Abstract
Objective: The purpose of this study was to investigate the in vitro inhibitory effects of the edible microalga Aphanizomenon flos-aquae (AFA) on human UDP-α-d-glucose 6-dehydrogenase (UGDH) activity, a cytosolic enzyme involved both in tumor progression and in phytochemical bioavailability.
Methods: Both the hydrophilic and ethanolic AFA extracts as well as the constitutive active principles phycocyanin (PC), phycocyanobilin (PCB) and mycosporine-like amino acids (MAAs) were tested.
Results: Among AFA components, PCB presented the strongest inhibitory effect on UGDH activity, acting as a competitive inhibitor with respect to UDP-glucose and a non-competitive inhibitor with respect to NAD+. In preliminary experiments, AFA PCB was also effective in reducing the colony formation capacity of PC-3 prostate cancer cells and FTC-133 thyroid cancer cells.
Conclusions: Overall, these findings confirmed that AFA and its active principles are natural compounds with high biological activity. Further studies evaluating the effects of AFA PCB in reducing tumor cell growth and phytochemical glucuronidation are encouraged.
Introduction
UDP-α-d-glucose 6-dehydrogenase (UGDH, EC 1.1.1.22) is a cytosolic enzyme catalyzing the conversion of UDP-glucose (UDP-Glc) to UDP-glucuronic acid (UDP-GlcA), using NAD+ as oxidantCitation1.
On the one hand, UDP-GlcA is an essential precursor for the synthesis of proteoglycans and extracellular matrix glycosaminoglycans, such as hyaluronan (HA)Citation2. It has been established that elevated levels of HA are directly implicated in the progression of various forms of epithelial cancerCitation3–6. In fact, HA fragments have potent angiogenic activity, and may promote invasion and metastasisCitation7,Citation8. Reduced formation of UDP-GlcA restricts HA production and this, in turn, slows down tumor growthCitation9,Citation10. Consequently, limiting UDP-GlcA availability in tumor cells by reducing UGDH expression or antagonizing UGDH activity might represent a useful therapeutic strategyCitation11,Citation12.
On the other hand, UDP-GlcA is involved in xenobiotic glucuronidation by UDP-glucuronosyltransferases (UGTs)Citation13. The human body uses glucuronidation to make a large variety of substances (such as drugs) more water-soluble, allowing, in this way, their elimination. Unfortunately, many phytochemicals also undergo extensive glucuronidation reactions, thus limiting their bioavailabilty and reducing their beneficial clinical effectsCitation14. There have been different attempts to decrease phytochemical metabolism through adjuvant therapies, in particular by using natural compounds inhibiting UGT activityCitation15,Citation16. Limiting UDP-GlcA availability by antagonizing UGDH activity might represent another helpful strategy that is not yet investigated.
Our interest in the development of non-toxic natural compounds that may regulate UGDH activity has led us to examine the effects of the food supplement Aphanizomen flos-aquae (AFA), a blue-green alga traditionally used for its nutritional value and health-enhancing propertiesCitation17,Citation18. In particular, AFA is a rich source of phycocyanin (PC) and of mycosporine-like amino acids (MAAs)Citation19,Citation20. AFA PC is a photosynthetic protein with well documented antioxidantCitation21,Citation22, anti-inflammatoryCitation23 and neuroprotective propertiesCitation24. The strong antioxidant activity of the protein originates from covalently attached linear tetrapyrrole prosthetic groups known as phycocyanobilins (PCB)Citation22. MAAs are water-soluble low molecular weight compounds, which have UV-absorbing properties and antioxidant activitiesCitation25,Citation26. In addition, we have recently demonstrated that AFA MAAs show a competitive type of inhibition towards monoamine oxidase B activityCitation20.
In this study, we tested for the first time the effects of both AFA extracts (hydrophilic and ethanolic) and constitutive active principles (PC, PCB and MAAs) on human UGDH activity in vitro.
Materials and methods
Preparation of the AFA extracts
Food-grade AFA powder from Klamath Lake (Oregon, USA) was kindly provided by Nutratec (Urbino, Italy) and stored in the dark at 4 °C. To obtain the hydrophilic extract, the AFA powder was dissolved in distilled water at the concentration of 100 mg/ml, homogenized by an Ika’s Ultra-Turrax (Staufen, Germany), and centrifuged at 4000g at 10 °C for 10 min to remove any insoluble material. The spectrophotometric analysis of the blue supernatant revealed the characteristic peaks of PC at 620 nm and of MAAs (principally porphyra-334) at 334 nm20. Similarly, to obtain the ethanolic extract, the AFA powder was dissolved in ethanol at the concentration of 100 mg/ml, homogenized by an Ika’s Ultra-Turrax (Staufen, Germany), and centrifuged at 4000g at 10 °C for 10 min to remove any insoluble material. The spectrophotometric analysis of the yellow supernatant revealed the characteristic peaks of carotenoids at 400 nm and of chlorophyll a at 664 nm. However, the presence of other -- spectrophotometrically unidentified -- compounds in the two AFA extracts cannot be excluded.
Extraction of AFA PC, PCB, and MAAs
Phycocyanin was extracted from the hydrophilic AFA extract (100 mg/ml) by protein precipitation with ammonium sulphate (50% saturation)Citation19. After an incubation of 60 min, the extract was centrifuged at 10 000g; the clear colorless supernatant was discarded and the blue precipitate was dissolved in a small volume of distilled water and finally dialyzed overnight against water to remove ammonium sulphate. PC was then purified by a single-step chromatographic run using a hydroxyapatite column as previously describedCitation19. PC concentration (A620/A280 ratio > 4) was determined spectrophotometrically at 620 nm (ɛ = 770 000 M−1 cm−1).
As regards PCB extraction from the hydrophilic AFA extract (100 mg/ml)Citation22, PC was first precipitated with 1% trichloroacetic acid and collected by centrifugation. PCB was then released from PC by methanolysis in the presence of mercuric chloride. PC was removed by centrifugation and mercuric chloride by β-mercaptoethanol addition. PCB was subsequently partitioned into methylene chloride/butanol (2:1, vol/vol), washed with water, and dried under nitrogen. Before use, PCB was dissolved in ethanol and its concentration was evaluated spectrophotometrically at 370 nm (ɛ = 47 900 M−1 cm−1). Finally, MAAs were extracted from the AFA powder by incubation in 20% (v/v) aqueous methanol as recently reportedCitation20. MAA concentration was assessed spectrophotometrically at 334 nm (ɛ = 42 300 M−1cm−1).
UGDH activity assay
UDP-α-d-glucose 6-dehydrogenase activity was measured in 100 mM Tris-HCl, pH 8.5 containing 2.5 μg/ml human UGDH, 1 mM UDP-Glc and 2 mM NAD+ in the presence of increasing concentrations of AFA extracts (up to160 μl/ml), PC (up to 7 μM), PCB (up to 16 nM) and MAAs (up to 5 μM)Citation27. Enzyme activity was monitored spectrophotometrically at 340 nm for 20 min at 37 °C. All reagents were from Sigma-Aldrich (Milan, Italy).
The 50% inhibitory concentration (IC50) values were calculated by adjusting the experimental data (% inhibition versus concentration of inhibitor) to non-linear regression curves. The mechanism of UGDH inhibition was assessed by analyzing the corresponding double reciprocal Lineweaver--Burk plots. The apparent inhibition constants (Ki) for the AFA extracts and constituents were calculated using Lineweaver--Burk plots, estimated from the secondary plot (slope versus concentration of inhibitor).
Effects of PCB on tumor cell growth
Prostate carcinoma cells (PC-3) and follicular thyroid carcinoma cells (FTC-133) were grown at 37 °C and 5% CO2 in RPMI 1640 or DMEM medium, respectively, supplemented with 10% fetal bovine serum, 1% l-glutamine, and 1% penicillin/streptomycin 100 U/ml. Cell culture materials and reagents were from VWR International (Milan, Italy).
Colony formation capacity of tumor cells after PCB administration was measured by the clonogenic assay. Briefly, cells were plated in 6-well plates (500 cells/well), treated for 24 h with increasing concentrations of PCB (1--100 nM), and allowed to grow for 10--14 days at 37 °C and 5% CO2. Colonies were then stained for 90 min at room temperature with 0.25% methylene blue in 50% ethanol; pictures were captured digitally and analyzed using a software for densitometric analysis (Bio-Rad Laboratories, Milan, Italy) to evaluate the colony volumes. Data were expressed as percentage of inhibition of colony formation compared to the control.
Statistics and data processing
Results are expressed as mean ± standard deviation (SD). Values are at least from duplicate experiments. Statistics and graphs were obtained using the software Microcal Origin 6.0 (Microcal Software, Inc., Northampton, MA).
Results and discussion
The present study investigated for the first time the effects of AFA and its constitutive active principles PC, PCB and MAAs on human UGDH activity in vitro. As regards the AFA extracts, we observed that both extracts (hydrophilic and ethanolic) reduced UGDH activity in a dose-dependent manner (, respectively), acting as mixed-type inhibitors with respect to both UDP-Glc and NAD+ (). This type of inhibition might be probably due to the complex nature of the extracts themselves; in fact, the hydrophilic AFA extract is mainly rich in PC and MAAs, and the ethanolic extract in carotenoids and chlorophyll; however, the presence and synergistic action of other unidentified compounds cannot be ruled out. As regards the AFA constituents, we observed that MAAs had no appreciable effects on UGDH at the concentrations tested; indeed, the enzyme activity remained almost unchanged in the presence of increasing doses of MAAs (not shown). On the contrary, AFA PC showed a dose-dependent inhibition of UGDH activity (), acting as a mixed-type inhibitor with respect to both UDP-Glc and NAD+ (, respectively). PC is a photosynthetic protein of large dimensions (121 000 Da in its trimeric form, and approximately 40 000 Da in its monomeric form)19; consequently, UGDH inhibition by AFA PC might be due at least in part to its steric volume. This was not the case of AFA PCB, which has a molecular weight of approximately 588 Da once detached from PCCitation28. AFA PCB presented a very strong inhibitory effect on UGDH activity (), showing a competitive inhibition with respect to UDP-Glc and a non-competitive inhibition with respect to NAD+ (, respectively). Referring to natural compounds regulating UGDH activity in vitro, the polyphenols gallic acid and quercetin were capable of inhibiting UGDH activity in a dose-dependent mannerCitation29. In detail, gallic acid was a non-competitive inhibitor with respect to UDP-Glc, whereas quercetin inhibited UGDH in a competitive manner with respect to UDP-Glc as did AFA PCB. However, quercetin acted in the micromolar range (Ki-UDPG 83 μM), while AFA PCB was effective in the nanomolar range (Ki-UDPG 1.34 nM). Overall, the kinetic studies show that AFA PCB strongly binds the UDP-Glc site; however, the two molecules do not show structural similarity; therefore, the mechanism of competitive inhibition is not easy to understand at this stage, probably an aspecific binding of PCB might exist.
Figure 1. Dose-dependent inhibition of UGDH activity by increasing concentrations of: (A) hydrophilic AFA extract (up to 160 μl/ml), (B) ethanolic AFA extract (up to 40 μl/ml to avoid aspecific enzyme inhibition by ethanol), (C) AFA PC (up to 7 μM) and (D) AFA PCB (up to 16 nM) (insert: PCB chemical structure).
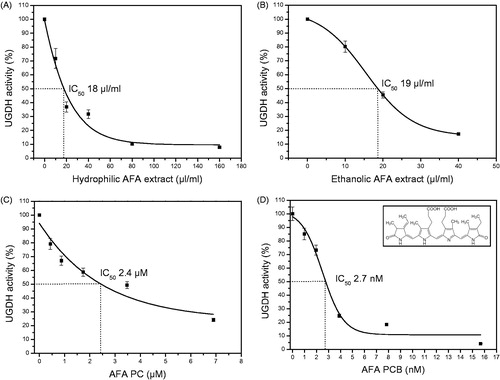
Figure 2. Lineweaver--Burk plots determined using increasing concentrations of UDP-Glc (5--40 μM) or NAD+ (6.25--200 μM). The hydrophilic AFA extract acted as a mixed-type inhibitor with respect to both UDP-Glc (A) and NAD+ (B), with Ki values of 3.26 and 3.55 μl/ml, respectively. Similarly, the ethanolic AFA extract showed a mixed-type inhibition with respect to both UDP-Glc (C) and NAD+ (D), with Ki values of 29 and 2.26 μl/ml, respectively. Values are expressed as the average from duplicate experiments.
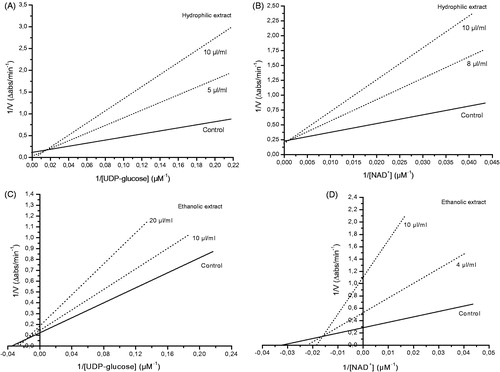
Figure 3. Lineweaver--Burk plots determined using increasing concentrations of UDP-Glc (5--40 μM) and NAD+ (6.25--200 μM). AFA PC acted as a mixed-type inhibitor with respect to both UDP-Glc (A) and NAD+ (B), with Ki values of 0.18 and 0.90 μM, respectively. Interestingly, AFA PCB acted as a competitive inhibitor with respect to UDP-Glc (C) and as a non-competitive inhibitor with respect to NAD+ (D), with Ki values of 1.34 and 1.81 nM, respectively. Values are expressed as the average from duplicate experiments.
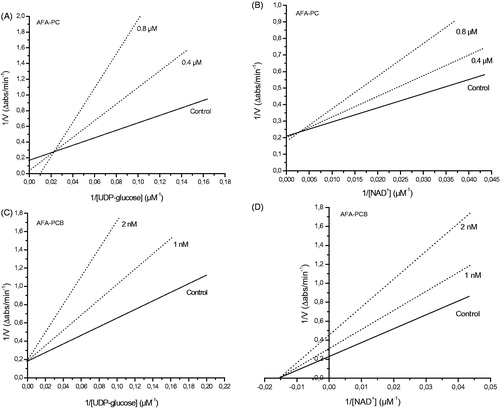
Antagonizing UGDH activity by natural occurring compounds, thus limiting UDP-GlcA and HA availability, might have significant clinical value in reducing tumor growth; in fact, overproduction of HA has been implicated in epithelial cancer progression, including those of prostate, ovarian, breast, and colon originsCitation3–6. Reduced formation of UDP-GlcA restricts HA production and this, in turn, was shown to slow tumor growthCitation9,Citation10. In this context, we preliminary demonstrated that AFA PCB was effective in reducing the colony formation capacity of PC-3 prostate cancer cells and FTC-133 thyroid cancer cells (), thus confirming the possibility of PCB to modulate cancer cell proliferation thanks to its ability to easily enter the cellsCitation30. This evidence opens the way to future more in-depth experiments aimed at the evaluation of the biochemical mechanisms involved in the antiproliferative properties of AFA PCB (via UGDH inhibition and/or other pathways).
Figure 4. Clonogenic assay in PC-3 and FTC-133 cancer cell lines treated with increasing concentration of AFA PCB (up to 100 nM). A dose-dependent inhibition of colony formation by PCB was evidenced.
Limiting UDP-GlcA availability by antagonizing UGDH activity might represent a useful strategy also in reducing phytochemical glucuronidation. Indeed, many phytochemicals (including curcumin and polyphenols) undergo extensive glucuronidation by UGTs, which reduces their bioavailabilty and beneficial clinical effectsCitation14. From this point of view, it would be worthwhile to investigate in vivo if the oral co-administration with AFA-based supplements might improve the bioavailabilty of such phytochemicals.
Conclusions
Herein, we show that AFA, and in particular AFA PCB, efficiently inhibits in a dose-dependent manner the activity of human UGDH, a cytosolic enzyme involved both in tumor progression and in phytochemical bioavailability. Our results confirm that the microalgae AFA and its constitutive active principles are natural compounds with high biological activity, as already observed in previous studies on in vitro and in vivo modelsCitation17–24. Overall, these findings further contribute to better understand the working mechanisms and the potential use of AFA-based food supplements.
Declaration of interest
No competing financial interests exist.
References
- Egger S, Chaikuad A, Kavanagh KL, et al. Structure and mechanism of human UDP-glucose 6-dehydrogenase. J Biol Chem 2011;286:23877–87
- Vigetti D, Karousou E, Viola M, et al. Hyaluronan: biosynthesis and signaling. Biochim Biophys Acta 2014;1840:2452–9
- Aaltomaa S, Lipponen P, Tammi R, et al. Strong stromal hyaluronan expression is associated with PSA recurrence in local prostate cancer. Urol Int 2002;69:266–72
- Anttila MA, Tammi RH, Tammi MI, et al. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res 2000;60:150–5
- Auvinen P, Tammi R, Parkkinen J, et al. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol 2000;156:529–36
- Ropponen K, Tammi M, Parkkinen J, et al. Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res 1998;58:342–7
- Bourguignon LY, Shiina M, Li JJ. Hyaluronan-CD44 interaction promotes oncogenic signaling, microRNA functions, chemoresistance, and radiation resistance in cancer stem cells leading to tumor progression. Adv Cancer Res 2014;123:255–75
- Singleton PA. Hyaluronan regulation of endothelial barrier function in cancer. Adv Cancer Res 2014;123:191–209
- Simpson MA, Wilson CM, McCarthy JB. Inhibition of prostate tumor cell hyaluronan synthesis impairs subcutaneous growth and vascularization in immunocompromised mice. Am J Pathol 2002;161:849–57
- Huh JW, Choi MM, Yang SJ, et al. Inhibition of human UDP-glucose dehydrogenase expression using siRNA expression vector in breast cancer cells. Biotechnol Lett 2005;27:1229–32
- Egger S, Chaikuad A, Kavanagh KL, et al. UDP-glucose dehydrogenase: structure and function of a potential drug target. Biochem Soc Trans 2010;38:1378–85
- Wang TP, Pan YR, Fu CY, Chang HY. Down-regulation of UDP-glucose dehydrogenase affects glycosaminoglycans synthesis and motility in HCT-8 colorectal carcinoma cells. Exp Cell Res 2010;316:2893–902
- Rowland A, Miners JO, Mackenzie PI. The UDP-glucuronosyltransferases: their role in drug metabolism and detoxification. Int J Biochem Cell Biol 2013;45:1121–32
- Li L, Hu H, Xu S, et al. Roles of UDP-glucuronosyltransferases in phytochemical metabolism of herbal medicines and the associated herb-drug interactions. Curr Drug Metab 2012;13:615–23
- Grancharov K, Naydenova Z, Lozeva S, Golovinsky E. Natural and synthetic inhibitors of UDP-glucuronosyltransferase. Pharmacol Ther 2001;89:171–86
- Grill AE, Koniar B, Panyam J. Co-delivery of natural metabolic inhibitors in a self-microemulsifying drug delivery system for improved oral bioavailability of curcumin. Drug Deliv Transl Res 2014;4:344–52
- Baroni L, Scoglio S, Benedetti S, et al. Effect of a Klamath algae product (“AFA-B12”) on blood levels of vitamin B12 and homocysteine in vegan subjects: a pilot study. Int J Vitam Nutr Res 2009;79:117–23
- Scoglio S, Benedetti S, Canino C, et al. Effect of a 2-month treatment with Klamin, a Klamath algae extract, on the general well-being, antioxidant profile and oxidative status of postmenopausal women. Gynecol Endocrinol 2009;25:235–40
- Benedetti S, Rinalducci S, Benvenuti F, et al. Purification and characterization of phycocyanin from the blue-green alga Aphanizomenon flos-aquae. J Chromatogr B Analyt Technol Biomed Life Sci 2006;833:12–18
- Scoglio S, Benedetti Y, Benvenuti F, et al. Selective monoamine oxidase B inhibition by an Aphanizomenon flos-aquae extract and by its constitutive active principles phycocyanin and mycosporine-like amino acids. Phytomedicine 2014;21:992–7
- Benedetti S, Benvenuti F, Pagliarani S, et al. Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenon flos-aquae. Life Sci 2004;75:2353–62
- Benedetti S, Benvenuti F, Scoglio S, Canestrari F. Oxygen radical absorbance capacity of phycocyanin and phycocyanobilin from the food supplement Aphanizomenon flos-aquae. J Med Food 2010;13:223–7
- Canestrari F, Benvenuti F, Benedetti S, et al Antinflammatory properties of phycocyanin from Aphanizomenon flos-aquae. Prog Nutr 2006;8:99–103
- Sedriep S, Xia X, Marotta F, et al Beneficial nutraceutical modulation of cerebral erythropoietin expression and oxidative stress: an experimental study. J Biol Regul Homeost Agents 2011;25:187–94
- Rastogi RP, Sinha RP, Moh SH, et al. Ultraviolet radiation and cyanobacteria. J Photochem Photobiol B 2014;141:154–69
- Wada N, Sakamoto T, Matsugo S. Multiple roles of photosynthetic and sunscreen pigments in cyanobacteria focusing on the oxidative stress. Metabolites 2013;3:463–83
- Sommer BJ, Barycki JJ, Simpson MA. Characterization of human UDP-glucose dehydrogenase. CYS-276 is required for the second of two successive oxidations. J Biol Chem 2004;279:23590–6
- Beale SI, Cornejo J. Biosynthesis of phycobilins. 3(Z)-phycoerythrobilin and 3(Z)-phycocyanobilin are intermediates in the formation of 3(E)-phycocyanobilin from biliverdin IX alpha. J Biol Chem 1991;266:22333–40
- Hwang EY, Huh JW, Choi MM, et al. Inhibitory effects of gallic acid and quercetin on UDP-glucose dehydrogenase activity. FEBS Lett 2008;582:3793–7
- Koníčková R, Vaňková K, Vaníková J, et al. Anti-cancer effects of blue-green alga Spirulina platensis, a natural source of bilirubin-like tetrapyrrolic compounds. Ann Hepatol 2014;13:273–83
