Abstract
Fourteen novel 4-aminoquinazoline derivatives 2–15 were designed and synthesized. The structure of the newly synthesized compounds was established on the basis of elemental analyses, IR, 1H-NMR, 13C-NMR, and mass spectral data. The compounds were evaluated for their potential cytoprotective activity in murine Hepa1c1c7 cells. All of the synthesized compounds showed concentration-dependent ability to induce the cytoprotective enzyme NAD(P)H: quinone oxidoreductase (NQO1) with potencies in the low- to sub-micromolar range. This approach offers an encouraging framework which may lead to the discovery of potent cytoprotective agents.
Introduction
Quinazolines are classes of fused heterocycles that are of considerable interest because of the diverse range of their biological properties, they play a vital role in synthetic drugs and biological processesCitation1–5. 4-Aminoquinazoline represents an important class of drugs having well-known inhibitory activity to the epidermal growth factor receptor (EGFR)Citation6,Citation7. A considerable amount of experimental studies have been carried out with 4-aminoquinazoline derivatives which are potent and highly selective inhibitors of epidermal growth factor (EGFR) phosphorylation at the ATP-binding site. These compounds cause inhibition of EGFR produced by abnormal signal transduction via hyperactivation of tyrosine protein kinases due to overexpression or mutation, thus leading to anticancer activities against human lung cancer, breast cancer, squamous head, and neck carcinomasCitation8.
Oxidative stress causes damage to multiple cellular components such as DNA, proteins, and lipids; and it is implicated in various human diseases including cancer, neurodegeneration, inflammatory diseases, and aging. In response to oxidative attack, cells have developed an antioxidant defense system both to maintain cellular redox homeostasis and to protect cells from damage. The thiol-containing small molecules (e.g. glutathione), reactive oxygen species-inactivating enzymes (e.g. glutathione peroxidase), and phase 2 detoxifying enzymes (e.g. NAD(P)H: quinone oxidoreductase 1 and glutathione-S-transferases) are members of this antioxidant systemCitation9. The transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) is a key oxidative stress response modifier that induces transcription of a variety of genes through binding to the antioxidant response element (ARE) in target gene promotersCitation10–12. Nrf2-dependent activation of ARE-driven gene promoters is generally understood to lead to induction of cytoprotective proteins, which enable cells to combat oxidative insultCitation11,Citation12. NAD(P)H: quinone oxidoreductase (NQO1), a cytosolic flavoprotein that catalyzes quinone detoxification, is induced in response to various types of endogenous and exogenous electrophiles at the transcriptional level. The NQO1 enzyme is implicated in protection against oxidative stressCitation9,Citation13 and carcinogenesis. The NQO1 gene promoter region contains several essential cis-elements including xenobiotic response element (XRE) and ARECitation14. Induction of NQO1 expression by various stimuli has been reported to be regulated by ARECitation15–17, which contains several receptors for several nuclear transcription factors such as Nrf2.
Keap1 reacts rapidly with electrophilic enones; certain monocyclic, tricyclic, and pentacyclic enones represent the most potent NQO1 inducers known to date both in cells and in animalsCitation18–20. Consequent to its modification by electrophiles, the substrate adaptor activity of Keap1 is impaired, Nrf2 is stabilized, and the expression of its target genes and related proteins, exemplified by NQO1, is enhanced. Such Nrf2-dependent induction has been shown to be protective in numerous animal models of chronic disease, including cancer, cardiovascular disease, and neurodegenerative conditionsCitation21. In our continuous efforts to provide biologically active heterocyclesCitation22–26, in this work we designed novel quinazoline derivatives endowed with improved electron affinity for better biological interactions by substituents of various mono- and bicyclic-fused heterocyclic ring systems which were introduced into the C-4 position of quinazoline ring to generate compounds 2–15 to study their possible role in inducing the cytoprotective enzyme NQO1.
Materials and methods
Chemistry
Melting points (uncorrected) were determined in open capillary on a Gallen Kamp melting point apparatus (Sanyo, Gallen Kamp, Southborough, UK). Precoated silica gel plates (Kieselgel 0.25 mm, 60 F254, Merck, Darmstadt, Germany) were used for thin layer chromatography. A developing solvent system of chloroform/methanol (8:2) was used, and the spots were detected by ultraviolet light. IR spectra (KBr disc) were recorded using an FT–IR spectrophotometer (Perkin Elmer, Norwalk, CT). 1H-NMR spectra were scanned on a NMR spectrophotometer (Bruker AXS Inc., Flawil, Switzerland), operating at 500 MHz for 1H-NMR and 125.76 MHz for 13C-NMR. Chemical shifts are expressed in δ-values (ppm) relative to TMS as an internal standard, using DMSO-d6 as a solvent. Elemental analyses were done on a model 2400 CHNSO analyzer (Perkin Elmer, Norwalk, CT). All the values were within ± 0.4% of the theoretical values. All reagents used were of AR grads. The starting material 4-chloro-2-phenylquinazoline 1 was purchased from sigma (St. Louis, MO) and was directly used for the preparation of target compounds.
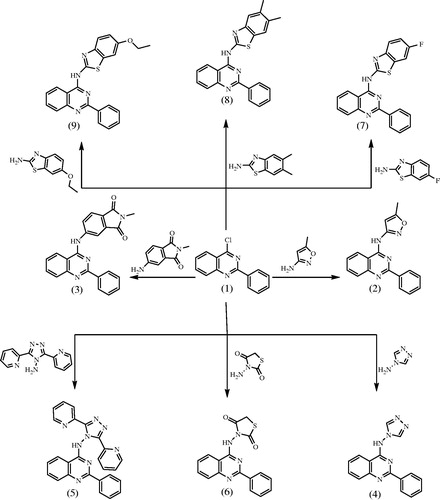
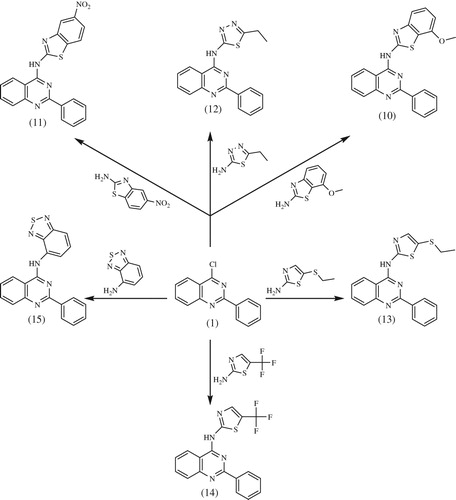
Synthesis of 2-phenyl-4-substitutedquinazoline derivatives (2–15)
General procedure
A mixture of 1 (2.40 g, 0.01 mol) and different amines (0.012 mol) in dry dimethylformamide (15 mL) containing triethylamine three drops was refluxed for 24 h, then left to cool. The solid product formed was collected by filtration and recrystallized from dioxane to give 2–15, respectively.
5-Methyl-N-(2-phenylquinazolin-4-yl)isoxazol-3-amine (2)
Yield, 91%; m.p. = 208.1 °C. IR (KBr, cm−1): 3422 (NH), 3060 (CH arom.), 2975, 2836 (CH aliph.), 1618 (C=N). 1H-NMR (DMSO-d6): 2.4 [s, 3H, CH3], 7.4–8.3 [m, 10H, Ar-H], 12.5 [s, 1H, NH exchangeable with D2O].13C-NMR (DMSO-d6): 12.0, 101.1, 114.8, 124.8, 126.5 (2), 127.9, 128.2, 128.4 (2), 129.0, 130.5, 134.0, 149.2, 152.7, 158.3. 162.7, 163.3. MS m/z (%): 302 (M+) (18.45), 220 (100). Anal. Calcd. For C18H14N4O(302): C, 71.51; H, 4.67; N, 18.53. Found: C, 71.22; H, 4.97; N, 18.88.
2-Methyl-4-(2-phenylquinazolin-4-ylamino)isoindoline-1,3-done (3)
Yield, 88%; m.p. = 218.0 °C. IR (KBr, cm−1): 3398 (NH), 3061 (CH arom.), 2956, 2855 (CH aliph.), 1758, 1670 (2C=O), 1602 (C=N). 1H-NMR (DMSO-d2): 3.05 [s, 3H, N-CH3], 7.4–8.7 [m, 12H, Ar-H], 12.5 [s, 1H, NH exchangeable with D2O]. 13C-NMR (DMSO-d6): 24.1, 114.5, 114.8, 115.5, 121.4, 123.8, 124.8, 125.3 (2), 126.8, 127.9 (2), 129.0, 130.5, 131.8, 133.2, 135.0, 145.5, 149.2, 152.7, 158.3, 168.4 (2). MS m/z (%): 380 (M+) (22.43), 294 (100). Anal. Calcd. For C23H16N4O2(380.40): C, 72.62; H, 4.24; N, 14.73. Found: C, 72.32; H, 4.54; N, 14.45.
2-Phenyl-N-(4H-1,2,4-triazol-4-yl)quinazolin-4-amine (4)
Yield, 92%; m.p. 313.4 °C. IR (KBr, cm−1): 3452 (NH), 3094 (CH arom.), 1605 (C=N). 1H-NMR (DMSO-d6): 7.6–8.9 [m, 11H, Ar-H], 12.3 [s, 1H, NH exchangeable with D2O]. Citation13C-NMR (DMSO-d6): 117.6, 123.7, 128.7 (2), 128.9, 129.2, 130.6 (2), 131.9, 132.0, 132.9, 142.6 (2), 153.1, 154.1 (2). MS m/z (%): 288 (M+) (16.45), 204 (100). Anal. Calcd. For C16H12N6 (288): C, 66.66; H, 4.20; N, 29.15. Found: C, 66.39; H, 3.92; N, 28.81.
N-(3,5-di(pyridine-2-yl)-4H-1,2,4-triazol-4-yl)-2-phenylquinqzolin-4-amine (5)
Yield, 84%; m.p. 225.2 °C. IR (KBr, cm−1): 3413 (NH), 3059 (CH arom.), 1617 (C=N). 1H-NMR (DMSO-d6): 7.3–8.5 [m, 17H, Ar-H], 12.6 [s, 1H, NH exchangeable with D2O]. Citation13C-NMR (DMSO-d6): 114.8, 121.4 (2), 123.7 (2), 124.8, 126.3 (2), 126.5, 127.0, 128.2 (2), 129.5, 130.5, 133.8, 135.0 (2), 149.2 (2), 149.9, 152.7 (2), 158.3 (2), 162.7, 163.3. MS m/z (%): 442 (M+) (27.35), 363 (100). Anal. Calcd. For C26H18N8 (442): C, 70.58; H, 4.10; N, 25.32. Found: C, 70.28; H, 3.83; N, 25.66.
3-(2-Phenylquinqzolin-4-ylamino)thiazolidine-2,4-dione (6)
Yield, 87%; m.p. 236.1 °C. IR (KBr, cm−1): 3167 (NH), 3058 (CH arom.), 1668 (C=O), 1601 (C=N), 1240 (C=S). 1H-NMR (DMSO-d6): 4.3 [s, 2H, CH2], 7.4–8.2 [m, 9H, Ar-H], 12.5 [s, 1H, NH exchangeable with D2O]. 13C-NMR (DMSO-d6): 36.2, 121.4, 126.3, 127.0, 127.9, 128.2, 128.6, 129.0 (2), 131.8, 133.1, 135.0, 149.2, 152.7 (2), 162.7 (2). MS m/z (%): 336 (M+) (19.34), 187 (100). Anal. Calcd. For C17H12N4O2S (336): C, 60.70; H, 3.60; N, 16.66. Found: C, 60.34; H, 3.92; N, 16.16.37.
6-Fluoro-N-(2-phenylquinazolin-4-yl)benzo[d]thiazol-2-amine (7)
Yield, 79%; m.p. 200.6 °C. IR (KBr, cm−1): 3170 (NH), 3100 (CH arom.), 1602 (C=N). 1H-NMR (DMSO-d6): 7.2–9.0 [m, 12H, Ar-H], 12.6 [s, 1H, NH exchangeable with D2O]. 13C-NMR (DMSO-d6): 108.5, 114.7, 114.9, 121.3, 123.3, 126.3 (2), 127.8, 128.2, 129.3 (2), 131.8, 133.1, 133.3, 135.6, 145.5, 149.0, 156.6, 160.1, 161.1, 162.7. MS m/z (%): 372 (M+) (14.08), 205 (100). Anal. Calcd. For C21H13FN4S (372): C, 67.73; H, 3.52; N, 15.04. Found: C, 67.47; H, 3.26; N, 16.32.
5,6-Dimethyl-N-(2-phenylquinazolin-4-yl)benzo[d]thiazol-2-amine (8)
Yield, 93%; m.p. 203.9 °C. IR (KBr, cm−1): 3163 (NH), 3069 (CH arom.), 2978, 2841 (CH aliph.), 1624 (C=N). 1H-NMR (DMSO-d6): 2.2, 2.3 [2s, 6H, 2CH3], 7.1–9.0 [m, 11H, Ar-H], 12.5 [s, 1H, NH exchangeable with D2O]. 13C-NMR (DMSO-d6): 19.7, 20.0, 121.5, 121.9, 122.2, 126.3, 127.8 (2), 128.2, 129.0 (2), 129.2 (2), 129.3 (2), 131.8, 133.1, 135.0, 147.4, 155.7 (2), 160.7 (2). MS m/z (%): 382 (M+) (6.54), 78 (100). Anal. Calcd. For C23H18N4S (382): C, 72.22; H, 4.74; N, 14.65. Found: C, 72.49; H, 4.36; N, 14.29.
6-Ethoxy-N-(2-phenylquinazolin-4-yl) benzo[d]thiazol-2-amine (9)
Yield, 86%; m.p. 214.7 °C. IR (KBr, cm−1): 3213 (NH), 3068 (CH arom.), 2954, 2876, (CH aliph.), 1618 (C=N). 1H-NMR (DMSO-d6): 1.3 [t, 3H, CH3], 4.1 [q, 2H, CH2], 7.0–9.0 [m, 12H, Ar-H], 12.1 [s, 1H, NH exchangeable with D2O]. 13C-NMR (DMSO-d6): 15.0, 64.2, 108.8, 112.3, 115.3, 115.6, 126.3, 127.1 (2), 127.5, 128.2, 129.3 (2), 129.8, 131.8, 132.9, 133.8, 141.7, 152.9, 154.8, 161.3, 162.7, 168.1. MS m/z (%): 398 (M+) (10.68), 352 (100). Anal. Calcd. For C23H18N4OS (398): C, 69.32; H, 4.55; N, 14.06. Found: C, 69.04; H, 4.26; N, 14.33.
4-Methoxy-N-(2-phenylquinazolin-4-yl)benzo[d]thiazol-2-amine (10)
Yield, 83%; m.p. 193.3 °C. IR (KBr, cm−1): 3413 (NH), 3063 (CH arom.), 2900, 2846 (CH aliph.), 1603 (C=N). 1H-NMR (DMSO-d6): 3.9 [s, 3H, OCH3], 7.0–8.5 [m, 12H, Ar-H], 12.5 [s, 1H, NH exchangeable with D2O]. 13C-NMR (DMSO-d6): 56.1, 108.1, 113.7, 114.8, 121.4, 124.7, 126.3 (2), 127.0, 128.2, 128.4 (2), 129.5, 130.5, 131.8, 133.4, 142.5, 149.2, 150.3, 158.3, 163.2, 165.7. MS m/z (%): 384 (M+) (4.65), 307 (100). Anal. Calcd. For C22H16N4OS (384): C, 68.73; H, 4.19; N, 14.57. Found: C, 68.97; H, 4.54; N, 14.28.
6-Nitro-N-(2-phenylquinazolin-4-yl)benzo[d]thiazol-2-amine (11)
Yield, 89%; m.p. 204.2 °C. IR (KBr, cm−1): 3401(NH), 3089 (CH arom.), 1598 (C=N). 1H-NMR (DMSO-d6): 7.2–8.6 [m, 12H, Ar-H], 12.8 [s, 1H, NH exchangeable with D2O].13C-NMR (DMSO-d6): 115.3, 116.1, 118.7, 119.9, 124.3, 125.6 (2), 126.8, 127.3, 128.6 (2), 129.8, 130.6, 131.4, 133.0, 142.6, 151.2, 161.3, 161.9, 163.7, 169.6. MS m/z (%): 399 (M+) (13.81), 321 (100). Anal. Calcd. For C21H13N5O2S (399): C, 63.15; H, 3.28; N, 17.53. Found: C, 63.44; H, 2.97; N, 17.21.
5-Ethyl-N-(2-phenylquinazolin-4-yl)-1,3,4-thiadiazol-2-amine (12)
Yield, 77%; m.p. 229.9 °C. IR (KBr, cm−1): 3432 (NH), 3087 (CH arom.), 2979, 2779 (CH aliph.), 1619 (C=N). 1H-NMR (DMSO-d6): 1.2 [t, 3H, CH3], 3.0 [q, 2H, CH2], 7.5–8.0 [m, 9H, Ar-H], 12.8 [s, 1H, NH exchangeable with D2O]. 13C-NMR (DMSO-d6): 13.6, 23.5, 112.2, 123.7, 126.9, 127.4, 127.7, 128.2, 129.3 (2), 129.7, 131.8, 133.6, 148.9, 152.0, 163.4, 165.2, 169.0. MS m/z (%): 333 (M+) (14.01), 218 (100). Anal. Calcd. For C18H15N5S (333): C, 64.84; H, 4.53; N, 21.01. Found: C, 64.48; H, 4.82; N, 21.33.
5-(ethylthio)-N-(2-phenylquinazolin-4-yl)-1,3,4-thiadiazol-2-amine (13)
Yield, 82%; m.p. 199.8 °C. IR (KBr, cm−1): 3176 (NH), 3076 (CH arom.), 2976, 2839 (CH aliph.), 1618 (C=N). 1H-NMR (DMSO-d6): 1.2 [t, 3H, CH3], 3.2 [q, 2H, CH2], 7.2–9.1 [m, 9H, Ar-H], 7.5 [s, 1H, CH thiazole], 12.5 [s, 1H, NH exchangeable with D2O]. 13C-NMR (DMSO-d6): 15.1, 29.2, 121.3, 123.7, 126.1 (2), 127.6, 127.9, 128.8 (2), 130.5, 131.8, 133.1, 150.4, 152.8, 162.7, 165.4, 170.0. MS m/z (%): 365 (M+) (18.63), 204 (100). Anal. Calcd. For C18H15N5S2 (365): C, 59.15; H, 4.14; N, 19.16. Found: C, 59.47; H, 4.51; N, 19.45.
N-(2-phenylquinazolin-4-yl)-5-(trifluoromethyl)-1,3,4-thiadiazol-2-amine (14)
Yield, 81%; m.p. 192.3 °C. IR (KBr, cm−1): 3231 (NH), 3098 (CH arom.), 1610 (C=N). 1H-NMR (DMSO-d6): 7.3–8.6 [m, 9H, Ar-H], 6.8 [s, 1H, CH thiazole], 12.6 [s, 1H, NH exchangeable with D2O]. 13C-NMR (DMSO-d6): 114.6, 121.4, 124.8, 125.3 (2), 126.5, 127.0, 128.2 (2), 130.6, 131.8, 133.1, 149.2, 152.7, 158.2, 162.7, 163.1. MS m/z (%): 373 (M+) (5.61), 303 (100). Anal. Calcd. For C17H10F3N5S (373): C, 54.69; H, 2.70; N, 18.76. Found: C, 54.33; H, 2.44; N, 18.38.
N-(2-phenylquinazolin-4-yl)benzo[c][1,2,5]thiadiazol-4-amine (15)
Yield, 85%; m.p. 192.8 °C. IR (KBr, cm−1): 3411 (NH), 3059 (CH arom.), 1619 (C=N). 1H-NMR (DMSO-d6): 7.4–8.5 [m, 12H, Ar-H], 12.3 [s, 1H, NH exchangeable with D2O]. 13C-NMR (DMSO-d6): 114.4, 117.5, 121.8, 123.4, 126.5 (2), 127.0, 128.3, 128.8 (2), 129.0, 130.7, 131.6, 132.5, 135.6, 139.4, 149.2, 158.7, 159.4, 162.8. MS m/z (%): 355 (M+) (6.34), 219 (100). Anal. Calcd. For C20H13N5S (355): C, 67.59; H, 3.69; N, 19.70. Found: C, 67.22; H, 3.33; N, 19.34.
Biological activity evaluation
The NQO1 inducer activity was determined using a quantitative microtiter plate assay in Hepa1c1c7 murine hepatoma cells as described previouslyCitation27. The results are plotted as the ratio of the specific enzyme activity in cell lysates prepared from treated over control wells. The CD value (Concentration of a compound required to Double the specific enzyme activity of NQO1) was used as a measure of inducer potency. The classical NQO1 inducer sulforaphane (SF) was included in each assay as a positive control.
Results and discussion
Chemistry
4-Chloro-2-phenyl quinazoline was used as excellent precursor for the synthesis of novel 4-aminoquinazoline derivatives by incorporating different monocyclic or fused bicyclic biologically active ring systems. Since development of facile preparative methods for title compounds is one of our main objectives, we herein report the synthesis of a novel series of 4-aminoquinazoline derivatives through simple and convenient method, the synthesis of target compounds is outlined in Schemes 1 and 2. 2-Phenyl-4-substituted quinazoline derivatives (2–15) were prepared via substitution of 4-chloro group in the starting (1) with different heterocyclic amines. The reactions proceeded using dimethylformamide as solvent in the presence of catalytic amount of trimethylamine. All structures of newly synthesized compounds were supported by physical, micro analytical, and spectral data. The structures of all the synthesized compounds were determined by IR, 1H-NMR, 13C-NMR, and mass spectral, and were in conformity with the assigned structures along with the elemental analyses which were found within the limit of 0.4% of theoretical values for all the synthesized compounds.
Biological activity
All of the synthesized compounds showed a concentration-dependent ability to induce NQO1 with high magnitude (up to 13-fold) and comparable potencies in the low- to sub-micromolar range ( and ).
Figure 1. Dose-response curves for NQO1 inducer activity of novel aminoquinazoline derivatives. Data are expressed as the ratio of treated/control (T/C) values.
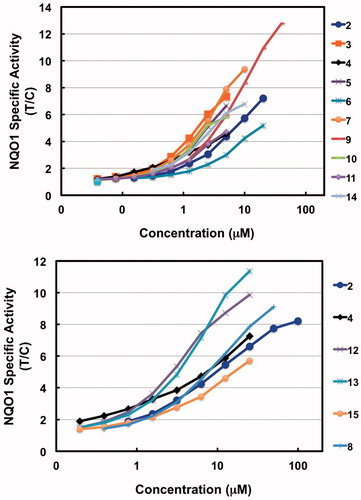
Table 1. NQO1 inducer potency of novel aminoquinazoline derivatives.
Conclusion
We have designed a series of 4-aminoquinazoline derivatives and analyzed their cytoprotective activities. All of the synthesized compounds showed concentration-dependent ability to induce NQO1 robustly with potencies in the low- to sub-micromolar range.
Declaration of interest
The authors declare that they have no conflict of interest.
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research through the Research Group Project no. RGP-VPP-302. Maureen Higgins and Albena T. Dinkova-Kostova are grateful to Cancer Research, UK, (C20953/A18644) for financial support.
References
- Kiruthiga B, Ilango K, Valentina P, Umarani N. Ritesh. Synthesis of some new 2-substituted quinazolin-4-one derivatives and their biological activities. Int J Pharm Tech Res 2009;1:1503–6.
- Rani P, Archana S, Srivastava VK, Kumar A. Synthesis and anti-inflammatory activity of some new 2,3-disubstituted-6-monosubstituted-quinazolin-4(3H)-ones. Ind J Heterocycl Chem 2002;41B:2642–6.
- Pandya T, Chaturvedi SC. QSAR study of a series of 2, 3, 6-substituted quinazolinones as AT1 selective angiotensin II receptor antagonists. Ind J Chem 2004;43B:2440–5.
- Chandra T, Garg N, Kumar A. Synthesis of sulpha drug quinazolin-4-one derivatives and their evaluation for anti-inflammatory activity. World J Chem 2009;4:210–18.
- Meyyanathan SN, Murali KE, Chandrashekhar HR, et al. Synthesis of some amino acids incorporated4(3H)-quinazolinones as possible antiherpes viral agents. Ind Drugs 2006;43:497–502.
- Michel J, Cedric L, Alexandra V, Besson T. Synthesis of novel -3H-Qinazoline-4-ones containing pyrazolinone, pyrazole and pyridinone moieties. Eur J Med Chem 2008;43:1469–77.
- Kalil A, Hamide S, El-Sabbagh HI. Synthesis of some novel oxadiazole and Quinazoline 4-one analogues for their biological activity. Arch Pharm Chem 2003;2:95–103.
- Abouzid K, Shouman S. Design, synthesis and in vitro antitumor activity of 4-aminoquinoline and 4-aminoquinazoline derivatives targeting EGFR tyrosine kinase. Bioorg Med Chem 2008;16:7543–51.
- Siegel D, Gustafson DL, Dehn DL, et al. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol 2004;65:1238–47.
- Kwak MK, Wakabayashi N, Itoh K, et al. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem 2003;278:8135–45.
- Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 2003;43:233–60.
- Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med 2004;10:549–57.
- Siegel D, Bolton EM, Burr JA, et al. The reduction of alpha tocopherolquinone by human NAD(P)H:quinone oxidoreductase: the role of alpha-tocopherolhydroquinone as a cellular antioxidant. Mol Pharmacol 1997;52:300–5.
- Begleiter A, Fourie J. Induction of NQO1 in cancer cells. Methods Enzymol 2004;382:320–51.
- Dhakshinamoorthy S, Jaiswal AK. Functional characterization and role of INrf2 in antioxidant response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. Oncogene 2001;20:3906–17.
- Nioi P, Hayes JD. Contribution of NAD(P)H:quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix-loop-helix transcription factors. Mutat Res 2004;555:149–71.
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 2004;36:1199–207.
- Dinkova-Kostova AT, Talalay P, Sharkey J, et al. An exceptionally potent inducer of cytoprotective enzymes: elucidation of the structural features that determine inducer potency and reactivity with Keap1. J Biol Chem 2010;285:33747–55.
- Honda T, Yoshizawa H, Sundararajan C, et al. Tricyclic compounds containing nonenolizable cyano enones. A novel class of highly potent anti-inflammatory and cytoprotective agents. J Med Chem 2011;54:1762–78.
- Zheng S, Santosh Laxmi YR, David E, et al. Synthesis, chemical reactivity as Michael acceptors, and biological potency of mono-cyclic cyanoenones, novel and highly potent anti-inflammatory and cytoprotective agents. J Med Chem 2012;55:4837–46.
- Dinkova-Kostova AT, Kostov RV. Glucosinolates and isothiocyanates in health and disease. Trends Mol Med 2012;18:337–47.
- AlSaid MS, Ghorab MM, Higgins M, Dinkova-Kostova AT. NAD(P)H:quinone oxidoreductase 1 inducer activity of some enaminone derivatives. Biomed Res 2015;26:7–12.
- Ghorab MM, Higgins M, Alsaid MS, et al. Synthesis, molecular modeling and NAD(P)H:quinone oxidoreductase 1 inducer activity of novel cyanoenone and enone benzenesulfonamides. J Enzyme Inhib Med Chem 2014;29:840–5.
- Ghorab MM, Ragab FA, Heiba HI, et al. Synthesis, anticancer and radiosensitizing evaluation of some novel sulfonamide derivatives. Eur J Med Chem 2015;92:682–92.
- Ghorab MM, Alsaid MS, Ceruso M, et al. Carbonic anhydrase inhibitors: synthesis, molecular docking, cytotoxic and inhibition of the human carbonic anhydrase isoforms I, II, IX, XII with novel benzenesulfonamides incorporating pyrrole, pyrrolopyrimidine and fused pyrrolopyrimidine moieties. Bioorg Med Chem 2014;14:3684–95.
- Ghorab MM, Ceruso M, Alsaid MS, et al. Novel sulfonamides bearing pyrrole and pyrrolopyrimidine moieties as carbonic anhydrase inhibitors: synthesis, cytotoxic activity and molecular modeling. Eur J Med Chem 2014;87:186–96.
- Fahey JW, Dinkova-Kostova AT, Stephenson KK, Talalay P. The “Prochaska” microtiter plate bioassay for inducers of NQO1. Methods Enzymol 2004;382:243–58.