaying the CA catalyzed CO2 hydration activityCitation34. Phenol red (at 0.2 mM) was used as an indicator, working at the absorbance maximum of 557 nm with 10 mM Hepes (pH 7.4) or TRIS (pH 8.3) as buffers and 0.1 M NaClO4 (for maintaining constant ionic strength), at 20 °C, following the CA-catalyzed CO2 hydration reaction for a period of 10–100 s (the uncatalyzed reaction needs around 60–100 s in the assay conditions, whereas the catalyzed ones are of around 6–10 s), as described earlierCitation35–43. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Enzyme concentrations in the assay system were in the range of 10 nM for all the enzymes considered in the present study, which have been obtained as recombinant proteins, in-house, as reported earlierCitation43–48.
Results and discussion
Characterization
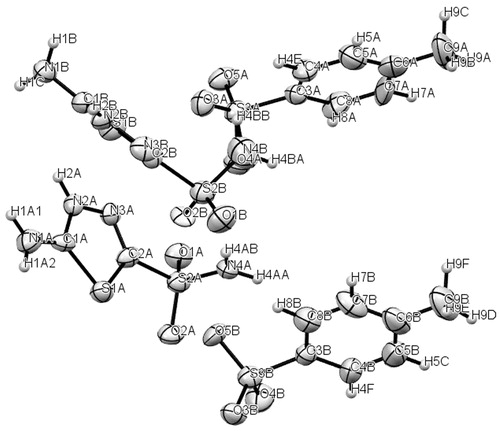
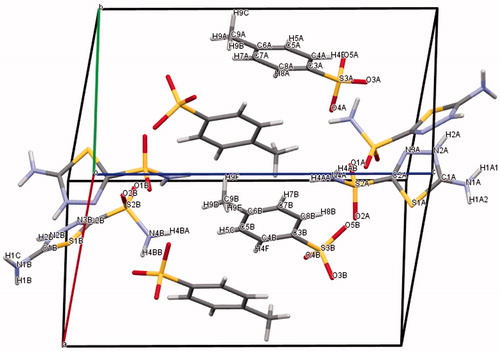
In a previous work, we have reported the crystal structures and spectroscopic characterization of Hats.MeSO3H and Hats.HClCitation22,Citation23. Crystal and experimental data for Hats.tosylate are listed in . shows ORTEP diagram of the studied compound, and shows the molecular packing. Hats.tosylate consists of two H2ats+ and two p-toluenesulfonate ions in the asymmetric unit. The anion p-toluensulfonate and cation H2ats+ are distributed in alternative parallel layers along with the b-axis and stabilized by hydrogen bonds between the 2-sulfonamido group and sulfonate ion, 5-amino and protonated N2 (azole) group and sulfonate group (). The π-stacking between phenyl groups are reinforced by interactions between these groups and terminal methyl moieties, which stabilize the crystal lattice.
Table 1. Crystal data of Hats.tosylate.
Table 2. H-bonds interactions in Hats.tosylate crystal structure.
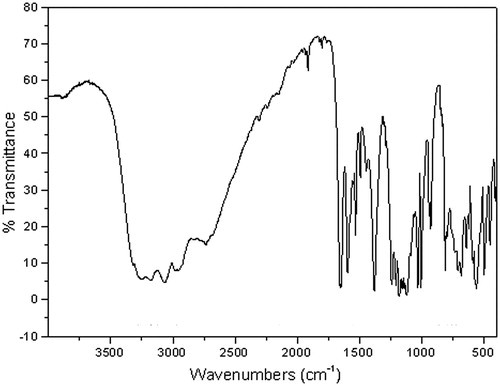
The complete assignment of the vibrational modes of Hats has been studied by our group23. Based on these studies and using appropriate literatureCitation35, it has been possible to assign the vibrational modes of Hats salts. In this work, the assignment of vibrational modes of Hats.tosylate has been done by comparison with the other two above mentioned salts and Hats. shows the FTIR spectra of Hats.tosylate and proposes assignation of vibrational modes.
Table 3. Proposed assignment of the FTIR spectrum and a comparison of selected.
Analyzing the vibrational modes mentioned above, it can be seen that NH2 stretching appears at lower frequencies and NH2 deformation at higher frequencies in the three salts with respect to Hats. These facts are consistent with the respective crystal structures, where these groups are interacting with the hydrogen bridge formation, stabilizing the crystal structure, weakening the NH bond. Four modes corresponding to NH stretching are observed. The values observed at 3319 and 3246 cm−1 correspond to the asymmetric and symmetric stretching of the –NH2 group attached to the carbon of the thiadiazole ring, at higher frequencies in Hats.tosylate with respect to Hats.MeSO3H and Hats.HCl, and these three frequencies are less than Hats (). The other two values observed at 3177 and 3067 cm−1 are very similar to those observed in Hats, Hats.MeSO3H and Hats.HCl. The NH deformations are observed at higher frequencies in the salts regarding Hats. These facts confirm the implication of the amino groups in the formation of hydrogen bonds to stabilize the crystal structure. It is also interesting to note that the corresponding modes νas SO2 groups and CN appear at higher frequencies than Hats, suggesting that these links are strengthened with respect to Hats (). This fact indicates that the S–O and C–N bonds are strengthened. The stretching N–N appears at similar frequencies in all cases.
The result of mass spectra shows that the molecular ions are not visible due to its relative abundance which is lower than 5% and mainly due to the ionic nature of the salts. In the three salts, the fragment of m/z 180 (compounds Hats.tosylate and Hats.MeSO3H) and m/z 181 (V) has a 100% relative abundance. This fragment corresponds to Hats and protonated Hats. In addition, compound Hats.tosylate appears to be a fragment of m/z 172 (55% relative abundance) due to p-toluenesulfonic acid. The Hats.MeSO3H shows a fragment at m/z 96 (40% relative abundance) corresponding to methylsulfonic acid. The mass spectra of three salts are presented in the Supplementary material.
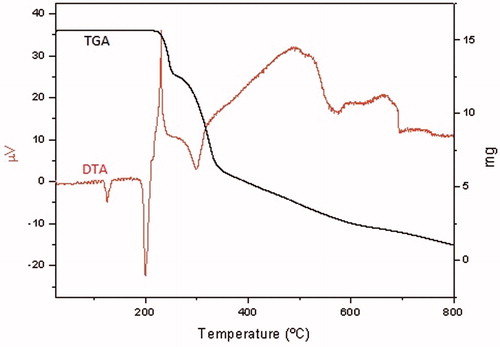
A very rich thermal behavior was observed in the studied compounds. Hats.tosylate is stable up to 120 °C. Starting at 120 °C associated to an endothermic process, a change of the crystalline phase occurred. This fact was observed in a heating microscope stage. Compound Hats.MeSO3H too changed its crystalline phase below 100 °C. Compound Hats.HCl decomposed at 120 °C (onset 118 °C) with loss of water of crystallization.
Immediately before decomposition, Hats.tosylate and Hats.MeSO3H showed a melting point near 200 °C [Hats.tosylate: 212 °C (onset 203 °C); Hats.MeSO3H 200 °C (onset 196 °C)]. Hats.HCl anhydrous melted at 194 °C (on set 187 °C). Subsequently the three salts decomposed in many endothermic and exothermic steps until complete calcinations occur at 700 °C (Hats.tosylate), 925 °C (Hats.MeSO3H), and 600 °C (Hats.HCl). shows thermal diagrams (TGA-DTA) of Hats.tosylate. Thermal diagrams of compounds Hats.MeSO3H and Hats.HCl are presented in the Supplementary material.
Biological assays
Diuretic effect
The diuretic effect is attributed to the ability of these salts to inhibit the renal CACitation1,Citation4. This effect is demonstrated by the alkalinity of the urine excreted. However, it has been suggested that the inhibition of sodium reabsorption in different parts of the nephron also contribute to this mechanism of action. To verify this effect, six batches were prepared with eight animals each. Batch 1 (negative control) received saline physiological solution; batch 2 (positive control) received acetazolamide (20 mg/kg); batch 3 (reference) received furosemide (Sigma Ch. Co., St. Louis, MO) (10 mg/kg); batch 4 received compound III (70 mg/kg); batch 5 received compound IV (70 mg/kg), and batch 6 received compound V (70 mg/kg). Urine fractions were collected each hour for a period of 5 h after the administration of each drug. The urinary excretion volume was calculated using the following relationship:
where UVE is the urinary volume excreted.
The recorded values were expressed as means ± SEM. It applies statistical analysis one-way ANOVA and subsequent comparisons used the Turkey test. Differences below a probability level of 5% (p < 0.05) were considered statistically significant. To carry out this study, we used the program GraphPad Prim (GraphPad Software Inc., La Jolla, CA)Citation40.
Diuresis has two components: an increment of the urine volume (water excretion) and a net loss of solutes (electrolytes)Citation31. shows comparative diuretic effect of Hats salts with respect to acetazolamide and furosemide. Hats.MeSO3H possesses a similar effect which Hats.HCl as to the excretion of electrolytes in the urine. However, the removal of liquid is important in the first 60 min of the test, subsequently removing liquid is similar to the batch control. Hats.MeSO3H exhibited a significant diuretic effect during the test. Diuresis was significant for the first 75 min of the test with respect to the control. Hats.tosylate also presented a diuretic effect, which was maintained throughout the test. Spectrophotometric data of urinary ions concentration yielded the results as exhibited in .
Figure 6. Diuresis of Hats salts (mL of urine versus time). From left to right (each set of columns): control, Aza, furosemide, p-toluensulfonate Hats, Hats methylsulfonate, Hats hydrochloride monohydrate.
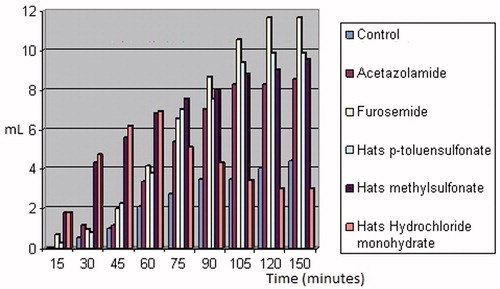
Table 4. Biological assays.
Anticonvulsant activity
In a first assay, physiological saline solution (negative control) was administered intraperitoneally to eight mice per group, whereas compounds Hats.tosylate, Hats.MeSO3H, Hats.HCl, and AZA at doses of 20, 50, and 90 mg/kg of live animal, in volumes of 0.1 mL per 30 g mouse, were administered to other animals. After 30 min of administration of the test compounds, nikethamide was injected by the same route at 0.1 mL (dose 20 mg/kg). It was observed that a dose of 20 mg/kg of test compound is not fully effective for convulsions protection; convulsions were observed in one or two mice per group. However, at higher doses (50 and 90 mg/kg of test compound), the convulsions protection was effective, since none of the mice suffered convulsions.
In a second assay, a dose of compound of 90 mg/100 g of animal weigh was given in a volume of 0.1 mL per 10 g mouse, intraperitoneally (using eight mice per group). After 30 min of administration, test compounds were injected by the same route of picrotoxine 0.1 mL per 30 g mouse (20 µg/100 g body weight).
In the control batch, we observed that in animals treated with picrotoxine the convulsions appeared after 4 min post-administration. The animals injected with compounds Hats.tosylate and Hats.MeSO3H were protected from convulsions during 20–25 min post-administration, whereas after longer periods of time, clonic convulsions appeared.
The animals injected with compound Hats.HCl were protected from convulsions during the 20 min post-administration. Tonic convulsions appear thereafter, but the animals survived. Acetazolamide showed a weaker protective effect than Hats salts since convulsions occurred within 15 min post-administration ().
Neurotoxicity (rota-rod test)
The rota-rod assay has been used to examine in vivo neurotoxicity of the sulfonamide salts. Five groups of eight animals each were used. For the negative control group (saline solution), one fall (mean) had been recorded during the first 15 min. In the positive control group, injected with phenobarbital (10 mg/kg), five falls were observed. In the third group previously injected with compound Hats.tosylate (90 mg/kg), two falls were observed during the first 15 min. In the fourth group, injected with compound Hats.MeSO3H (90 mg/kg), two falls were observed during the first 15 min. Finally, in the fifth group, injected with compound Hats.HCl (90 mg/kg), two falls during the first 15 min were observed. The results showed no adverse effects on motor coordination with the tested drugs compared with control animals (p > 0.05, one-way ANOVA and subsequent Turkey test) at the doses here assayed. shows these results.
Effect of Hats salts on locomotor activity of mice. Chimney test
Five groups of eight animals each were employed. Animals used in the first group (saline solution, negative control) showed a delay of 6 s to pass through the glass tube. The group injected with phenobarbital (positive control) did not perform the test within the considered time. The group intraperitoneally injected with compound Hats.tosylate (0.1 mL/15 g mouse) led to a delay of 8 s. The group intraperitoneally injected with compound Hats.MeSO3H (0.1 mL/15 g mouse) led to a delay of 6 s. The group intraperitoneally injected the compound Hats.HCl (0.1 mL/15 g mouse) led to a delay of 7 s. The results show that there is no sedation or ataxia drug tested, compared with those animals of the control batch (p > 0.05, one-way ANOVA and subsequent Turkey test).
CA inhibition
Inhibition data with Hats salts against various human (h) CAs, hCA I, II, IV, VII, IX, and XII, and pathogenic beta and gamma CAs have been obtained. AZA, a well-known CAI, was used as a standard drug for comparison (). As in other CAs inhibition assays with sulfonamides and their metal complexes, phenols and thiophenols, the enzymes were incubated for 10–15 min with test compounds for allowing the formation of the enzyme-inhibitor adductCitation41–45,Citation49,Citation50. Working under similar conditions, Hats salts inhibited both hCAs and micro-organisms CAs in the micromolar concentration range, although they exhibited a weaker inhibitory power compared with AZA (). These results show that although the solubility in water is improved for Hats by salt formation, the interaction of the sulfonamide with the active site of the enzyme is weaker (than for acetazolamide) probably due to the fact that the acetamido moiety present in the drug allows the stabilization of the adduct through additional interactions in which Hats cannot participate. This is consistent with the X-ray crystal structures, in which the deprotonated sulfonamido group appears bound to the Zn(II) ion from the enzyme active siteCitation7,Citation44,Citation45.
Table 5. Inhibition of human (h) isoforms hCA I – XII with hats salts and AZA as standard drug.
In previous studies, it was found that by inhibiting CAs from bacterial or other pathogens, a growth inhibition of the studied micro-organisms occurredCitation37,Citation45,Citation46. shows the values of enzyme inhibition for CAs in Porphyromonas gingivalis (responsible of periodontal pathogenicity) and Cryptococcus neoformans (responsible of serious, systemic fungal diseases)Citation1,Citation7,Citation8. According to these values, the best results were obtained against PgiCAgamma (). Thus, the Hats salts can be considered as interesting candidates for possible application in designing anti-infective agents with a novel mechanism of action.
Table 6. Inhibition of PgiCA from porphyromona sgingivalis; and Can2, from cryptococcus neoformans with hats salts investigated in this paper.
Conclusions
In this work, we obtained new salts of Hats: 5-amino-1,3,4-thiadiazole-2-sulfonamide. The crystal structure was determined for one of them, whereas spectroscopic, spectrometric, and thermal analysis showed that the new salts differentiate with respect to similar salts previously obtained in our group. The results of preclinical assays suggest that the effect of the studied sulfonamides on electrolytes excretion is similar with the thiadiazide diuretics due to the significant removal of Na+ and K+ ions. Hats and AZA showed the same anticonvulsant protection effects against intraperitoneal administration of niketamide and picrotoxin. The sulfonamide salts were most effective as anticonvulsants in the niketamide than in the picrotoxin-induced convulsions assay. These facts suggest that they are effective to protect against mild convulsions. The tested compounds showed no effects of neurotoxicity in the full range of effective doses in the study by the rota-rod test and chimney test. Hats salts inhibited human, bacterial, and fungal CAs. These facts led us to study more deeply these compounds as potential antiinfective agents. This work stimulates further study for their possible pharmacological applications.
Supplementary material available online
Supplementary Figure S1–S7
IENZ_1096270_Supp.pdf
Download PDF (242.4 KB)Acknowledgements
The authors thank Dr. C. Ardanaz (UNSL) for mass spectra facilities. They also thank S.C.S.I.E. (X-ray section) of University of Valencia for provision of the X-ray crystallographic facilities.
Declaration of interest
This work is supported by CONICET-PIP 6246 and UNSL. J. C. P. and D. R. V. are members of CONICET.
References
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77
- Supuran CT, Scozzafava A, Conway J, eds. Carbonic anhydrase—its inhibitors and activators. Boca Raton, New York, London: CRC Press; 2004:1–363
- Temperini C, Cecchi A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Sulfonamide diuretics revisited-old leads for new applications? Org Biomol Chem 2008;6:2499–506
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229
- Supuran CT, Scozzafava A, Casini A. Development of sulfonamide carbonic anhydrase inhibitors. In: Supuran CT, Scozzafava A, Conway J, eds. Carbonic anhydrase—its inhibitors and activators. Boca Raton: CRC Press; 2004:67–147
- Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72
- Supuran CT. Carbonic anhydrases: from biomedical applications of the inhibitors and activators to biotechnological use for CO(2) capture. J Enzyme Inhib Med Chem 2013;28:229–30
- Nishimori I, Onishi S, Takeuchi H, Supuran CT. The alpha and beta classes carbonic anhydrases from Helicobacter pylori as novel drug targets. Curr Pharm Des 2008;14:622–30
- Casini A, Antel J, Abbate F, et al. Carbonic anhydrase inhibitors: SAR and X-ray crystallographic study for the interaction of sugar sulfamates/sulfamides with isozymes I, II and IV. Bioorg Med Chem Lett 2003;13:841–5
- De Simone G, Di Fiore A, Menchise V, et al. Carbonic anhydrase inhibitors. Zonisamide is an effective inhibitor of the cytosolic isozyme II and mitochondrial isozyme V: solution and X-ray crystallographic studies. Bioorg Med Chem Lett 2005;15:2315–20
- Winum JY, Dogne JM, Casini A, et al. Carbonic anhydrase inhibitors: synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, IX, and XII with N-hydroxysulfamides – a new zinc-binding function in the design of inhibitors. J Med Chem 2005;48:2121–5
- Scozzafava A, Passaponti M, Supuran CT, Gulcin I. Carbonic anhydrase inhibitors: guaiacol and catechol derivatives effectively inhibit certain human carbonic anhydrase isoenzymes (hCA I, II, IX and XII). J Enzyme Inhib Med Chem 2015;30:586–91
- De Simone G, Alterio V, Supuran CT. Exploiting the hydrophobic and hydrophilic binding sites for designing carbonic anhydrase inhibitors. Expert Opin Drug Discov 2013;8:793–810
- Cecchi A, Hulikova A, Pastorek J, et al. Carbonic anhydrase inhibitors. Design of fluorescent sulfonamides as probes of tumor-associated carbonic anhydrase IX that inhibit isozyme IX-mediated acidification of hypoxic tumors. J Med Chem 2005;48:4834–41
- De Simone G, Alterio V, Supuran CT. Exploiting the hydrophobic and hydrophilic binding sites for designing carbonic anhydrase inhibitors. Expert Opin Drug Discov 2013;8:793–810
- Pedregosa JC, Alzuet G, Borrás J, et al. Structure of 5-amino-1,3,4-thiadiazole-2-sulfonamide, an inhibitor of the enzyme carbonic anhydrase. Acta Cryst 1993;C49:630–3
- Pedregosa JC, Casanova J, Alzuet G, et al. Metal complexes of 5-tertbutyloxycarbonylamido-1,3,4-thiadiazole-2-sulfonamide (B-H2ats), a carbonic anhydrase inhibitor. Synthesis and characterization of the copper(II) complex. Crystal structures of B-H2ats and the [Cu(B-ats)(NH3)2]2 dimer complex. Inorg Chim Acta 1995;232:117–24
- Camí GE, Server-Carrió J, Fustero S, Pedregosa JC. A novel heterocyclic sulfonamide: N-benzyl-5-[N-benzyl-N-(tert-butyloxycarbonyl)amino]-N-(tert-butyloxycarbonyl)-1,3,4-thiadiazole-2-sulfonamide. Acta Crystallogr C 2000;56:E209–10
- Camí GE, Ramírezde Arellano MC, Supuran CT, et al. Optimal synthetic route and structural characterization of new 1,3,4-thiadiazole-5-N-substituted-sulfonamides. J Arg Chem Soc 2006;94:5–17
- Chufán EE, Pedregosa JC, Baldini OL, Bruno-Blanch L. Anticonvulsant activity of analogues of acetazolamide. Il Farmaco 1999;54:838–41
- Diaz JRA, Camí GE, Liu-González M, et al. Two novel potential anticonvulsant salts of 5-amino-2-sulfonamide-1,3,4-thiadiazole. J Chem Crystallogr 2011;41:1114–19
- Camí GE, Chufán EE, Pedregosa JC, Varetti EL. Infrared and Raman spectra of 5-amino-1,3,4-thiadiazole-2-sulfonamide (Hats). Experimental data and quantum chemistry calculations. J Mol Struct 2001;570:119–27
- Nonius (1997–2000). COLLECT. Delft, The Netherlands: Nonius BV
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. In: Carter CW Jr, Sweet RM, eds. DENZO and SCALEPACK. Methods in enzymology, vol 276, Macromolecular crystallography, PartA. San Diego: Academic Press; 1997:307–26
- Sheldrick GM. SHELXL97 and SHELXS97 programs for crystal structure refinement and solution. University of Göttingen, Germany. Acta Cryst 2008;A64:112–22
- Farrugia LJ. Win GX. J Appl Cryst 1999;32:837–8
- Farrugia LJ. ORTEP-3 for Windows. J Appl Cryst 1997;30:565–6
- Administración Nacionalde Medicamentos,A limentosy Tecnología (ANMAT) Disposición 6344/96. Reglamentaciónpara Bioteriosdelaboratorioselaboradoresdeespecialidadesmedicinalesy/odeanálisisdeterceros
- Lipschitz WL, Hadidian Z, Kerpesar A. Bioassay of diuretics. J Pharmacol Exp Ther 1943;79:97–110
- Jackson EK. Drugs affecting renal and cardiovascular function. In: Hardman JC, Gilman AG, Limbird LE, eds. Goodman and Gilman’s the pharmacological basis of therapeutics, 10th ed. New York: Pergamon Press; 1996:685–713
- Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc 1957;46:208–10
- Boissier JR, Tardy J, Diverres JC. Une novelle methode simple pour explorer l′action �tranquillisante�: le test de la cheminee. Med Exp 1960;3:81–4
- Khalifah RG. The carbon dioxide hydration activity of Carbonic Anhidrase. I. Stop flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73
- Maresca A, Temperini C, Vu H, et al. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. J Am Chem Soc 2009;131:3057–62
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–74
- Alafeefy AM, Ceruso M, Al-Jaber NA, et al. A new class of quinazoline-sulfonamides acting as efficient inhibitors against the α-carbonic anhydrase from Trypanosoma cruzi. J Enzyme Inhib Med Chem 2015;30:581–5
- Carta F, Di Cesare Mannelli L, Pinard M, et al. A class of sulfonamide carbonic anhydrase inhibitors with neuropathic pain modulating effects. Bioorg Med Chem 2015;23:1828–40
- Prestch E, Clerc T, Seibl J, Simon W. Tablasparaladilucidaciónestructuraldecompuestosorgánicospormétodosespectroscópicos:13CRMN,1H-RMN,IR,EM,UV-Vis. Barcelona, España: Singer Verlag-Ibérica; 1998
- GraphPad Prism, Graphpad Software Inc., Copyright 1994–2000v3.02
- Carta F, Aggarwal M, Maresca A, et al. Dithiocarbamates: a new class of carbonic anhydrase inhibitors. Crystallographic and kinetic investigations. Chem Commun (Camb) 2012;48:1868–70
- Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: interactions of phenols with the 12 catalytically active mammalian isoforms (CA I-XIV). Bioorg Med Chem Lett 2008;18:1583–7
- Innocenti A, Vullo D, Scozzafava A, et al. Carbonic anhydrase inhibitors. Interaction of isozymesI, II, IV, V, and IX with carboxylates. Bioorg Med Chem Lett 2005;15:573–8
- Di Fiore A, Monti SM, Hilvo M, et al. Crystal structure of human carbonic anhydrase XIII and its complex with the inhibitor acetazolamide. Proteins 2009;74:164–75
- Supuran CT. Carbonic anhydrase inhibition/activation: trip of a scientist around the world in the search of novel chemotypes and drug targets. Curr Pharm Des 2010;16:3233–45
- Supuran CT. Inhibition of bacterial carbonic anhydrases and zinc proteases: from orphan targets to innovative new antibiotic drugs. Curr Med Chem 2012;19:831–44
- Sahin H, Can Z, Yildiz O, et al. Inhibition of carbonic anhydrase isozymes I and II with natural products extracted from plants, mushrooms and honey. J Enzyme Inhib Med Chem 2012;27:395–402
- Scozzafava A, Menabuoni L, Mincione F, et al. Carbonic anhydrase inhibitors. Synthesis of sulfonamides incorporating dtpa tails and of their zinc complexes with powerful topical antiglaucoma properties. Bioorg Med Chem Lett 2001;11:575–82
- Supuran CT, Casini A, Mastrolorenzo A, Scozzafava A. COX-2 selective inhibitors, carbonic anhydrase inhibition and anticancer properties of sulfonamides belonging to this class of pharmacological agents. Mini Rev Med Chem 2004;4:625–32
- De Simone G, Di Fiore A, Supuran CT. Are carbonic anhydrase inhibitors suitable for obtaining antiobesity drugs? Curr Pharm Des 2008;14:655–60