Abstract
The synthesis of 14-(aryl)-14H-naphto[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine-2-yl) acetamidoximes 2a–e has been accomplished by reaction of 2-acetonitrile derivatives 1a–e with hydroxylamine. Cyclocondensation reaction of precursors 2a–e with some elctrophilic species such as ethylorthoformate, acetic anhydride, and methyl-acetoacetate provided the new oxadiazole derivatives 3a–e, 4a–e, and 5a–e, respectively. On the other hand, the reaction of precursors 2a–e with 2-chloropropanoyl chloride afforded the new acetimidamides 6a–e which evolve under reflux of toluene to the new oxadiazoles 7a–e. The synthetic compounds were screened for their anti-xanthine oxidase, anti-soybean lipoxygenase, and cytotoxic activities. Moderate to weak xanthine oxidase and soybean lipoxygenase inhibitions were obtained but significant cytotoxic activities were noted. The most cytotoxic activities were recorded mainly (i) 5a was the most active (IC50 = 4.0 μM) and selective against MCF-7 and (ii) 2a was cytotoxic against the four cell lines with selectivity for MCF-7 and OVCAR-3 (IC50 = 17 and 12 μM, respectively) while 2e is highly selective against OVCAR-3 (IC50 = 10 μM).
Introduction
In recent years, pyrimidine derivatives attracted organic chemists due to their widespread potential biological and chemotherapeutic activitiesCitation1. In this framework, triazolopyrimidines, as well as their condensed derivatives are very attractive targets on account of their diverse pharmacological activities, such as antimicrobialCitation2, antimalariaCitation3, antifungalCitation4, macrophage activationCitation5, xanthine oxidase inhibitors ()Citation6, anti-inflammatory ()Citation7, and they have proved to be promising cytotoxic agents ()Citation8.
On the other hand, oxadiazole derivatives possess various biological activities such as immunosuppressiveCitation9, antimicrobialCitation10, and antitumorCitation11. Especially, 1,2,4-oxadiazole fragments are beneficial for interacting with DNACitation12, antibacterialCitation13, antioxidant ()Citation14, anti-inflammatory ()Citation15 and cytotoxic ()Citation16. This phenomenon gives us an important information that 1,2,4-oxadiazole may be a powerful core to construct functional groups in the drug design.
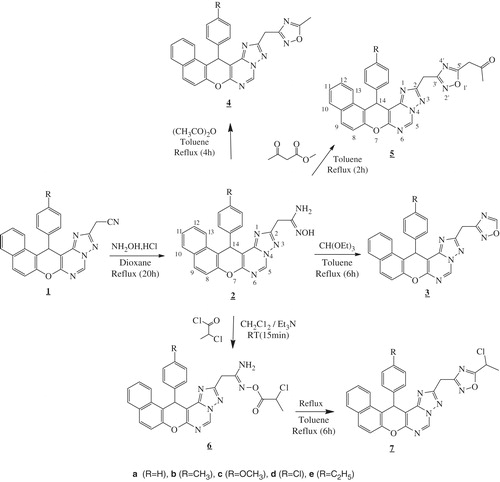
In addition, association of triazolopyrimidine ring with different heterocyclic fragments gives rise to a new class of hybrid molecules possessing improved activityCitation17–21. In this context and as a continuation of our previous work on the synthesis of new fused pyrimidin scaffoldsCitation22,Citation23 and wishing to access to bioactive and why not multi-bioactive molecules, we report here the synthesis of amidoximes 2a–e, which allied to a class of precursors having proven to exhibit numerous biological activities such as antimalarialCitation24, antileishmanialCitation25, antioxidantCitation26, anti-inflammatoryCitation27, and cytotoxic activitiesCitation28, using the naphtopyranyl moiety as a support, and their utility as building blocks in the synthesis of some novel hybrid molecules 3, 4, 5, and 7 (Scheme 1) bearing in their structures fragments described as antioxidant, anti-inflammatory, and cytotoxic agents, as indicated above, such triazolopyrimidine and 1,2,4-oxadiazole systems.
Scheme 1. The design of target compounds. a (R = H), b (R = CH3), c (R = OCH3), d (R = Cl), e (R = C2H5).
The synthetic compounds were then screened for their in vitro xanthine oxidase, soybean lipoxygenase inhibition, and cytotoxic activity.
Materials and methods
General experimental procedures
All reactions were monitored by thin layer chromatography (TLC) using aluminum sheets of Merck silica gel 60 F254, 0.2 mm. Melting temperatures were determined on an electrothermal 9002 apparatus and were reported uncorrected. NMR spectra were recorded on a Bruker AC-300 spectrometer at 300 MHz (1H) and 75 MHz (13C). All chemical shifts were reported as δ values (ppm) relative to residual non deuterated solvent. Mass spectra were acquired with a LCT Premier XE (Waters, ESI technique, positive mode) mass spectrometer. The starting materials 1 were prepared according to the literature and their structures were established on the basis of their spectroscopic dataCitation22,Citation23.
Synthesis
Synthesis of amidoximes 2a–e
The cyano compounds 1a–e (10 mmol) were added to a solution of NH2OH.HCl (12 mmol in 2 ml water) and Na2CO3 (12 mmol in 2 ml water) in dioxane (15 ml). The resulting mixture was heated under reflux for 20 h. After cooling, the mixture was diluted with water and left to stand at room temperature for 1 h. The precipitated solid was collected by filtration, washed with water, then dried and crystallized from ethanol to afford the amidoximes 2a–e.
2–(14-phenyl-14H-naphto[2,1-b]pyrano[3,2,e][1,2,4]triazolo[1,5-c]pyrimidin-2-yl) acetamidoxime 2a
Gray solid, Yield: 65%; m.p.: >300 °C; 1H NMR (DMSO-d6, 300 MHz): δ 9.59 (s, 1H, -OH), 9.10 (s, 1H, H5), 7.06–8.11 (m, 11 H, Harom), 6.30 (s, 1H, H14), 5.52 (s, 2H, -NH2), 3.63 (s, 2H, -CH2); 13C NMR (DMSO-d6, 75 MHz): δ 165.7, 153.1, 152.3, 148.9, 147.9, 143.1, 139.8, 131.1, 130.2, 129.8, 128.5, 128.1, 127.3, 126.9, 125.1, 123.5, 117.5, 115.0, 102.1, 36.8, 31.0. ESI-HRMS [M + H]+ calcd. for (C24H19N6O2)+: 423.1569, found: 423.1579.
2–(14-(4-methylphenyl)-14H-naphto[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c] pyrimidin-2-yl) acetamidoxime 2b
Gray solid, Yield: 59%; m.p.: >300 °C; 1H NMR (C5D5N-d5, 300 MHz): δ 10.81 (s, 1H, -OH), 7.96 (s, 1H, H5), 6.20–7.50 (m, 10H, Harom), 5.71 (s, 1H, H14), 5.59 (s, 2H, -NH2), 3.43 (s, 2H, -CH2-), 1.26 (s, 3H, -CH3); 13C NMR (C5D5N-d5, 75 MHz): δ 168.2, 155.4, 155.0, 150.7, 142.4, 141.0, 138.5, 133.6, 133.1, 131.8, 131.2, 130.7, 130.4, 129.3, 127.1, 125.9, 125.7, 119.6, 117.5, 105.1, 39.2, 33.8, 22.3. ESI-HRMS [M + H]+ calcd. for (C25H21N6O2)+: 437.1726, found: 437.1724.
2–(14-(4-methoxylphenyl)-14H-naphto[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c] pyrimidin-2-yl) acetamidoxime 2c
Gray solid, Yield: 66%; m.p.: >300 °C; 1H NMR (DMSO-d6, 300 MHz): δ 9.57 (s, 1H, -OH), 9.08 (s, 1H, H5), 6.75–8.10 (m, 10 H, Harom), 6.25 (s, 1H, H14), 5.51 (s, 2H, -NH2), 3.63 (s, 2H, -CH2-), 3.61 (s, 3H, -OCH3); 13C NMR (DMSO-d6, 75 MHz): δ 165.7, 158.0, 152.9, 152.3, 149.0, 147.8, 139.7, 135.2, 131.1, 130.3, 129.7, 129.1, 128.5, 127.3, 125.0, 123.5, 117.5, 115.2, 113.9, 102.4, 54.9, 36.0, 31.0,. ESI-HRMS [M + H]+ calcd. for (C25H21N6O3)+: 453.1675, found: 453.1685.
2–(14-(4-chlorophenyl)-14H-naphto[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c] pyrimidin-2-yl) acetamidoxime 2d
Gray solid, Yield: 70%; m.p.: >300 °C; 1H NMR (DMSO-d6, 300 MHz): δ 9.59 (s, 1H, -OH), 9.07 (s, 1H, H5), 7.26–8.08 (m, 10 H, Harom), 6.35 (s, 1H, H14), 5.52 (s, 2H, -NH2), 3.63 (s, 2H, -CH2-); 13C NMR (DMSO-d6, 75 MHz): δ 165.7, 153.0, 152.2, 148.9, 147.9, 141.9, 140.0, 131.1, 130.2, 130.1, 130.0, 128.6, 128.5, 127.4, 125.2, 123.4, 117.5, 114.4, 101.6, 36.3, 31.0. ESI-HRMS [M + H]+ calcd. for (C24H18ClN6O2)+: 457.1180, found: 457.1169.
2–(14-(4-ethylphenyl)-14H-naphto[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c] pyrimidin-2-yl) acetamidoxime 2e
Gray solid, Yield: 62%; m.p.: >300 °C; 1H NMR (DMSO-d6, 300 MHz): δ 9.59 (s, 1H, -OH), 9.09 (s, 1H, H5), 7.00–8.12 (m, 10H, Harom), 6.28 (s, 1H, H14), 5.52 (s, 2H, -NH2), 3.61 (s, 2H, -CH2-), 2.43 (q, 2H, -CH2-CH3, J = 8.7 Hz), 1.03 (t, 3H, -CH3, J = 8.7 Hz); 13C NMR (DMSO-d6, 75 MHz): δ 165.7, 153.0, 152.3, 148.9, 147.9, 142.3, 140.4, 139.8, 131.1, 130.2, 129.8, 128.5, 127.9, 127.3, 125.1, 123.5, 117.5, 115.2, 102.3, 36.4, 31.0, 27.5, 15.2. ESI-HRMS [M + H]+ calcd. for (C26H23N6O3)+: 451.1882, found: 451.1871.
Synthesis of 2-((1,2,4-oxadiazol-3′-yl)methyl)-14-aryl-14H-naphto[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidines 3a-c and 3e
A dispersion of compounds “2a–c and 2e” (0.5 mmol) and ethyl orthoformate (3 mmol) in toluene (5 ml) was refluxed for 96 h. The solvent was evaporated and the residue was separated by column chromatography (silica gel, CH2Cl2/EtOAc 9:1) to afford compounds 3a–c and 3e.
2-((1,2,4-oxadiazol-3′-yl)methyl)-14-phenyl-14H-naphto [2,1-b]pyrano[3,2-e] [1,2,4]triazolo [1,5-c]pyrimidine 3a
White solid, Yield: 40%; m.p.: 252 °C; 1H NMR (CDCl3, 300 MHz): δ 9.05 (s, 1H, H5), 8.75 (s, 1H, H5′), 7.13–8.00 (m, 11H, Harom), 6.37 (s, 1H, H14), 4.55 (s, 2H, -CH2-); 13C NMR (CDCl3, 75 MHz): δ 166.0, 165.1, 164.6, 154.0, 153.5, 148.6, 142.4, 138.1, 131.6, 130.8, 129.9, 128.6, 128.3, 127.3, 127.2, 125.2, 123.5, 117.5, 114.9, 103.6, 37.6, 26.5. ESI-HRMS [M + H]+ calcd. for (C25H17N6O2)+: 433.1413, found: 433.1420.
2-((1,2,4-oxadiazol-3′-yl)methyl)-14-(4-methylphenyl)-14H-naphto[2,1-b] pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine 3b
White solid, Yield: 45%; m.p.: 254 °C; 1H NMR (CDCl3, 300 MHz): δ 9.05 (s, 1H, H5), 8.76 (s, 1H, H5′), 7.00–8.00 (m, 10H, Harom), 6.35 (s, 1H, H14), 4.55 (s, 2H, -CH2-), 2.21 (s, 3H, -CH3); 13C NMR (CDCl3, 75 MHz): δ 166.0, 165.1, 164.6, 154.0, 153.5, 148.6, 139.5, 138.0, 136.9, 131.6, 130.9, 129.8, 129.3, 128.6, 128.2, 127.3, 125.2, 123.5, 117.5, 115.1, 103.8, 37.2, 26.5, 20.9. ESI-HRMS [M + H]+ calcd. for (C26H19N6O2)+: 447.1569, found: 447.1580.
2-((1,2,4-oxadiazol-3′-yl)methyl)-14-(4-methoxyphenyl) -14H-naphto[2,1-b] pyrano[3,2-e] [1,2,4]triazolo[1,5-c] pyrimidine 3c
White solid, Yield: 41%; m.p.: 260 °C; 1H NMR (CDCl3, 300 MHz): δ 9.05 (s, 1H, H5), 8.76 (s, 1H, H5′), 6.71–8.00 (m, 10H, Harom), 6.33 (s, 1H, H14), 4.55 (s, 2H, -CH2-), 3.68 (s, 3H, -OCH3); 13C NMR (CDCl3, 75 MHz): δ 166.0, 165.1, 164.6, 158.6, 153.9, 153.5, 148.6, 138.0, 134.7, 131.6, 130.8, 129.8, 129.4, 128.6, 127.3, 125.1, 123.5, 117.5, 115.1, 114.0, 103.8, 55.1, 36.7, 26.5. ESI-HRMS [M + H]+ calcd. for (C26H19N6O3)+: 463.1519, found: 463.1515.
2-((1,2,4-oxadiazol-3′-yl)methyl)-14-(4-ethylphenyl) -14H-naphto[2,1-b]pyrano[3,2-e] [1,2,4]triazolo[1,5-c] pyrimidine 3e
White solid, Yield: 39%; m.p.: 222 °C; 1H NMR (CDCl3, 300 MHz): δ 9.05 (s, 1H, H5), 8.75 (s, 1H, H5′), 7.00–8.00 (m, 10H, Harom), 6.37 (s, 1H, H14), 4.55 (s, 2H, -CH2-),2.43 (q, 2H, -CH2-CH3, J = 8.7 Hz), 1.03 (t, 3H, -CH3, J = 8.7 Hz); 13C NMR (CDCl3, 75 MHz): δ 166.0, 165.0, 164.5, 154.1, 153.4, 148.6, 143.1, 139.7, 138.0, 131.6, 130.9, 129.8, 129.1, 128.6, 128.2, 128.1, 127.3, 125.2, 123.5, 117.5, 115.2, 103.8, 37.2, 28.3, 26.4, 15.1. ESI-HRMS [M + H]+ calcd. for (C27H21N6O2)+: 461.1726, found: 461.1715.
Synthesis of 2-((5′-methyl-1,2,4-oxadiazol-3′-yl)methyl)-14-aryl-14H-naphto[2,1-b]pyrano[3,2-e][1,2,4]triazolo [1,5-c] pyrimidines 4a-e
A mixture of 2a–e (0.5 mmol) and excess of acetic anhydride (2 ml) in toluene (10 ml) was refluxed for 4 h. The solvent was evaporated and the residue was separated by column chromatography (silica gel, CH2Cl2/EtOAc 9.5:0.5) to give compounds 4a–e.
2-((5′-methyl-1,2,4-oxadiazol-3′-yl)methyl)-14-phenyl-14H-naphto[2,1-b] pyrano[3,2-e] [1,2,4]triazolo[1,5-c]pyrimidine 4a
Yellow solid, Yield: 51%; m.p.: 196 °C; 1H NMR (CDCl3, 300 MHz): δ 9.03 (s, 1H, H5), 7.06–7.95 (m, 11H, Harom), 6.37 (s, 1H, H14), 4.44 (s, 2H, -CH2-), 2.59 (s, 3H, -CH3); 13C NMR (CDCl3, 75 MHz): δ 176.5, 166.1, 164.2, 153.5, 152.8, 148.1, 141.9, 137.6, 131.1, 130.3, 129.4, 128.1, 127.8, 126.9, 126.7, 124.7, 123.0, 117.0, 114.4, 103.1, 37.1, 26.1, 11.8. ESI-HRMS [M + H]+ calcd. for (C26H19N6O2)+: 447.1569, found: 447.1580.
2-((5′-methyl-1,2,4-oxadiazol-3′-yl)methyl)-14-(4-methylphenyl)-14H-naphto[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c] pyrimidine 4b
Yellow solid, Yield: 56%; m.p.: 194 °C; 1H NMR (CDCl3, 300 MHz): δ 9.05 (s, 1H, H5), 7.02–7.99 (m, 10H, Harom), 6.37 (s, 1H, H14), 4.46 (s, 2H, -CH2-),2.62 (s, 3H, -CH3), 2.21 (s, 3H, -CH3); 13C NMR (CDCl3, 75 MHz): δ 176.6, 166.1, 164.1, 153.6, 152.8, 148.0, 139.0, 137.6, 136.4, 131.1, 130.3, 129.3, 128.8, 128.1, 127.7, 126.9, 124.7, 123.0, 117.0, 114.6, 103.2, 36.6, 26.0, 20.5, 11.9,. ESI-HRMS [M + H]+ calcd. for (C27H21N6O2)+: 461.1726, found: 461.1733.
14-(4-methoxyphenyl)-2-((5′-methyl-1,2,4-oxadiazol-3′-yl)methyl)-14H-naphto[2,1-b] pyrano[3,2-e][1,2,4]triazolo [1,5-c]pyrimidine 4c
Yellow solid, Yield: 59%; m.p.: 172 °C; 1H NMR (CDCl3, 300 MHz): δ 9.04 (s, 1H, H5), 6.71–7.96 (m, 10H, Harom), 6.33 (s, 1H, H14), 4.45 (s, 2H, -CH2-), 3.68 (s, 3H, -OCH3), 2.61 (s, 3H, -CH3); 13C NMR (CDCl3, 75 MHz): δ 176.5, 166.2, 164.4, 158.0, 153.3, 153.0, 148.0, 137.5, 134.3, 131.1, 130.4, 129.2, 128.9, 128.1, 126.8, 124.6, 123.0, 117.0, 114.6, 103.3, 54.6, 36.2, 26.1, 11.8. ESI-HRMS [M + H]+ calcd. for (C27H21N6O3)+: 477.1675, found: 477.1661.
14-(4-chlorophenyl)-2-((5′-methyl-1,2,4-oxadiazol-3′-yl)methyl)-14H-naphto[2,1-b] pyrano[3,2-e][1,2,4]triazolo[1,5-c] pyrimidine 4d
Yellow solid, Yield: 49%; m.p.: 160 °C; 1H NMR (CDCl3, 300 MHz): δ 9.07 (s, 1H, H5), 7.14–7.93 (m, 10H, Harom), 6.37 (s, 1H, H14), 4.45 (s, 2H, -CH2-), 2.62 (s, 3H, -CH3); 13C NMR (CDCl3, 75 MHz): δ 177.1, 166.6, 164.9, 153.9, 153.3, 148.6, 140.8, 138.3, 133.1, 131.6, 130.7, 130.1, 129.7, 128.7, 128.1, 127.5, 125.3, 123.3, 117.5, 114.3, 103.0, 37.0, 26.6, 12.3. ESI-HRMS [M + H]+ calcd. for (C26H18ClN6O2)+: 481.1180, found: 481.1165.
2-((5′-methyl-1,2,4-oxadiazol-3′-yl)methyl)-14-(4-ethylphenyl)-14H-naphto[2,1-b] pyrano[3,2-e][1,2,4]triazolo[1,5-c] pyrimidine 4e
Yellow solid, Yield: 56%; m.p.: 178 °C; 1H NMR (CDCl3, 300 MHz): δ 8.97 (s, 1H, H5), 6.93–7.92 (m, 10H, Harom), 6.29 (s, 1H, H14), 4.38 (s, 2H, -CH2-), 2.63 (s, 3H, -CH3), 2.45 (q, 2H, -CH2-CH3, J = 8.7 Hz), 1.04 (t, 3H, -CH3, J = 8.7 Hz); 13C NMR (CDCl3, 75 MHz): δ 176.6, 166.1, 164.1, 148.1, 142.6, 139.2, 137.5, 131.1, 130.4, 129.2, 128.1, 127.7, 127.6, 126.8, 124.7, 123.0, 117.0, 114.7, 103.3, 36.6, 27.8, 26.0, 14.6, 11.9. ESI-HRMS [M + H]+ calcd. for (C28H23N6O2)+: 475.1882, found: 475.1893.
Synthesis of 1–(3-((14-aryl-14H-naphto[2,1-b]pyrano [3,2-e][1,2,4]triazolo[1,5-c] pyrimidin-2-yl)methyl)-1,2,4-oxadiazol-5′-yl) propan-2-ones 5a-e
A dispersion of compounds 2a–e (0.5 mmol) and methyl acetoacetate (1 mmol) in toluene (7 ml) was refluxed for 2 h. The solvent was evaporated and the residue was chromatographed (column, silica gel, CH2Cl2/EtOAc, 9:1) to give compounds 5a–e.
1–(3-((14-phenyl-14H-naphto[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidin-2-yl) methyl)-1,2,4-oxadiazol-5′-yl) propan-2-one 5a
Brown solid, Yield: 49%; m.p.: 210 °C; 1H NMR (CDCl3, 300 MHz): δ 9.02 (s, 1H, H5), 7.06–7.94 (m, 11H, Harom), 6.35 (s, 1H, H14), 4.47 (s, 2H, -CH2-),4,05 (s, 2H, -CH2-CO), 2.29 (s, 3H, -CH3); 13C NMR (CDCl3, 75 MHz): δ 198.0, 173.0, 166.4, 164.1, 153.5, 152.9, 148.1, 141.9, 137.6, 131.1, 130.3, 129.3, 128.1, 127.8, 126.8, 126.7, 124.7, 123.0, 117.0, 114.4, 103.1, 41.0, 37.1, 29.4, 26.1. ESI-HRMS [M + H]+ calcd. for (C28H21N6O3)+: 489.1675, found: 489.1683.
1–(3-((14-(4-methylphenyl)-14H-naphto[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c] pyrimidin-2-yl)methyl)-1,2,4-oxadiazol-5′-yl) propan-2-one 5b
Brown solid, Yield: 40%; m.p.: 210 °C; 1H NMR (CDCl3, 300 MHz): δ 8.94 (s, 1H, H5), 6.89–7.87 (m, 10H, Harom), 6.23 (s, 1H, H14), 4.47 (s, 2H, -CH2-), 3,98 (s, 2H, -CH2-CO), 2.22 (s, 3H, -CO-CH3), 2.10 (s, 3H, -CH3); 13C NMR (CDCl3, 75 MHz): δ 198.5, 173.5, 167.0, 164.7, 153.9, 153.5, 148.6, 139.6, 138.0, 136.9, 131.6, 130.9, 129.8, 129.3, 128.6, 128.2, 127.3, 125.1, 123.5, 117.5, 115.1, 103.8, 41.5, 37.1, 29.9, 26.7, 21.0. ESI-HRMS [M + H]+ calcd. for (C29H23N6O3)+: 503.1832, found: 503.1819.
1–(3-((14-(4-methoxyphenyl)-14H-naphto[2,1-b]pyrano [3,2-e][1,2,4]triazolo[1,5-c] pyrimidin-2-yl)methyl)-1,2,4-oxadiazol-5′-yl) propan-2-one 5c
Brown solid, Yield: 44%; m.p.: 202 °C; 1H NMR (CDCl3, 300 MHz): δ 9.03 (s, 1H, H5), 6.70–7.88 (m, 10H, Harom), 6.31 (s, 1H, H14), 4.48 (s, 2H, -CH2-), 4,07 (s, 2H, -CH2-CO), 3.67 (s, 3H, -OCH3), 2.31 (s, 3H, -CO-CH3); 13C NMR (CDCl3, 75 MHz): δ 198.5, 173.5, 166.9, 164.6, 153.8, 153.5, 148.5, 138.0, 134.7, 131.6, 130.8, 129.7, 129.4, 128.6, 128.5, 127.3, 125.1, 123.5, 117.5, 115.1, 113.9, 103.8, 55.0, 41.5, 36.7, 29.8, 26.6. ESI-HRMS [M + H]+ calcd. for (C29H23N6O4)+: 519.1781, found: 519.1793.
1–(3-((14-(4-chlorophenyl)-14H-naphto[2,1-b]pyrano [3,2-e][1,2,4]triazolo[1,5-c]pyrimidin-2-yl)methyl)-1,2,4-oxadiazol-5′-yl) propan-2-one 5d
Brown solid, Yield: 44%; m.p.: 224 °C; 1H NMR (CDCl3, 300 MHz): δ 9.07 (s, 1H, H5), 7.14–7.92 (m, 10H, Harom), 6.37 (s, 1H, H14), 4.49 (s, 2H, -CH2-),4,09 (s, 2H, -CH2-CO), 2.33 (s, 3H, -CO-CH3); 13C NMR (CDCl3, 75 MHz): δ 198.0, 173.0, 166.4, 164.1, 153.5, 148.1, 140.3, 137.8, 132.6, 131.1, 130.2, 129.6, 129.2, 128.3, 128.2, 127.0, 124.8, 122.8, 117.0, 113.8, 102.5, 41.0, 36.5, 29.4, 26.1. ESI-HRMS [M + H]+ calcd. for (C28H20ClN6O3)+: 523.1285, found: 523.1292.
1–(3-((14-(4-ethylphenyl)-14H-naphto[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c] pyrimidin-2-yl)methyl)-1,2,4-oxadiazol-5′-yl) propan-2-one 5e
Brown solid, Yield: 53%; m.p.: 198 °C; 1H NMR (CDCl3, 300 MHz): δ 9.02 (s, 1H, H5), 7.00–7.98 (m, 10H, Harom), 6.35 (s, 1H, H14), 4.48 (s, 2H, -CH2-),4,09 (s, 2H, -CH2-CO), 2.48 (q, 2H, -CH2-CH3, J = 8.7 Hz), 2.30 (s, 3H, -CO-CH3), 1.11 (t, 3H, -CH3, J = 8.7 Hz); 13C NMR (CDCl3, 75 MHz): δ 197.9, 173.0, 166.4, 163.9, 153.6, 152.8, 148.1, 142.6, 139.2, 137.5, 131.1, 130.4, 129.2, 128.0, 127.7, 127.6, 126.8, 124.7, 123.0, 117.0, 114.7, 103.3, 41.0, 36.6, 29.4, 27.7, 26.1, 14.6. ESI-HRMS [M + H]+ calcd. for (C30H25N6O3)+: 517.1988, found: 517.1979.
Synthesis of N′-((2-chloropropanoyl)oxy)-2–(14-aryl-14H-naphto[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidin-2-yl) acetimidamides 6a–e
2-chloropropanoyl chloride (1 mmol) was added dropwise during 1 h to a stirred dispersion of compounds 2a–e (1 mmol) and Et3N (1 mmol) in CH2Cl2 (10 ml) and the mixture was stirred vigorously at room temperature for 15 min. the filtrate after removing of the Et3N.HCl was concentrated under reduced pressure then crystallized from petroleum ether/CH2Cl2 (1v:1v) to give compounds 6a–e.
N′-((2-chloropropanoyl)oxy)-2–(14-phenyl-14H-naphto[2,1-b]pyrano[3,2-e][1,2,4] triazolo[1,5-c]pyrimidin-2-yl) acetimidamide 6a
Creamy white solid, Yield: 71%; m.p.: 135 °C; 1H NMR (CDCl3, 300 MHz): δ 9.05 (s, 1H, H5), 7.10–7.97 (m, 11H, Harom), 6.31 (s, 1H, H14), 5.70 (s, 2H, -NH2), 4.56 (q, 1H, CH, J = 6.9 Hz), 3.97 (s, 2H, -CH2-), 1.78 (d, 3H, -CH3, J = 6.9 Hz); 13C NMR (CDCl3, 75 MHz): δ 166.6, 163.6, 155.1, 153.5, 152.7, 147.9, 142.9, 137.7, 131.1, 130.3, 129.5, 128.1, 127.9, 126.9, 124.8, 122.9, 117.0, 114.1, 102.8, 51.1, 37.4, 29.7, 21.1. ESI-HRMS [M + H]+ calcd. for (C27H22ClN6O3)+: 513.1442, found: 513.1449.
N′-((2-chloropropanoyl)oxy)-2–(14-(4-methylphenyl)-14H-naphto[2,1-b]pyrano[3,2-e] [1,2,4]triazolo[1,5-c]pyrimidin-2-yl) acetimidamide 6b
Creamy white solid, Yield: 72%; m.p.: 188 °C; 1H NMR (CDCl3, 300 MHz): δ 8.98 (s, 1H, H5), 6.93–7.91 (m, 10H, Harom), 6.22 (s, 1H, H14), 5.67 (s, 2H, -NH2), 4.48 (q, 1H, CH, J = 6.9 Hz), 3.89 (s, 2H, -CH2-), 2.13 (s, 3H, -CH3), 1.70 (d, 3H, -CH3, J = 6.9 Hz); 13C NMR (CDCl3, 75 MHz): δ 167.0, 163.6, 155.5, 154.3, 148.3, 139.5, 138.1, 137.2, 131.7, 130.8, 130.0, 129.4, 128.7, 128.2, 127.5, 125.3, 123.4, 117.5, 114.8, 103.5, 51.6, 37.5, 30.0, 21.6, 20.9. ESI-HRMS [M + H]+ calcd. for (C28H24ClN6O3)+: 527.1598, found: 527.1585.
N′-((2-chloropropanoyl)oxy)-2–(14-(4-methoxyphenyl)-14H-naphto[2,1-b]pyrano[3,2-e] [1,2,4]triazolo[1,5-c]pyrimidin-2-yl) acetimidamide 6c
Creamy white solid, Yield: 80%; m.p.: 140 °C; 1H NMR (CDCl3, 300 MHz): δ 9.08 (s, 1H, H5), 6.74–8.00 (m, 10H, Harom), 6.32 (s, 1H, H14), 5.76 (s, 2H, -NH2), 4.54 (q, 1H, CH, J = 6.9 Hz), 4.00 (s, 2H, -CH2-),3.69 (s, 3H, -OCH3), 1.79 (d, 3H, -CH3, J = 6.9 Hz); 13C NMR (CDCl3, 75 MHz): δ 167.1, 164.2, 158.0, 155.4, 153.8, 153.5, 148.5, 138.0, 134.7, 131.6, 130.8, 129.7, 129.4, 128.6, 128.5, 127.3, 125.1, 123.5, 117.5, 115.1, 113.9, 103.8, 54.9, 51.6, 37.3, 30.3, 21.6. ESI-HRMS [M + H]+ calcd. for (C28H24ClN6O4)+: 543.1548, found: 543.1537.
N′-((2-chloropropanoyl)oxy)-2–(14-(4-chlorophenyl)-14H-naphto[2,1-b]pyrano[3,2-e] [1,2,4]triazolo[1,5-c]pyrimidin-2-yl) acetimidamide 6d
Creamy white solid, Yield: 79%; m.p.: 170 °C; 1H NMR (CDCl3, 300 MHz): δ 9.08 (s, 1H, H5), 7.18–7.93 (m, 10H, Harom), 6.30 (s, 1H, H14), 5.61 (s, 2H, -NH2), 4.55 (q, 1H, CH, J = 6.9 Hz), 3.97 (s, 2H, -CH2-), 1.80 (d, 3H, -CH3, J = 6.9 Hz); 13C NMR (CDCl3, 75 MHz): δ 167.1, 164.3, 155.5, 154.0, 153.2, 148.4, 140.9, 138.3, 133.2, 131.7, 130.6, 130.3, 129.7, 128.9, 128.8, 127.5, 125.4, 123.2, 117.5, 114.0, 102.9, 51.6, 37.3, 30.3, 21.6. ESI-HRMS [M + H]+ calcd. for (C27H21Cl2N6O3)+: 547.1052, found: 547.1062.
N′-((2-chloropropanoyl)oxy)-2–(14-(4-ethylphenyl)-14H-naphto[2,1-b]pyrano[3,2-e] [1,2,4]triazolo[1,5-c]pyrimidin-2-yl) acetimidamide 6e
Creamy white solid, Yield: 79%; m.p.: 170 °C; 1H NMR (CDCl3, 300 MHz): δ 9.03 (s, 1H, H5), 7.01–7.97 (m, 10H, Harom), 6.26 (s, 1H, H14), 5.67 (s, 2H, -NH2), 4.53 (q, 1H, CH, J = 6.9 Hz), 3.94 (s, 2H, -CH2-),2.50 (q, 2H, -CH2-CH3, J = 8.7 Hz), 1.76 (d, 3H, -CH3, J = 6.9 Hz), 1.10 (t, 3H, -CH3, J = 8.7 Hz); 13C NMR (CDCl3, 75 MHz): δ 166.6, 163.6, 155.2, 153.1, 152.8, 147.9, 142.8, 139.3, 137.6, 131.1, 130.3, 129.4, 128.1, 127.8, 127.6, 126.9, 124.7, 123.0, 117.0, 114.3, 103.1, 51.1, 37.0, 29.7, 27.7, 21.1, 14.6. ESI-HRMS [M + H]+ calcd. for (C29H26ClN6O3)+: 541.1755, found: 541.1765.
Synthesis of 2-((5′-(1-chloroethyl)-1,2,4-oxadiazol-3′-yl)methyl)-14-aryl-14H-naphto[2,1-b]pyrano[3,2-e][1,2,4]triazolo [1,5-c]pyrimidines 7a–e
A solution of compound 6a–e (0.5 mmol) in toluene (5 ml) was refluxed for 6 h. The solvent was evaporated and the residue was crystallized from Hexane/CH2Cl2 (1v:1v) to give compounds 7a–e
2-((5′-(1-chloroethyl)-1,2,4-oxadiazol-3′-yl)methyl)-14-phenyl-14H-naphto[2,1-b] pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine 7a
Creamy white solid, Yield: 89%; m.p.: 128 °C; 1H NMR (CDCl3, 300 MHz): δ 9.05 (s, 1H, H5), 7.09–7.98 (m, 11H, Harom), 6.40 (s, 1H, H14), 4.20 (q, 1H, CH, J = 6.9 Hz), 4.50 (s, 2H, -CH2-), 1.98 (d, 3H, -CH3, J = 6.9 Hz); 13C NMR (CDCl3, 75 MHz): δ 177.6, 166.4, 163.8, 148.1, 141.8, 137.6, 131.1, 130.3, 129.4, 128.1, 127.8, 126.9, 126.7, 124.7, 122.9, 117.0, 114.4, 103.1, 45.1, 37.0, 26.1, 21.9. ESI-HRMS [M + H]+ calcd. for (C27H20ClN6O2)+: 495.1336, found: 495.1324.
2-((5′-(1-chloroethyl)-1,2,4-oxadiazol-3′-yl)methyl)-14-(4-methylphenyl)-14H-naphto [2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine 7b
Creamy white solid, Yield: 87%; m.p.: 204 °C; 1H NMR (CDCl3, 300 MHz): δ 9.04 (s, 1H, H5), 6.99–7.97 (m, 10H, Harom), 6.33 (s, 1H, H14), 5.20 (q, 1H, CH, J = 6.9 Hz), 4.49 (s, 2H, -CH2-), 2.16 (s, 3H, -CH3), 1.98 (d, 3H, -CH3, J = 6.9 Hz); 13C NMR (CDCl3, 75 MHz): δ 178.0, 166.8, 164.5, 154.3, 153.8, 148.3, 139.5, 138.1, 137.2, 131.7, 130.8, 130.0, 129.4, 128.7, 128.2, 127.5, 125.3, 123.4, 117.5, 114.8, 103.5, 45.1, 37.1, 26.2, 21.8,20.9. ESI-HRMS [M + H]+ calcd. for (C28H22ClN6O2)+: 509.1493, found: 509.1482.
2-((5′-(1-chloroethyl)-1,2,4-oxadiazol-3′-yl)methyl)-14-(4-methoxyphenyl)-14H-naphto [2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine 7c
Creamy white solid, Yield: 79%; m.p.: 162 °C; 1H NMR (CDCl3, 300 MHz): δ 9.05 (s, 1H, H5), 6.71–7.97 (m, 10H, Harom), 6.33 (s, 1H, H14), 5.20 (q, 1H, CH, J = 6.9 Hz), 4.50 (s, 2H, -CH2-), 3.68 (s, 3H, -OCH3), 1.99 (d, 3H, -CH3, J = 6.9 Hz); 13C NMR (CDCl3, 75 MHz): δ 178.1, 166.9, 164.3, 158.6, 153.9, 153.4, 148.5, 138.3, 138.0, 134.7, 131.6, 130.8, 129.8, 129.3, 128.8, 128.6, 127.3, 125.2, 123.5, 117.5, 115.1, 114.0, 103.8, 55.1, 45.6, 36.7, 26.6, 22.4. ESI-HRMS [M + H]+ calcd. for (C28H22ClN6O3)+: 525.1442, found: 525.1430.
2-((5′-(1-chloroethyl)-1,2,4-oxadiazol-3′-yl)methyl)-14-(4-chlorophenyl)-14H-naphto [2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine 7d
Creamy white solid, Yield: 88%; m.p.: 230 °C; 1H NMR (CDCl3, 300 MHz): δ 9.05 (s, 1H, H5), 7.14–7.91 (m, 10H, Harom), 6.33 (s, 1H, H14), 5.20 (q, 1H, CH, J = 6.9 Hz), 4.50 (s, 2H, -CH2-), 2.00 (d, 3H, -CH3, J = 6.9 Hz); 13C NMR (CDCl3, 75 MHz): δ 178.1, 167.0, 164.5, 153.8, 153.4, 148.6, 140.8, 138.3, 133.0, 131.6, 130.7, 130.1, 129.7, 128.8, 128.7, 127.5, 125.3, 123.3, 117.5, 114.2, 103.0, 45.6, 37.0, 26.7, 22.4. ESI-HRMS [M + H]+ calcd. for (C27H19Cl2N6O2)+: 529.0947, found: 529.0948.
2-((5′-(1-chloroethyl)-1,2,4-oxadiazol-3′-yl)methyl)-14-(4-ethylphenyl)-14H-naphto[2,1-b]pyrano[3,2-e][1,2,4]triazolo [1,5-c]pyrimidine 7e
Creamy white solid, Yield: 89%; m.p.: 140 °C; 1H NMR (CDCl3, 300 MHz): δ 9.04 (s, 1H, H5), 7.10–7.99 (m, 10H, Harom), 6.37 (s, 1H, H14), 5.20 (q, 1H, CH, J = 6.9 Hz), 4.50 (s, 2H, -CH2-), 2.50 (q, 2H, -CH2-CH3, J = 8.7 Hz), 1.99 (d, 3H, -CH3, J = 6.9 Hz), 1.12 (t, 3H, -CH3, J = 8.7 Hz); 13C NMR (CDCl3, 75 MHz): δ 178.1, 166.9, 164.3, 154.1, 153.4, 148.6, 143.1, 139.7, 138.0, 131.6, 130.9, 129.7, 128.6, 128.2, 128.1, 127.3, 125.1, 123.5, 117.5, 115.2, 103.8, 45.6, 37.1, 28.2, 26.6, 22.4, 15.0. ESI-HRMS [M + H]+ calcd. for (C29H24ClN6O2)+: 523.1649, found: 523.1639.
Biological evaluation
Xanthine oxidase inhibition
A solution of 50 μL of each compound, 60 μL of 0.1 mM phosphate buffer (pH = 7.5) and 30 μL of enzyme (xanthine oxidase from bovine milk) solution (0.1 u/ml) prepared from phosphate buffer (pH = 7.5) were prepared before useCitation29. The mixture was added in a 96-well microplate and incubated at 25 °C for 15 min, then 60 μL of substrate solution (150 mM xanthine in the same buffer). The assay mixture was incubated at 25 °C for 30 min and the absorbance was determined at 290 nm. A blank was prepared in the same manner. Result is expressed as the percentage inhibition of xanthine oxidase in the above system, calculated as (1-B/A) × 100, where A and B are the activities of the enzyme without and with test material. The tested compounds were dissolved initially in DMSO, followed by dilution with the buffer; the final concentration of DMSO was less than 0.5%. Allopurinol was used as positive control.
Soybean lipoxygenase inhibition
The anti-soybean lipoxygenase activity of the compounds was determined as described by Bekir et al.Citation30 with some modifications. Various concentrations of 20 μL of each compound was mixed individually with sodium phosphate buffer (pH 7.4) containing soybean lipoxygenase and 60 μL of linoleic acid (3.5 mM). However, the blank does not contain the substrate but will be added 30 μL of buffer solution. All compounds were re-suspended in the DMSO followed by dilution in the buffer so that the DMSO does not exceed 1%. The mixture was incubated at 25 °C for 10 min, and the absorbance was determined at 234 nm. The absorption change with the conversion of linoleic acid to 13-hydroperoxyoctadeca-9, 11-dienoate (characterized by the appearance of the conjugated diene at 234 nm) was flowed for 10 min at 25 °C. Nordihydroguaiaretic acid (NDGA) was used as positive control. The percentage of enzyme activity was plotted against concentration of each compound.
Cytotoxic activity
The human cancer cell lines (HCT-116, MCF-7, IGROV-1, and OVCAR-3) were used for cytotoxic assayCitation30. The cells were grown in RPMI-1640 medium supplemented with 10% faetal calf serum (Gibco, Grand Island, NY), air and 5% CO2. The tested compound was added to a medium containing 1 × 106 cells/ml, L-glutamine (2 mM) and gentamycin (50 μg/ml), and kept at 37 °C in a fully humidified atmosphere. After 18 h of incubation at 37 °C in 5% CO2 incubator, the tubes were centrifuged at 8000 g for 10 min. The supernatant was decanted, and the pellets were taken and washed with 20 mM of phosphate buffered saline solution. Each pellet was dissolved in 100 μL (2 mg/ml) MTT solution in a tube, incubated at 22 °C for 4 h and centrifuged at 8000 g for 10 min. All the pellets were dissolved in 500 μL DMSO and read spectrophotometrically at 500 nm. Doxorubicin (HCT116 and MCF-7) and tamoxifen (IGROV-1 and OVCAR-3) were used as positive control.
Results and discussion
Synthesis
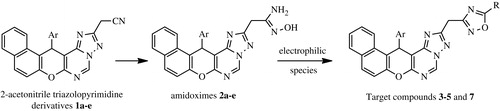
In this work, we prepared naphtopyranotriazolopyrimidine amidoximes 2a–e using cyano compounds 1a–e which was synthesized in three steps from the key intermediates 2-amino-naphto[2,1-b]pyrane-3-carbonitriles according to some previous worksCitation22,Citation23. Initially, we carried out the reaction of 2-acetonitrilestriazolopyrimidine derivatives 1a–e with hydroxylamine hydrochloride as a model reaction, in various solvents (ethanol, methanol, and dioxane) and bases (piperidine, triethylamine, and sodium carbonate)Citation31. The progress of the reaction was monitored by TLC and the best yields were obtained when compounds 1a–e, hydroxylamine hydrochloride, and aqueous solution of Na2CO3 were heated under reflux of dioxane (Scheme 2).
The formed amidoximes 2a–e were characterized by their 1H NMR and 13C NMR spectra. In fact, the 1H NMR spectra of compounds 2a–e showed the presence of new signals at 5.52–5.59 ppm and 9.55–10.81 ppm relative to mobile protons -NH2 and -OH, respectively. The 13C NMR spectra of 2a–e showed the appearance of a new signal at 147.8–147.9 ppm due to amidoxime carbon and the disappearance of the signal relative to the -CN carbon.
On the other hand, amidoximes 2a–c and 2e were treated with ethylorthoformate to afford the 1,2,4-oxadiazoles 3a–c and 3eCitation32. The reaction was conducted until TLC indicated that the starting materials have been completely converted. The analytical and spectral data are in good agreement with the proposed structures. The 1H NMR spectra of compounds 3a–c and 3e displayed a singlet in the region 8.75-8.76 ppm corresponding to H5′ of the oxadiazole ring, also showed the disappearance of signals related to mobile protons -OH and -NH2 (of compounds 2) (Scheme 2). Treatment of amidoximes 2a–e with acetic anhydride under reflux of toluene gave the 5-methyl-1,2,4-oxadiazoles 4a–e. Analog reaction of compounds 2a–e with an excess of methyl acetoacetate gave the corresponding acetonyl compounds 5a–e. The 1H NMR spectra of compounds 4a–e and 5a–e showed the disappearance of signals relative to mobile protons -NH2 and -OH, the presence of new signals at 2.59–2.63 ppm (s, 3H, CH3) (for compounds 4), 2.22–2.33 ppm (s, 3H, -CO-CH3) and 3.98–4.09 ppm (s, 2H, -CH2-CO-) (for compounds 5), while the 13C NMR spectra of compounds 4a–e showed new signals at 11.8–12.3 ppm and at 176.5–177.1 ppm relative to carbons -CH3 et C5′, respectively. Analysis of 13C NMR spectra of compounds 5a–e showed the appearance of signals at 26.1–26.7, 41.0–41.5, 173.0–173.5, and 197.9–198.5 ppm relative to carbons (COCH3), (-CH2CO), C5′, and (C=O), respectively. Reactions of amidoximes 2a–e with 2-chloropropanoyl chloride in the presence of Et3N resulted to O-acyl derivatives 6a–e. The 1H NMR spectra of compounds 6a–e showed, in addition to the signals relative to protons introduced by the 2-chloropropanoyl chloride, the presence of new signals at 1.70–1.80 ppm (d, 3H, J = 6.9 Hz) and 4.48-4.56 ppm (q, 1H, J = 6.9 Hz) relative to protons -CH3 and -CH, respectively and the disappearance of the signal related to the mobile proton -OH. The structures of compounds 6a–e has been also characterized by 1H and 13C NMR data. The exploration of 13C NMR spectra allowed to note the appearance of new signals at 21.1–21.6, 51.1–51.6, and 166.6–167.1 ppm attributed to carbons -CH3, -CH, and C=O, respectively. Heating of the toluene solution of compounds 6a–e resulted to the condensation products 7a–e as indicated from 1H NMR (no signal of -NH2 protons) and supported by ESI-HRMS (Scheme 2).
Biological activity
Most of the synthetic compounds were screened for the first time for their xanthine oxidase and soybean lipoxygenase inhibition and cytotoxic activity.
Xanthine oxidase inhibition
According to the results obtained (), most of the tested compounds have displayed weak anti-xanthine oxidase activity (4.4–25.4% inhibition) compared to allopurinol (81% inhibition at 100 μM) used as a reference compound, while the remaining compounds were inactive. Particularly, compounds 6 are all inactive at the used concentration (100 μM). Despite the presence of the triazolopyrimidine moiety in all tested molecules and the presence of the oxadiazole ring in compounds 3, 4, 5, and 7, no significant activity was obtained. This finding could be explained by the common naphtopyranyl system that may reduce the activity in this case and by the position of the oxadiazole ring in compounds 3, 4, 5, and 7, which probably was not in favor of this activity.
Table 1. Anti-xanthine oxidaseand anti-soybean lipoxygenase activities of compounds 2a–e, 3a–c, 6e, 4a, 4b, 4e, 5a–e, 6a, 6d, 6e, and 7a–e.
Soybean lipoxygenase inhibition
Soybean lipoxygenase catalyzes deoxygenation of polyunsaturated fatty acids to yield cis, trans-conjugated dienehydroperoxides. The results for soybean lipoxygenase inhibitory () revealed that the synthesized compounds showed variable degrees of in vitro percentage inhibition. In fact, the acetimidamide 6a was found to be the most active compound (71% inhibition at 100 μM). The compounds 3a, 3e, 4a, 4b, 4e, 5e, and 6d exhibited a moderate inhibitory activity (40.3–50.2% inhibition at 100 μM). The remaining compounds showed weak inhibitory effects.
This limited activity fails to understand the structure–activity relationship and consider unambiguously that the system attached at C-2 of the triazole ring alone manages the behavior of these compounds against soybean lipoxygenase. The diversification of fragments borne by the triazole ring in a larger series of derivatives could lead to a plausible conclusion.
Cytotoxic activity
Cytotoxic activity of the most synthesized products tested against the human cell lines HCT-116 (human colon carcinoma), MCF-7 (breast carcinoma), IGROV-1 (human ovarian carcinoma), and OVCAR-3 (human ovarian carcinoma) () was assessed using MTT assay, which is reliable to detect proliferation of cells. The cytotoxic effect of the compounds 2c, 2d, 3c, 3e, 4a, 4b, 4e, 6a, 7a, 7b, 7c, 7d, 7e was found limited against the four cell lines (IC50 ≥ 94 μM). The other compounds were active against at least one cell line.
Table 2. Cytotoxic activity of compounds 2a–e, 3a–c, 6e, 4a, 4b,4e, 5a–e, 6a, 6d, 6e, and 7a–e.
The results obtained showed that the coexistence of the amidoxime function at C-2 of the triazole and the unsubstituted phenyl at C-14 (compound 2a) of para-methylphenyl (compound 2b) and para-ethylphenyl (compound 2e) appears to the origin of the activity of these compounds against most cancer cell lines used. We find that the presence of the methoxy and chlorine both in the para position of the phenyl-C at C-14 are responsible for the loss of activity of the corresponding compounds (2c and 2d, respectively) against all cell lines used.
It appears from our findings that the conversion of the amidoxime function into unsubstituted oxadiazole in compounds 3 theoretically carrying a cytotoxic activityCitation16 and independently of the substituent in the para position on the aromatic ring attached at C-14 resulted in the loss of activity toward all cell lines used.
The presence of the methyl group at C-5′ in oxadiazoles 4a (R = H) and 4b (R = CH3) attenuated the cytotoxic activity compared to the corresponding derivatives 3a and 3b where the oxadiazole is unsubstituted. The activity of the compound 4e (R = C2H5) is held against the cell line OVCAR-3 compared to the third derivative (R = C2H5).
Unlike derivatives 3 and 4, the presence of the 2-oxopropyl group at C-5′ of the oxadiazole ring could explain the activity of compound 5a toward OVCAR-3 (IC50 = 28 ± 3 μM), the compounds 5a, 5b, 5c, and 5e against HCT-116 (IC50 = 31 ± 3, 34 ± 2, 38 ± 1, and 25 ± 2 μM, respectively) and 5a and 5e toward MCF-7. In the case of HCT-116 cell line, the 2-oxopropyl group in 5c (IC50 = 38 ± 1 μM) appears impose its effect on that of the methoxy group which appears to be one of responsible of the loss of activity of the analog 3c where the oxadiazole ring is unsubstituted.
In the case of compounds 6, the acylation of the hydroxyl group of the amidoxime function in compounds 2 with the 2-chloropropionic acid chloride did not improve the activity of compounds 2d (R = Cl) and 2e (R = C2H5) except against the OVCAR-3 cell line which the IC50 values passes from 10 ± 1 μM (2e) to 16 ± 2 μM (6e). The total inactivity of the derivative 6a compared to the starting amidoxime 2a against all the cell lines used shows the difficulty of explaining the structure–activity relationship, taking into account both the introduced acyl and variable R in para position of the aromatic ring at C-14. In addition, the limited number of derivatives 6 synthesized in this work seems insufficient to advance a plausible explanation.
In the case of compounds 7, the 1-chloroethyl group attached at C-5′ of the oxadiazole and independently of the nature of the R group attached in para position of the aromatic ring at C-14 seems to have no effect against all the cell lines used in comparison to the compounds 3 wherein the oxadizole ring is unsubstituted.
Our findings point out that the chlorine atom in para position of the phenyl at C-14 in compounds 2d, 5d, and 7d also appears responsible for the loss in cytotoxic activity of these compounds.
The compounds 2a, 2e, and 5a that were found to be the most cytotoxics deserve to be tested against other cancer cell lines and to be chemically modified by thinking to replace naphthalene used as a support by other systems that could play a dual role (support and activity carrier).
Conclusion
In this paper, we designed and synthesized 24 novel acetimidamides 6a–e and oxadiazoles 3a–c and 3e, 4a–e, 5a–e, and 7a–e. Their in vitro xanthine oxidase, soybean lipoxygenase inhibition and cytotoxic activities (against four cancer cell lines: HCT-116, MCF-7, IGROV-1, and OVCAR-3) were also evaluated. A moderate xanthine oxidase and soybean lipoxygenase inhibition were obtained but the good cytotoxic activities were recorded. Most compounds bearing a free or esterified amidoxime function exhibited an interesting cytotoxic effect, hence its contribution in this activity. On the other hand, it is well known from the literature that triazolopyrimidines and isoxazoles are among the systems responsible for the cytotoxic activity of several molecules but their coexistence in a single molecule in a determined sequence can eliminate or at least mitigate this activity. The significant cytotoxic activity of compounds 2a, 2e, and 5a may open new ways of research on the amidoxime function, the oxadiazole bearing a 2-oxopropyl fragment without neglecting the triazolopyrimidine moiety and the naphthalene which could be replaced by other systems.
Declaration of interest
The authors report no declarations of interest.
References
- Rajendran A, Raghupathy D, Priyadarshini M. Green synthesis of biologically active pyrazolopyrimidine derivatives using an ionic liquid 2-methyl-3-butyl imidazolium chloride. Int J Chem Tech Res 2011;3:293–7
- Yin L, Shuai Z, Zhi-Jun L, Hai-Liang Z. Synthesis and antimicrobical evaluation of a novel class of 1,3,4-thiadiazole: derivatives bearing 1,2,4-triazolo[1,5-a]pyrimidine moiety. Eur J Med Chem 2013;64:54–61
- Marwaha J, White F, El Mazouni SA, et al. Bioisosteric transformations and permutations in the triazolopyrimidine scaffold to identify the minimum pharmacophore required for inhibitory activity against Plasmodium falciparum dihydroorotate dehydrogenase. J Med Chem 2012;55:7425–36
- Chen Q, Zhu XL, Jiang LL, et al. Synthesis, antifungal activity and CoMFA analysis of novel 1,2,4-triazolo[1,5-a]pyrimidine derivatives. Eur J Med Chem 2008;43:595–603
- Uryu S, Tokuhiro S, Murasugi T. A novel compound, RS-1178, specifically inhibits neuronal cell death mediated by beta-amyloid-induced macrophage activation in vitro. Brain Res 2002;946:298–306
- Tomohisa N, Takayuki F, Kazuki EJ. Novel xanthine oxidase inhibitor studies. Part 3. Convenient and general syntheses of 3-substituted 7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H)-ones as a new class of potential xanthine oxidase inhibitors. Chem Soc Perkin Trans 2000;1:33–42
- Said SA, Amr AEE, Sabry NM, Abdalla MM. Analgesic, anticonvulsant and anti-inflammatory activities of some synthesized benzodiazipine, triazolopyrimidine and bis-imide derivatives. Eur J Med Chem 2009;44:4787–92
- Ghorab MM, Al-Dhfyan A, Al-Dosari MS, et al. Antiproliferative activity of novel thiophene and thienopyrimidine derivatives. Drug Res 2014;64:313–20
- Bokach NA, Khripoun AV, Kukushkin VY, et al. A route to 1,2,4-oxadiazoles and their complexes via platinum-mediated 1,3-dipolar cycloaddition of nitrile oxides to organonitriles. Inorg Chem 2003;42:896–993
- Rai NP, Narayanaswamy VK, Govender T, et al. Design, synthesis characterization, and antibacterial activity of {5-chloro-2-[(3-substitutedphenyl-1,2,4-oxadiazol-5-yl)-methoxy]-phenyl}-(phenyl)-methanones. Eur J Med Chem 2010;45:2677–82
- Kiss LE, Ferreira HS, Torrao L, et al. Discovery of a long-acting, peripherally selective inhibitor of catechol-O-methyltransferase. J Med Chem 2010;53:3396–410
- Terenzi G, Barone AP, Piccionello G, et al. Synthesis, characterization, cellular uptake and interaction with native DNA of a bis(pyridyl)-1,2,4-oxadiazole copper(II) complex. Dalton Trans 2010;39:9140–5
- Piccionello AP, Musumeci R, Cocuzza C, et al. Pace A. Synthesis and preliminary antibacterial evaluation of linezolid-like 1,2,4-oxadiazole derivatives. Eur J Med Chem 2012;50:441–8
- Ningaiah S, Bhadraiah UK, Keshavamurthy S. Novel pyrazoline amidoxime and their 1,2,4-oxadiazole analogues: synthesis and pharmacological screening. Bioorg Med Chem Lett 2013;23:4532–9
- Farooqui M, Bora R, Patil CR. Synthesis, analgesic and anti-inflammatory activities of novel 3-(4-acetamido-benzyl)-5-substituted-1,2,4-oxadiazoles. Eur J Med Chem 2009;44:794–9
- Barros CJP, De Souza CZ, De Fritas JJRDA, et al. A convenient synthesis and cytotoxic activity of 3-aryl-5-phenyl-1,2,4-oxadiazoles from carboxylic acid esters andarylamidoximes under solvent-free conditions. J Chil Chem 2014;59:2359–62
- El-Agrody AM, El-Hakim MH, Abd El-Latif MS, et al. Synthesis pyrano[2,3-d]pyrimidine and pyrano[3,2-e][1,2,4]triazolo[2,3-c]pyrimidine derivatives with promising antibacterial activities. Acta Pharmaceut 2000;2:111–20
- Bedair AH, Emam HA, El-Hady NA, et al. Synthesis and antimicrobial activities of novel naphtho[2,1-b]pyran, pyrano[2,3-d]pyrimidine and pyrano[3,2-e][1,2,4]triazolo[2,3-c]-pyrimidine derivatives. Farmaco 2001;56:965–73
- Eid FA, Abdel-Wahab AHF, Al-Hag Ali GAM, Khalagy MM. Synthesis and antimicrobial evaluation of naphtho[2,1-b]pyrano[2,3-d]pyrimidine and pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives. Acta Pharmaceut 2004;54:13–26
- Mehdi F, Hedi CM, Leila BM, Mansour CGS. Synthesis and antigenotoxic activity of some naphtho[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives. Eur J Med Chem 2007;42:715–18
- Abd El-Wahab AHF. Synthesis, reactions and evaluation of the antimicrobial activity of some 4-(p-Halophenyl)-4H-naphthopyran, pyranopyrimidine and pyranotriazolopyrimidine derivatives. Pharmaceut 2012;5:745–57
- Romdhane A, Gallard JF, Hamza MA, Jannet HB. Synthesis of new phosphonate derivatives of naphto[2,1-b]pyran[3,2-e][1,2,4]triazolo[1,5-c]pyrimidines. Phosphorus Sulfur Silicon Relat Elem 2012;187:612–18
- Ben said B, Romdhane A, Elie N, et al. Synthesis of novel fused coumarine and naphtho[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives. Lett Org Chem 2013;10:185–90
- Degardin M, Wein S, Durand T, et al. N-substituted bis-C-alkyloxadiazolones as dual effectors: efficient intermediates to amidoximes or amidines and prodrug candidates of potent antimalarials. Bioorg Med Chem Lett 2009;19:5233–6
- Bouhlel A, Curti C, Dumètre A, et al. Synthesis and evaluation of original amidoximes as antileishmanial agents. Bioorg Med Chem 2010;18:7310–20
- Gosenca M, Marvljak J, Gasperlin M, Obreza A. The design, synthesis, and antioxidant activity of amphiphilic oximes and amidoximes. Acta Chim Slov 2013;60:310–22
- Doulou I, Kontogiorgis C, Koumbis AE, et al. Synthesis of stable aromatic and heteroaromatic sulfonyl-amidoximes and evaluation of their antioxidant and lipid peroxidation activity. Eur J Med Chem 2014;80:145–53
- Kalvinsh I, Abele R, Golomba L, et al. Synthesis and cytotoxicity of N-hydroxy–ω-(hetarylmethoxy or hetarylthio)-alkanamidines. Heterocycl Lett 2011;1:47–54
- Chen CH, Chan HC, Chu YT, et al. Antioxidant activity of some plant extracts towards xanthine oxidase, lipoxygenase and tyrosinase. Molecules 2009;14:2947–58
- Bekir J, Mars M, Souchard JP, Bouajila J. Assessment of anti-oxidant, anti-inflammatory, anti-cholinesterase and cytotoxic activities of pomegranate (Punicagranatum) leaves. Food Chem Toxicol 2013;55:470–5
- Oresmaa L, Kotikoski H, Haukka M, et al. Synthesis, ocular effects, and nitric oxide donation of imidazole amidoximes. Eur J Med Chem 2006;41:1073–9
- Nicolaides DN, Fylaktakidou KC, Litinas KE, Hadjipavlou-litina D. Synthesis and biological evaluation of several coumarin-4-carboxamidoxime and 3-(coumarin-4-yl)-1,2,4-oxadiazole derivatives. Eur J Med Chem 1998;33:715–24