Abstract
New phenolic mono and bis Mannich bases incorporating benzimidazole, such as 2-(aminomethyl)-4-(1H-benzimidazol-2-yl)phenol and 2,6-bis(aminomethyl)-4-(1H-benzimidazol-2-yl)phenol were synthesized starting from 4-(1H-benzimidazol-2-yl)phenol. Amines used for the synthesis included dimethylamine, pyrrolidine, piperidine, N-methylpiperazine and morpholine. The CA inhibitory properties of these compounds were tested on the human carbonic anhydrase (CA, EC 4.2.1.1) isoforms hCA I and hCA II. These new compounds, as many phenols show moderate CA inhibitory properties.
Introduction
Mannich bases are an important group of compounds in medicinal chemistry and they are synthesized by the Mannich reactionCitation1,Citation2. Mannich bases have a wide range of biological activities, such as cytotoxicCitation3–6, anti-inflammatoryCitation7 and anticonvulsantCitation8 activities. Several types of phenolic Mannich bases were also reported with cytotoxicCitation6,Citation9 and carbonic anhydrase (CA) inhibitoryCitation10 activities. The reported biological mechanisms of action of Mannich bases include thiol alkylationCitation11–13; inhibition of mitochondrial respirationCitation14,Citation15; topoisomerase inhibitionCitation16 and tubulin polymerizationCitation17.
One of the ring systems that is present in many pharmacological agents is the benzimidazole. The importance of this ring system has been the subject of many reviewsCitation18,Citation19. One of these reviews summarized the anticancer, protein kinase, tyrosine kinase, antihelmintic, antidiabetic, anticoagulant, analgesic, anti-inflammatory, antiallergic and antihormonal activities of benzimidazole derivativesCitation19.
Carbonic anhydrase (CA, EC 4.2.1.1) enzymes play important roles in physiological and pathological processesCitation20–26. Sixteen CA isoforms have been identified in mammals. CA isoforms take part in several vital biological processes, such as electrolyte secretion, acid-base balance, ion transport and lipogenesis, ureagenesis and bone resorption. Inhibitors or activators of these enzymes have medical applications, such as diuretics, in the treatment of glaucoma, neurological disorders including epilepsy and Alzheimer’s diseaseCitation27–29. Phenols also constitute an interesting class of CA inhibitors (CAIs)Citation30–34.
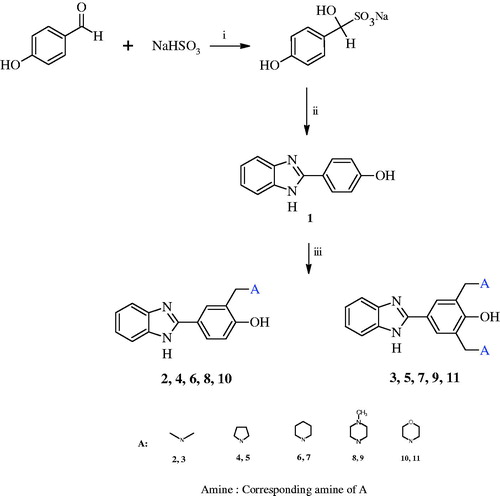
Based on these informations, in this study, it was aim to synthesize 2-(aminomethyl)-4-(1H-benzimidazol-2-yl)phenol and 2,6-bis(aminomethyl)-4-(1H-benzimidazol-2-yl)phenols incorporating benzimidazole moieties () and to investigate their CA inhibitory properties on hCA I and II.
Experimental
Materials
1H NMR (400 MHz) spectra was taken using a Bruker DPX-500FT-NMR (Billerica, MA) and 13C NMR (100 MHz) spectra was taken using a Varian spectrometer (Palo Alto, CA) Chemical shifts (δ) were reported in ppm. Melting points were determined using an Electrothermal 9100 (IA9100, Bibby Scientific Limited, Staffordshire, UK) instrument and are uncorrected. Mass specta was taken using a Thermo Electron Coop. Finnigor LTQ (IT-MS/ESI-MS).
Methods
Synthesis of 4-(1H-benzimidazol-2-yl)phenol (1)
The mixture of 4-hydroxybenzaldehyde (24.6 mmol) and sodium hydrogen sulphide (24.6 mmol, 40%) in ethanol-water (3:1, 60 mL) was stirred at room temperature for 120 min. The white solid precipitated, sodiumhydroxy(4-hydroxyphenyl)methanesulfonate, was filtered, and driedCitation35. The mixture of sodiumhydroxy(4-hydroxyphenyl)methanesulfonate (4.4 mmol) and o-phenylendiamine (4.4 mmol) in acetonitrile (15 mL) was heated in a microwave oven (13.8 barr, 145 °C, 200 W) for 10 min. Reaction was monitored by TLC. Reaction was stopped when sodiumhydroxy(4-hydroxyphenyl)methanesulfonate in reaction medium finished. The solid precipitated was seperated, dried and crystallized from the mixture of methanol water (3:1). The dark-brown colored compound (1) was obtained (74%)Citation36,Citation37. 1H NMR (400 MHz, ppm, δ) 6.92 (dd, J = 6.8, 2.0 Hz, 2H), 7.16–7.19 (m, 2H), 7.51–7.55 (m, 2H), 7.92 (dd, J = 6.8, 2.0 Hz, 2H). 13C NMR (100 MHz, ppm, δ) 114.3, 115.7, 120.9, 122.3, 128.3, 152.7, 159.8. M++1 Calculated: 211.2; M++1 Found: 211.2.
Synthesis of 4-(1H-benzimidazol-2-yl)-2-[(dimethylamino)methyl]phenol (2)
The mixture of the compound 1 (2.4 mmol), paraformaldehyde (9.5 mmol) and water solution of dimethylamine (40%, 2.4 mmol) in acetonitrile (10 mL) was refluxed for 90 min. The reaction was monitored by TLC (chloroform:methanol (80:20)). When the compound 1 finished, reaction was stopped. The reaction solvent was removed under vacuum. The residue was purified by column chromatography [silicagel 60 (70–230 mesh) and choloroform: methanol (80:20)]. The white solid compound 2 was obtained (37%); m.p.: 187–189 °C. 1H NMR (400 MHz, ppm, δ) 2.13 (s, 6H), 3.33 (s, 2H), 6.77 (d, J = 8.4 Hz, 1H), 7.20 (dd, J = 5.9, 3.1 Hz, 2H), 7.60 (dd, J = 5.9, 3.1 Hz, 2H), 7.96 (s, 1H), 8.07 (dd, J = 8.5, 1.5 Hz, 1H). 13C NMR (100 MHz, ppm, δ) 44.4, 62.4, 115.1, 116.8, 120.9, 122.7, 122.7, 127.9, 139.5, 153.4, 160.6. M++1 Calculated: 268.3; M++1 Found: 268.2.
Synthesis of 4-(1H-benzimidazol-2-yl)-2,6-bis[(dimethylamino)methyl]phenol (3)
The mixture of the compound 1 (14.3 mmol), paraformaldehyde (57.1 mmol) and water solution of dimethylamine (40%, 57.1 mmol) in acetonitrile (40 mL) was heated in a microwave oven (13.8 barr, 110 °C, 150 W) for 5 min. The reaction was monitored by TLC. Reaction was stopped when compound 1 was consumed. The solvent was removed under vacuum and the residue was purified by column chromatography [silicagel 60 (70–230 mesh) and chloroform:methanol:ammonium hydroxyde (80:20:2)]. The light yellow colored solid compound 3 was obtained (26%); m.p.: 170–172 °C. 1H NMR (400 MHz, ppm, δ) 2.05 (s, 12H), 3.30 (s, 4H), 7.10 (dd, J = 6.0, 3.1 Hz, 2H), 7.52 (dd, J = 6.0, 3.1 Hz, 2H), 7.92 (s, 2H). 13C NMR (100 MHz, ppm, δ) 44.7, 60.0, 115.0, 120.5, 122.4, 123.7, 128.2, 139.6, 153.3, 159.3 M++1 Calculated: 325.2; M++1 Found: 325.2.
Synthesis of 4-(1H-benzimidazol-2-yl)-2-[(pyrrolidin-1-yl)methyl]phenol (4)
The mixture of the compound 1 (2.4 mmol), paraformaldehyde (9.5 mmol) and pyrrolidine (2.4 mmol) in acetonitrile (7 mL) was heated in a microwave oven (13.8 barr, 120 °C, 100 W) for 5 min. The reaction was monitored by TLC. Reaction was stopped when compound 1 was consumed. The solvent was removed under vacuum and the residue was purified by column chromatography [silicagel 60 (70–230 mesh) and chloroform:methanol: ammonium hydroxyde (70:30:1)]. The orange coloured solid 4 was obtained (26%); m.p.: 200–203 °C. 1H NMR (400 MHz, ppm, δ) 1.73 (s, 4H), 2.42 (s, 4H), 3.53 (s, 2H), 6.76 (d, J = 8.4 Hz, 1H), 7.19 (dd, J = 5.9, 3.1 Hz, 2H), 7.58 (dd, J = 5.9, 3.1 Hz, 2H), 7.92 (d, J = 1.5 Hz, 1H), 8.01 (dd, J = 8.4, 1.5 Hz, 1H). 13C NMR (100 MHz, ppm, δ) 23.8, 53.4, 58.4, 115.0, 116.6, 120.8, 122.6, 123.2, 127.5, 127.6, 139.5, 153.3, 160.6. M++1 Calculated: 294.3; M++1 Found: 294.2.
Synthesis of 4-(1H-benzimidazol-2-yl)-2,6-bis[(pyrrolidin-1-yl)methyl]phenol (5)
The mixture of the compound 1 (9.5 mmol), paraformaldehyde (38 mmol) and pyrrolidine (38 mmol) in acetonitrile (30 mL) was heated in a microwave oven (13.8 barr, 110 °C, 100 W) for 5 min. The reaction was followed by TLC. Reaction was stopped when compound 1 was consumed. The solvent was removed under vacuum. The viscous residue was triturated with diethyl ether and it was solidified. The crude compound was purified by crystallization from methanol:diethylether. The brown colored solid compound 5 was obtained (44%), m.p.: 189–191 °C. 1H NMR (400 MHz, ppm, δ) 1.71 (s, 8H), 2.52 (s, 8H), 3.68 (s, 4H), 7.18 (dd, J = 5.9, 3.3 Hz, 2H), 7.56 (bs, 2H), 7.83 (s, 2H). 13C NMR (100 MHz, ppm, δ) 23.7, 53.9, 56.4, 120.3, 122.4, 124.5, 127.1, 152.9, 158.9. M++1 Calculated: 377.4; M++1 Found: 377.3.
Synthesis of 4-(1H-benzimidazol-2-yl)-2-[(piperidin-1-yl)methyl]phenol (6)
The mixture of the compound 1 (2.9 mmol), formaldehyde solution (37%, 11.4 mmol) and piperidine (2.9 mmol) in acetonitrile (15 mL) was heated in a microwave oven (13.8 barr, 120 °C, 100 W) for 5 min. The reaction was followed by TLC. Reaction was stopped when compound 1 was consumed. The solvent was removed under vacuum. The residue was purified by column chromatography [silicagel 60 (70–230 mesh) and chloroform:methanol (70:30)]. The suitable fraction was crystallized from methanol three times. The white solid compound 6 was obtained (39%); m.p.: 254–256 °C. 1H NMR (400 MHz, ppm, δ) 1.59 (bs, 10H), 3.56 (s, 2H), 6.83 (d, J = 8.4 Hz, 1H), 7.22 (dd, J = 5.9, 3.3 Hz, 2H), 7.58 (bs, 2H), 7.78 (dd, J = 8.4, 1.8 Hz, 1H), 7.84 (s, 1H). 13C NMR (100 MHz, ppm, δ) 25.9, 53.9, 61.9, 76.9, 116.7, 120.8, 122.6, 122.8, 126.9, 127.9, 152.5, 127.9. M++1 Calculated: 308.3; M++1 Found: 308.3.
Synthesis of 4-(1H-benzimidazol-2-yl)-2,6-bis[(piperidin-1-yl)methyl]phenol (7)
The mixture of the compound 1 (4.8 mmol), paraformaldehyde (19 mmol) and piperidine (14.3 mmol) in acetonitrile (15 mL) was heated in a microwave oven (13.8 barr, 120 °C, 100 W) for 5 min. The reaction was monitored by TLC. Reaction was stopped when the compound 1 was consumed. The solvent was removed under vacuum. The viscous residue obtained was trituated with diethylether and solidified. It was filtered and washed with acetone (2 × 10 mL) and dried. The crude compound was crystallized from ethylacetate:methanol. The white colored compound 7 was obtained (16%); m.p.: 238–240 °C. 1H NMR (400 MHz, ppm, δ) 1.52 (bs, 4H), 1.58–1.62 (m, 6H), 2.49 (bs, 6H), 3.63 (s, 4H), 7.23 (dd, J = 5.9, 2.9 Hz, 2H), 7.50 (bs, 2H), 7.82 (s, 2H). 13C NMR (100 MHz, ppm, δ) 24.2, 25.9, 54.4, 59.4, 94.6, 120.3, 122.6, 123.5, 127.5, 159.3. M++1 Calculated: 405.5; M++1 Found: 405.3.
Synthesis of 4-(1H-benzimidazol-2-yl)-2-[(4-methylpiperazin-1-yl)methyl]phenol (8)
The mixture of the compound 1 (1.4 mmol), formaldehyde solution (37%, 5.7 mmol) and N-methlypiperazine (1.4 mmol) in acetonitrile (7 mL) was heated in a microwave oven (13.8 barr, 120 °C, 100 W) for 5 min. The reaction was monitored by TLC. Reaction was stopped when compound 1 was consumed. The solvent was removed under vacuum. The crude compound was purified by column chromatography [silicagel 60 (70–230 mesh) and choloroform:methanol:ammonium hydroxyde (70:30:1)]. The yellow solid compound 8 was obtained (21%); m.p.:116–118 °C. 1H NMR (400 MHz, ppm, δ) 2.15 (s, 3H), 2.39–2.16 (m, 8H), 3.38 (s, 2H), 6.73 (d, J = 8.6 Hz, 1H), 7.16 (dd, J = 5.9, 3.2 Hz, 2H), 7.55 (dd, J = 5.9, 3.2 Hz, 2H), 7.82 (d, J = 1.8 Hz, 1H), 7.95 (dd, J = 8.6, 1.8 Hz, 1H). 13C NMR (100 MHz, ppm, δ) 45.9, 52.2, 54.8, 60.8, 115.0, 116.8, 121.2, 121.9, 122.7, 127.8, 128.1, 139.4, 152.9, 160.0. M++1 Calculated: 323.4; M++1 Found: 323.3.
Synthesis of 4-(1H-benzimidazol-2-yl)-2,6-bis[(4-methylpiperazin-1-yl)methyl]phenol (9)
The mixture of the compound 1 (4.8 mmol), paraformaldehyde (19 mmol) and N-methlypiperazine (19 mmol) in acetonitrile (20 mL) was heated in a microwave oven (13.8 barr, 120 °C, 100 W) for 5 min. The reaction was monitored by TLC. Reaction was stopped when compound 1 was consumed. The solvent was removed under vacuum. The viscous residue was triturated with diethylether to solidify. The solid obtained was crystallized from methanol:diethylether. The white solid compound 9 was obtained (47%); m.p.: 231–233 °C. 1H NMR (400 MHz, ppm, δ) 2.19 (s, 6H), 2.22–2.42 (m, 16H), 3.54 (s, 4H), 7.20 (d, J = 4.8 Hz, 2H), 7.41 (bs, 2H), 7.75 (dd, J = 7.0 Hz, 2H). 13C NMR (100 MHz, ppm, δ) 46.0, 52.9, 54.9, 58.9, 111.0, 119.2, 120.7, 122.5, 122.8, 123.3, 127.9,152.5, 158.7. M++1 Calculated: 435.5; M++1 Found: 435.3.
Synthesis of 4-(1H-benzimidazol-2-yl)-2-[(morpholin-4-yl)methyl]phenol (10)
The mixture of the compound 1 (2.4 mmol), paraformaldehyde (9.5 mmol) and morpholine (2.4 mmol) in acetonitrile (7 mL) was heated in a microwave oven (13.8 barr, 120 °C, 100 W) for 5 min. The reaction was monitored by TLC. Reaction was stopped when compound 1 was consumed. The solvent was removed under vacuum. The residue was purified by column chromatography [silicagel 60 (70–230 mesh) and diisopropylether:methanol (80:20)]. The white colored solid 10 was obtained (64%); m.p.: 120–123 °C. 1H NMR (400 MHz, ppm, δ) 2.42 (bs, 4H), 3.54 (s, 2H), 3.70 (bs, 4H), 6.83 (d, J = 9.1 Hz, 1H), 7.23 (dd, J = 6.0, 3.0 Hz, 2H), 7.60 (dd, J = 6.0, 3.0 Hz, 2H), 7.89–7.91 (m, 2H). 13C NMR (100 MHz, ppm, δ) 52.9, 61.5, 66.8, 115.1, 116.8, 121.2, 121.7, 122.9, 127.5, 128.3, 152.5, 160.0. M++1 Calculated: 310.3; M++1 Found: 310.2.
Synthesis of 4-(1H-benzimidazol-2-yl)-2,6-bis[(morpholin-4-yl)methyl]phenol (11)
The mixture of compound 1 (2.4 mmol), paraformaldehyde (9.5 mmol) and morpholine (9.5 mmol) in acetonitrile (10 mL) was heated in a microwave oven (13.8 barr, 80 °C, 200 W) for 20 min. Heating was continued for additional 90 min (at 1 atm, 80 °C, 300 W). The reaction was monitored by TLC. Although 110 min heating period was over and since reaction did not go further, reaction was stopped. The solvent was removed under vacuum and the residue was purified by column chromatography [silicagel 60 (70–230 mesh) and diisopropylether:methanol (80:20)]. The white colored solid 11 was obtained (26%); m.p.:209–210 °C. 1H NMR (400 MHz, ppm, δ) 2.28 (s, 8H), 3.39 (s, 4H), 3.51 (s, 8H), 7.14 (dd, J = 5.7, 3.1 Hz, 2H), 7.51 (bs, 2H), 7.97 (s, 2H). 13C NMR (100 MHz, ppm, δ) 53.2, 59.2, 66.8, 120.8, 122.7, 123.1, 128.3, 153.0, 158.5. M++1 Calculated: 409.4; M++1 Found: 409.3.
Carbonic anhydrase inhibition assay
An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity by using method given by KhalifahCitation38. Phenol red (at a concentration of 0.2 mM) has been used as an indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5) as buffer, and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalyzed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled-deionized water and dilutions up to 0.01 nM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. The inhibition constants were obtained by non-linear least-squares methods using PRISM (www.graphpad.com), and non-linear least squares methods, values representing the mean of at least three different determinations, as described earlier by usCitation39. All CA isoforms were recombinant ones obtained in-house as reported earlierCitation21. The cell pellets were lysed, and hCA II and hCA I were purified through affinity chromatography using pAMBS resin.
Results and discussion
Synthesis of the starting compound 1 (4–(1H-benzimidazol-2-yl)-phenol), was initially achieved according to literature procedureCitation36,Citation37. In this study, compounds 2–11 () were successfully synthesized by the reaction of different and suitable ratios of compound 1, paraformaldehyde/formaldehyde solution, and amine in acetonitrile by the conventional method (2) or microwave irradiation method (the other compounds except 2) with yields of 16–63%. Their chemical structures were confirmed by 1H NMR, 13C NMR and MS spectra. Experimental and spectral details were presented in the “Experimental” section. Amines used were dimethylamine (2, 3), pyrrolidine (4, 5), piperidine (6, 7), N-methylpiperazine (8, 9), morpholine (10, 11). Different amines having different pKa values were considered since it may govern the deamination ratio of the Mannich bases, thiol alkilating potential of them, and their bioactivities. Carbonic anhydrase inhibiting properties of the compounds on hCA I and hCA II isoenzymes were investigated at 10−6 M concentration and the results are reported as percent inhibition in .
Table 1. Percent inhibition of hCA I and hCA II by the synthesized compounds at 1 μM concentration of inhibitor in the assay system.
The compounds inhibited hCA I (18–26%) and hCA II (7–25%) izoenzymes. The hCA I inhibiting properties of the compounds were higher values than the ones on hCA II, except 10 and 11, which are morpholine containing Mannich bases. Especially, in the case of the compounds 1 (starting compound, 2 times), 3 (bis Mannich base with dimethylamine, 2.9 times), 4 (mono Mannich base with pyrrolidine, 1.8 times), 7 (bis Mannich base with piperidine, 1.7 times) increases in CA inhibition percentage of the compounds on hCA I were remarkable compared with hCA II. As seen from the data given in , as many phenols the Mannich bases reported here are poor hCA I/II inhibitors.
Conclusion
In this study, new phenolic mono and bis Mannich bases were prepared, and their CA inhibiting properties against hCA I and hCA II were investigated. In our previous study, mono and bis Mannich bases of [2–(4-hydroxybenzylidene)-2,3-dihydro-1H-inden-1-one] were synthesized and their CA inhibitory properties had been investigatedCitation10. In that study, preparation of Mannich bases had decreased the CA inhibiting properties of the compounds comparing to the ones they are derived from 2–(4-hydroxybenzylidene)-2,3-dihydro-1H-inden-1-one. This situation had been attributed to bulky functionalities available at the ortho position of phenol, which may affect the interaction of OH group with the active site of the enzymes hCA I and hCA IICitation10. The increases in CA inhibition profile of the series reported here may result from the benzimidazole moiety, which may interact with the active site of CA enzyme in a hydroxybenzylidene manner.
Declaration of interest
The authors report no conflicts of interest. They are responsible for the contents and writing of the paper.
Prof. H. Inci Gul thanks Ataturk University, BAP Department, Turkey for the financial supports to this study.
References
- Dimmock JR, Shyam K, Logan BM, et al. Syntheses and evaluation of some Mannich bases derived from acetophenones against P388 lymphocytic leukemia and toxicological assessment of 3-dimethyl-amino-2-dimethylaminomethyl-1-(4-methoxyphenyl)-1-propanone dihydrochloride in rats. J Pharm Sci 1984;73:471–7
- Roman G. Mannich bases in medicinal chemistry and drug design. Eur J Med Chem 2015;89:743–816
- Gul HI, Gul M, Erciyas E. Toxicity of some bis Mannich bases and corresponding piperidinols in the brine shrimp (Artemia salina) bioassay. J Appl Toxicol 2003;23:53–7
- Gul HI, Yerdelen KO, Gul M, et al. Synthesis of 4′-hydroxy-3′-piperidinomethylchalcone derivatives and their cytotoxicity against PC-3 cell lines. Arch Pharm (Weinheim) 2007;340:195–201
- Gul HI, Yerdelen KO, Das U, et al. Synthesis and cytotoxicity of novel 3-aryl-1-(3′-dibenzylaminomethyl-4′-hydroxyphenyl)-propenones and related compounds. Chem Pharm Bull (Tokyo) 2008;56:1675–81
- Tugrak M, Yamali C, Sakagami H, Gul HI. Synthesis of mono Mannich bases of 2-(4-hydroxybenzylidene)-2,3-dihydroinden-1-one and evaluation of their cytotoxicities. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. DOI: 10.3109/14756366.2015.1070263
- Gul HI, Suleyman H, Gul M. Evaluation of the anti-inflammatory activity of N,N′-bis(3-dimethylamino-1-phenyl-propylidene)hydrazinedihydrochloride. Pharm Biol 2009;47:968–72
- Gul HI, Calis U, Ozturk Z, et al. Evaluation of anticonvulsant activities of bis(3-aryl-3-oxo-propyl) ethylamine hydrochlorides and 4-aryl-3-arylcarbonyl-1-ethyl-4-piperidinol hydrochlorides. Arzneimittelforschung 2007;57:133–6
- Yerdelen KO, Gul HI, Sakagami H, Umemura N. Synthesis and biological evaluation of 1,5-bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one and its aminomethyl derivatives. J Enzyme Inhib Med Chem 2015;30:383–8
- Yamali C, Tugrak M, Gul HI, et al. The inhibitory effects of phenolic Mannich bases on carbonic anhydrase I and II isoenzymes. J Enzyme Inhib Med Chem 2016. [Epub ahead of print]. DOI:10.3109/14756366.2015.1126715
- Gul M, Gul HI, Hanninen O. Effects of Mannich bases on cellular glutathione and related enzymes of Jurkat cells in culture conditions. Toxicol In Vitro 2002;16:107–12
- Gul M, Gul HI, Vepsalainen J, et al. Effect of acetophenone derived Mannich bases on cellular glutathione level in Jurkat cells. A possible mechanism of action. Arzneimittelforschung 2001;51:679–82
- Gul M, Atalay M, Gul HI, et al. The effects of some Mannich bases on heat shock proteins HSC70 and GRP75, and thioredoxin and glutaredoxin levels in Jurkat cells. Toxicol In Vitro 2005;19:573–80
- Dimmock JR, Hamon NW, De Gooijer CA, et al. Effect of some 4-alkyl-5-dimethylamino-1-phenyl-1-penten-3-one hydrochlorides and related compounds on respiration in mouse liver mitochondria. Pharmazie 1989;44:560–2
- Kucukoglu K, Gul HI, Cetin-Atalay R, et al. Synthesis of new N,N′-bis[1-aryl-3-(piperidine-1-yl)propylidene]hydrazine dihydrochlorides and evaluation of their cytotoxicity against human hepatoma and breast cancer cells. J Enzyme Inhib Med Chem 2014;29:420–6
- Canturk P, Kucukoglu K, Topcu Z, et al. Effect of some bis Mannich bases and corresponding piperidinols on DNA topoisomerase I. Arzneimittelforschung 2008;58:686–91
- Das U, Gul HI, Alcorn J, et al. Cytotoxic 5-aryl-1-(4-nitrophenyl)-3-oxo-1,4-pentadienes mounted on alicyclic scaffolds. Eur J Med Chem 2006;41:577–85
- Keri RS, Hiremathad A, Budagumpi S, Nagaraja BM. Comprehensive review in current developments of benzimidazole-based medicinal chemistry. Chem Biol Drug Des 2015;86:19–65
- Balasubramanian Narasimhan DS, Pradeep K. Benzimidazole: a medicinally important heterocyclic moiety. Med Chem Res 2012;21:269–83
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- D'Ambrosio K, Smaine FZ, Carta F, et al. Development of potent carbonic anhydrase inhibitors incorporating both sulfonamide and sulfamide groups. J Med Chem 2012;55:6776–83
- Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72
- Supuran CT. Carbonic anhydrases: from biomedical applications of the inhibitors and activators to biotechnological use for CO(2) capture. J Enzyme Inhib Med Chem 2013;28:229–30
- Capasso C, Supuran CT. Sulfa and trimethoprim-like drugs-antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J Enzyme Inhib Med Chem 2014;29:379–87
- Capasso C, Supuran CT. An overview of the alpha-, beta- and gamma-carbonic anhydrases from bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32
- Supuran CT, Capasso C. The η-class carbonic anhydrases as drug targets for antimalarial agents – carbonic as targets antimalarial. Expert Opin Ther Targets 2015;19:551–63
- Supuran CT. Carbonic anhydrase inhibitors and activators for novel therapeutic applications. Future Med Chem 2011;3:1165–80
- Scozzafava A, Supuran CT. Glaucoma and the applications of carbonic anhydrase inhibitors. Subcell Biochem 2014;75:349–59
- McKenna R, Supuran CT. Carbonic anhydrase inhibitors drug design. Subcell Biochem 2014;75:291–323
- Davis RA, Innocenti A, Poulsen SA, Supuran CT. Carbonic anhydrase inhibitors. Identification of selective inhibitors of the human mitochondrial isozymes VA and VB over the cytosolic isozymes I and II from a natural product-based phenolic library. Bioorg Med Chem 2010;18:14–18
- Innocenti A, Hilvo M, Scozzafava A, et al. Carbonic anhydrase inhibitors: inhibition of the new membrane-associated isoform XV with phenols. Bioorg Med Chem Lett 2008;18:3593–6
- Gulcin I, Beydemir S. Phenolic compounds as antioxidants: carbonic anhydrase isoenzymes inhibitors. Mini Rev Med Chem 2013;13:408–30
- Innocenti A, Gulcin I, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Antioxidant polyphenols effectively inhibit mammalian isoforms I-XV. Bioorg Med Chem Lett 2010;20:5050–3
- Senturk M, Gulcin I, Dastan A, et al. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–11
- Alpan AS, Gunes HS, Topcu Z. 1H-Benzimidazole derivatives as mammalian DNA topoisomerase I inhibitors. Acta Biochim Pol 2007;54:561–5
- Lewis JC, Berman AM, Bergman RG, Ellman JA. Rh(I)-catalyzed arylation of heterocycles via C-H bond activation: expanded scope through mechanistic insight. J Am Chem Soc 2008;130:2493–500
- Yu CG, Peng G, Jin C, Su W. The synthesis of benzimidazole derivatives in the absence of solvent and catalyst. J Chem Res 2009;5:333–6
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73
- Maresca A, Temperini C, Pochet L, et al. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem 2010;53:335–44