Abstract
Cyclohexyliden- and 2-methylcyclohexyliden-hydrazo-4-arylthiazoles were synthesized and tested as antifungal agents. All compounds exhibited minimal inhibitory concentration (MIC) values comparable with those of fluconazole (FLC). Moreover, some compounds showed fungicidal activity at low concentration. Worth noting five out of nine compounds were active towards Candida albicans 25 FLC resistant isolated from clinical specimens. The cellular toxicity was evaluated and none of the compounds is toxic at the MIC. On the basis of our data we can conclude that these derivatives are promising agents for the treatment of resistant C. albicans.
Introduction
Over the past 20 years the incidence of invasive fungal infections and associated mortality have increased significantly as a consequence of the rising number of immunocompromised patients, the wide development of organ transplantation, tracheal intubation and endoscopic techniques, and the extensive application of broad-spectrum antibiotics, immunosuppressant drugs and corticosteroidsCitation1,Citation2. In this respect, Candida albicans accounts for a large proportion of invasive fungal infections, placing itself at the first place as the major fungal pathogen in humansCitation3.
This organism, together with related Candida species, has become one of the commonest agents of hospital-acquired infection and in AIDS patientsCitation2–4.
So far there are four classes of antifungal agents, (polyenes, 5-fluorocytosine, azoles and echinocandins) that can be used for the treatment of systemic infectionsCitation5–8. Nevertheless, these antifungal agents have achieved limited success in term of severe resistance, limited efficacy and spectrum, drug-related toxicity, non-optimal pharmacokinetics and serious drug–drug interactions. Azoles, and in particular fluconazole (FLC), have been extensively used in clinical practice because of their great efficacy and low toxicityCitation3,Citation9. However, due to the excessive exposure to FLC, FLC-resistant C. albicans species have emergedCitation2,Citation10,Citation11.
In order to contrast this tendency, different efforts have been made to overcome the emergence of resistant fungi by using multi drugs therapy. Unfortunately, high costs and serious side effects have put limitation on the combinations of antifungal drugsCitation12,Citation13. In addition, contradictory results of the synergistic or antagonistic action of various antifungals combination have been reportedCitation9,Citation14,Citation15.
FLC-like drugs inhibit the cytochrome P450 sterol 14α-demethylase (14DM, CYP51) by coordinating the heme iron atom in the catalytic site with the azole nitrogen atom (N-3 and N-4 in the case of imidazole and triazole respectively). Inhibition of the cytochrome P450 3 A-dependent lanosterol 14-α-demethylase leads to accumulation of 14-α-methylsterol and depletion of ergosterolCitation16 resulting in plasma membrane structure disruptionCitation11. The efficacy and selectivity of azoles depends on the strength of the binding to heme iron as well as the affinity of the N-1 substituent for the CYP51Citation17.
It has been reported that the presence of iron chelators influences the growth of C. albicansCitation18,Citation19. In this respect, thiosemicarbazones iron chelators have been reported as antifungal agents and their activity compared with that of FLCCitation20.
On the basis of the above, the focus of our research is the design of a new class of anti-Candida agents characterized by a different scaffold and mechanism of action, with respect to the azole derivatives, that might be active towards FLC resistant Candida species. Indeed, iron chelators could represent a valuable therapeutic approach to Candida infection.
Our research group has already studied similar scaffolds as antifungal and antibacterial agents and some of these compounds exhibited potent activity towards several species of CandidaCitation21–24. Moreover, very similar compounds were reported as potent and selective monoamine oxidase inhibitorsCitation25,Citation26.
Methods
Materials and apparatus
Starting materials and reagents were obtained from commercial suppliers and were used without purification. All melting points were determined on a Stuart SMP11 melting points apparatus and are uncorrected. Electron ionization mass spectra were obtained by a Fisons QMD 1000 mass spectrometer (70 eV, 200 mA, ion source temperature 200 °C). Samples were directly introduced into the ion source. Found mass values are in agreement with theoretical ones. Melting points, yield of reactions and the analytical and descriptive data of derivatives are reported in and .
Table 1. Chemical, analytical and physical data of derivatives EMAC2098–2100–2101–2103–2104–2105–2107–2109–2110.
Table 2. Chemical and descriptive data of derivatives EMAC 2098–2100–2101–2103–2104–2105–2107–2109–2110.
1H NMR were registered on a Bruker AVANCE III 500 MHz spectrometer. All samples were measured in CDCl3. Chemical shifts are reported referenced to the solvent in which they were measured. Coupling constants J are expressed in Hertz (Hz). Elemental analyses were obtained on a Perkin–Elmer 240 B microanalyser. Analytical data of the synthesized compounds are in agreement within ± 0.4% of the theoretical values. TLC chromatography was performed using silica gel plates (Merck F 254), spots were visualized by UV light.
General procedure for the synthesis of compound derivatives EMAC2098–2100-2101–2103–2104–2105–2107–2109–2110
In a typical experiment, 2 mmol of cycloalkyl-thiosemicarbazone were reacted at room temperature (rt) in 20 ml of tap water with 2 mmol of α-halogen ketone The mixture was stirred for a period ranging between 24 and 32 h and the formation of a forming product is observed. The stirring was stopped and the solid filtered. The crude product was crystallized from ethanol, water/ethanol.
Biological assay
Strains
Antifungal susceptibility studies. The in vitro activity of compounds EMAC2098–2100–2101–2103–2104–2105–2107–2109–2110 was assessed against C. albicans ATTC 10231 and against a strain of C. albicans FLC resistant isolated from clinical specimen identified by standard methods. Prior to testing, strains were subcultured on Potato Dextrose Agar plates (Difco, BD Diagnostics, Sparks, MD).
Testing compounds
Compounds EMAC2098–2100–2101–2103–2104–2105–2107–2109–2110 were dissolved in dimethyl sulfoxide (Sigma-Aldrich, Saint Louis, MO) at concentrations ranging between 2 and 25 mg/ml depending on their specific solubility. FLC (Sigma-Aldrich), employed as reference drug, was dissolved in dimethyl sulfoxide at 50 mg/ml. Solutions of the testing compounds and FLC were stored at −20 °C until use.
Antifungal susceptibility assays
Minimal inhibitory concentrations (MICs) were determined by the broth microdilution method according to the Clinical and Laboratory Standard Institute reference document M27-A3. RPMI 1640 medium (Sigma-Aldrich) without sodium bicarbonate, supplemented with l-glutamine (Gibco, Life Technologies, Grand Island, NY) and buffered with 0.165 M MOPS (Sigma-Aldrich) at pH 7.0 was used as test medium. Two-fold dilutions of the testing compounds at concentration ranging between 0.048 and 200 µg/ml were obtained in RPMI 1640 and 100 µl of the obtained solutions were dispensed into the wells of microdilutions trays. 100 µl inoculum containing 2 × 104 cells/ml of C. albicans was added to each well to obtain final concentrations of the testing compounds ranging between 0.024 and 100 µg/ml and final inocula of 1 × 104 cells/ml. Plates were incubated at 35 °C for 24–48 h and then read visually. MICs were determined as the lowest concentration at which a 100% inhibition of growth compared with drug-free control was observed. Minimal fungicidal concentrations (MFCs) were determined by seeding a 100 -µl sample obtained from the wells for the MIC determination on plates of Sabouraud Dextrose agar. Plates were incubated at 35 °C for 72 h. MCF was defined as the lowest as the minimum concentration of compound that resulted in the growth of less than two colonies representing the killing of >99% of the original inoculum.
Cytotoxicity studies
Vero cells lines (African green monkey kidney, BS CL 86) used in this study were obtained from IZSLER (Brescia, Italy). Cells were grown on RPMI 1540 medium supplemented with 2 Mm l-glutamine, penicillin 50 IU/ml, streptomycin 50 mg/l and 10% fetal bovine serum. Cells were seeded in 96-well plastic microtiter plates (Falcon, BD Biosciences, Bedford, MD) at a density of 1 × 104 cells/ml in RPMI 1640 containing no antibiotics and the testing compounds at concentrations ranging between 0.78 and 500 µg/ml depending on their solubility in DMSO. To avoid interference by the solvent, the maximum concentration of DMSO was 2%. Cells were incubated at 37 °C in 5% CO2 and observed for morphological changes at 24, 48 and 72 h of incubation. After 72 h the effects on the proliferation of Vero cells were determined by the tetrazolium-based colorimetric MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. The 50% cell-inhibitory concentration (IC50) reduced by 50% the optical density values (OD540 690) with respect to control untreated cells.
Coordination studies
The interaction between EMAC 2104 and the Fe2+ or Fe3+ ions was checked by recording the UV/vis absorbance spectra of EMAC2104 in absence and in presence either of FeCl3 or FeSO4 using FLC as positive control. Compounds were solubilized in DMSO then diluted to 100 µM in water. The UV/vis spectrum of the solution was recorded before and after addition of 10 mM final concentration either of FeCl3 or FeSO4. The spectra were recorded with a Ultrospec 2100 pro (Amersham Biosciences) and analyzed with SWIFT II–METHOD software (GE Healthcare).
Results and discussion
As a continuation of our previous work on the discovery of new anti-Candida agents, we decided to focus our attention on the synthesis and biological evaluation of thiazole derivatives and test screen them toward FLC resistant C. albicans. Thus, to achieve more information on the SARs of these compounds and to evaluate their activity toward FLC resistant Candida species, we have synthesized differently substituted cycloalkylidenhydrazo-4-arylthiazoles.
Desired compounds were synthesized starting from different cyclic ketones (1.2 mol) with thiosemicarbazide (1 mol) in n-propanol with catalytic amounts of acetic acid at reflux condition for 12–24 h to obtain the corresponding thiosemicarbazones that were crystallized from ethanol. 4-Substituted thiazole derivatives were obtained by reaction of differently substituted α-halogenoketones with the previously obtained thiosemicarbazones as shown in Scheme 1.
Scheme 1. Synthesis of EMAC compounds. Reagents and conditions: (i) n-propanol, acetic acid; (ii) water.
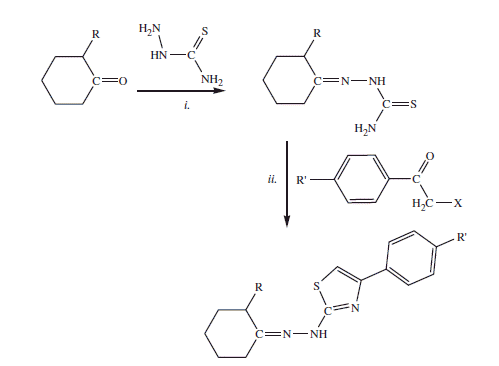
The reaction was performed using water as a solvent. Indeed water proved to be a more efficient, cheaper, and green solvent in comparison with both ethanol and isopropanol. The reaction was performed at rt and the formation of a foamy precipitate is observed. All derivatives were crystallized from ethanol or water/ethanol.
The structures were confirmed by means of analytical and spectroscopic methods. 1H NMR chemical shifts of all the synthesized compounds are reported in .
Table 3. 1H NMR data of derivatives EMAC2098, EMAC2100, EMAC2101, EMAC2103, EMAC2104, EMAC2105, EMAC2107, EMAC2109, EMAC2110.
Compounds EMAC2098–2100–2101–2103–2104–2105–2107–2109–2110 were assayed for their inhibitory activity toward C. albicans (). All tested compounds exhibit a remarkable activity towards C. albicans ATCC 10231. Noteworthy two of the synthesized compounds exhibit a very interesting activity towards C. albicans 25 FLC-resistant.
Table 4. MIC, MCF and vero cells toxicity of EMAC compounds against C. albicans ATCC 10231 and C. Albicans 25 FLC resistant.
Compounds EMAC2098, EMAC2100, EMAC2101 and EMAC2103 exhibit an interesting activity toward C. albicans ATCC 10231. MIC values range from 0.19 to 3.12 µg/ml. Interestingly, all compounds, with the exception of EMAC2100, exhibit fungicidal activity when tested on C. albicans ATCC 10231.
A different behavior was observed when compounds EMAC2098, EMAC2100, EMAC2101 and EMAC2103 were tested against C. albicans 25 FLC-resistant. In this case, only compounds EMAC2098 and EMAC2100 show remarkable MIC values (0.39 and 0.78 µg/ml, respectively), while almost no activity is observed for EMAC2101 and EMAC2103. Unfortunately, none of the tested compounds have interesting fungicidal activity when tested on C. albicans 25 FLC resistant, with the exception of EMAC2104 and EMAC2110 which show values of MCF at 1.56 and 3.12 µg/ml, respectively.
The introduction of a methyl substituent in the position 2 of the cyclohexylidene moiety leads to a different behavior: compound EMAC2105, bearing a 4-bromophenyl moiety in the position 4 of the thiazole ring is active only toward C. albicans ATCC 10231. Although its activity is comparable with that of FLC, but less potent than its homologous EMAC2098, it has neither fungicidal activity on C. albicans ATCC 10231, nor efficacy against C. albicans 25 FLC-resistant.
On the contrary, the 4-nitro-substituted derivative EMAC2107 exhibits a similar activity profile with respect to its homologous EMAC2100. Both compounds only exhibit fungi-static activity on both Candida species.
On the contrary, compounds EMAC2104 and EMAC2110 exhibit a very potent activity with respect to FLC.
The 4-phenylthiazole derivative EMAC2104 is particularly active against C. albicans 25 FLC resistant (MIC 0.19 µg/ml; MFC 1.56 µg/ml).
Although slightly less potent than EMAC2104, compound EMAC2110 is active toward both the tested strains. Moreover, its fungicidal activity is comparable to that of EMAC2104.
The cellular toxicity of all compounds was evaluated. CC50 values were generally lower than that of the reference FLC. The size of the substituent in the position 4 of the phenyl ring appeared as relevant in determining the toxicity. Thus, either the introduction of a nitrile (EMAC2103 and 2109) or a nitro group (EMAC2107 and 2100) leaded to a reduction of the toxicity with respect to fluorine (EMAC2110) and un-substituted (EMAC2104) derivatives.
In order to achieve a preliminary elucidation of the possible mechanism of action of such compounds, we verify the possibility that EMAC2104 could chelate the divalent and trivalent iron ions. Therefore, we determined its spectrum of absorbance in the absence and the presence of both Fe2+ and Fe3+ and compared this behavior with that exhibited by FLC ().
Figure 1. Effect of Fe2+ or Fe3+ ions on the spectrum of absorbance of EMAC2104 and FLC compounds. UV/vis spectrum was measured with 100 µM of compound alone (unbroken line) or in the presence of 10 mM FeCl3 or FeSO4 (dotted line).
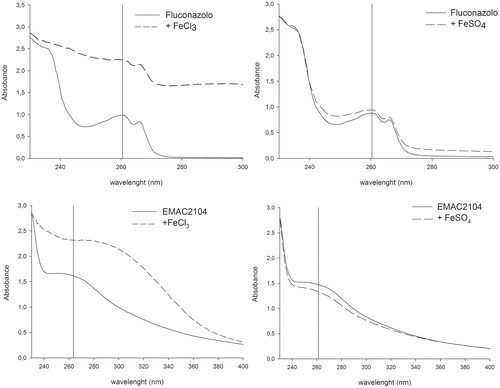
Interestingly neither EMAC2104 nor FLC maximum absorbance was modified by the addition of Fe2+. On the contrary, when Fe3+ was added either to EMAC2104 or to FLC a notable increase of maximum absorbance was observed, indicating that both compounds interact with ferric ions.
These data corroborate the hypothesis that EMAC derivatives might inhibit the growth of C. albicans either by chelating the Fe3+ ions inside the 14α-demethylase catalytic pocket or by Fe3+ withdrawal in the cell cytosol. Further studies are in progress to better understand the mode of action of cycloalkylidenhydrazo-4-arylthiazoles.
Conclusions
The purpose of this study was the synthesis of a series of cycloalkylidenhydrazo-4-arylthiazoles to examine whether molecular modification on the thiazole core might lead to the identification of new potential C. albicans FLC resistant inhibitors. All the compounds were obtained in good yields. With respect to FLC EMAC compounds exhibited comparable and, in some cases (EMAC 2098, 2101 and 2104), lower MIC values. Moreover, most of the compounds exhibited fungicidal activity. When tested against FLC resistant C. albicans isolated from clinical specimens, EMAC 2098, 2100, 21042107 and 2110 retained their antifungal activity. The cellular toxicity was evaluated and none of the compounds is toxic at the MIC. All together these data indicated that the thiazole scaffold could be relevant date have been obtained and will constitute the basis for the future work.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Kriengkauykiat J, Ito JI, Dadwal SS. Epidemiology and treatment approaches in management of invasive fungal infections. J Clin Epidemiol 2011;3:175–91
- Mulu A, Kassu A, Anagaw B, et al. Frequent detection of ‘azole' resistant Candida species among late presenting AIDS patients in northwest Ethiopia. BMC Infect Dis 2013;13:82
- Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol 2010;48:1366–77
- Fridkin SK, Jarvis WR. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev 1996;9:499–511
- Roemer T, Krysan DJ. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med 2014;4:a019703
- Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 years of clinical experience. Rev Infect Dis 1990;12:308–29
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev 1999;12:40–79
- Denning DW. Echinocandins: a new class of antifungal. J Antimicrob Chemother 2002;49:889–91
- Lignell A, Löwdin E, Cars O, et al. Voriconazole-induced inhibition of the fungicidal activity of amphotericin B in Candida strains with reduced susceptibility to voriconazole: an effect not predicted by the MIC value alone. Antimicrob Agents Chemother 2011;55:1629–37
- Arendrup MC, Dzajic E, Jensen RH, et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin Microbiol Infect 2013;19:e343–53
- Lupetti A, Danesi R, Campa M, et al. Molecular basis of resistance to azole antifungals. Trends Mol Med 2002;8:76–81
- Marchetti O, Moreillon P, Entenza JM, et al. Fungicidal synergism of fluconazole and cyclosporine in Candida albicans is not dependent on multidrug efflux transporters encoded by the CDR1, CDR2, CaMDR1, and FLU1 genes. Antimicrob Agents Chemother 2003;47:1565–70
- Tragiannidis A, Tsoulas C, Kerl K, Groll AH. Invasive candidiasis: update on current pharmacotherapy options and future perspectives. Expert Opin Pharmacother 2013;14:1515–28
- Karlowsky JA, Hoban DJ, Zhanel GG, Goldstein BP. In vitro interactions of anidulafungin with azole antifungals, amphotericin B and 5-fluorocytosine against Candida species. Int J Antimicrob Agents 2006;27:174–7
- Chaturvedi V, Ramani R, Andes D, et al. Multilaboratory testing of two-drug combinations of antifungals against Candida albicans, Candida glabrata, and Candida parapsilosis. Antimicrob Agents Chemother 2011;55:1543–8
- Aperis G, Mylonakis E. Newer triazole antifungal agents: pharmacology, spectrum, clinical efficacy and limitations. Expert Opin Invest Drugs 2006;15:579–602
- Monk BC, Tomasiak TM, Keniya MV, et al. Architecture of a single membrane spanning cytochrome P450 suggests constraints that orient the catalytic domain relative to a bilayer. Proc Natl Acad Sci U S A 2014;111:3865–70
- Holbein BE, Mira de Orduña R. Effect of trace iron levels and iron withdrawal by chelation on the growth of Candida albicans and Candida vini. FEMS Microbiol Lett 2010;307:19–24
- Santos ALS, Sodre CL, Valle RS, et al. Antimicrobial action of chelating agents: repercussions on the microorganism development, virulence and pathogenesis. Curr Med Chem 2012;19:2715–37
- Opletalová V, Kalinowski DS, Vejsová M, et al. Identification and characterization of thiosemicarbazones with antifungal and antitumor effects: cellular iron chelation mediating cytotoxic activity. Chem Res Toxicol 2008;21:1878–89
- De Logu A, Saddi M, Cardia MC, et al. In vitro activity of 2-cyclohexylidenhydrazo-4-phenyl-thiazole compared with those of amphotericin B and fluconazole against clinical isolates of Candida spp. and fluconazole-resistant Candida albicans. J Antimicrob Chemother 2005;55:692–8
- Cardia MC, Begala M, Delogu A, et al. Synthesis and antimicrobial activity of novel arylideneisothiosemicarbazones. Farmaco 2000;55:93–8
- Maccioni E, Cardia MC, Bonsignore L, et al. Synthesis and anti-microbial activity of isothiosemicarbazones and cyclic analogues. Farmaco 2002;57:809–17
- Chimenti F, Bizzarri B, Maccioni E, et al. Synthesis and in vitro activity of 2-thiazolylhydrazone derivatives compared with the activity of clotrimazole against clinical isolates of Candida spp. Bioorg Med Chem Lett 2007;17:4635–40
- Chimenti F, Maccioni E, Secci D, et al. Synthesis, stereochemical identification, and selective inhibitory activity against human monoamine oxidase-B of 2-methylcyclohexylidene-(4-arylthiazol-2-yl)hydrazones. J Med Chem 2008;51:4874–80
- Chimenti F, Secci D, Bolasco A, et al. Synthesis, semipreparative HPLC separation, biological evaluation, and 3D-QSAR of hydrazothiazole derivatives as human monoamine oxidase B inhibitors. Bioorg Med Chem 2010;18:5063–70
