Abstract
A new series of 1,3,5-triaryl-4,5-dihydro-1H-pyrazole derivatives 13a–p were synthesized via aldol condensation of 3/4-nitroacetophenones with appropriately substituted aldehydes followed by cyclization of the formed chalcones with 4-methanesulfonylphenylhydrazine hydrochloride. All the synthesized compounds were evaluated for their cyclooxygenase (COX) inhibition, anti-inflammatory activity and ulcerogenic liability. All compounds were more potent inhibitors for COX-2 than COX-1. While most compounds showed good anti-inflammatory activity, compounds 13d, 13f, 13k and 13o were the most potent derivatives (ED50 = 66.5, 73.4, 79.8 and 70.5 μmol/kg, respectively) in comparison with celecoxib (ED50 = 68.1 μmol/kg). Compounds 13d, 13f, 13k and 13o (ulcer index = 3.89, 4.86, 4.96 and 3.92, respectively) were 4–6 folds less ulcerogenic than aspirin (ulcer index = 22.75) and showed approximately ulceration effect similar to celecoxib (ulcer index = 3.35). In addition, molecular docking studies were performed for compounds 13d, 13f, 13k and 13o inside COX-2 active site which showed acceptable binding interactions (affinity in kcal/mol −2.1774, −6.9498) in comparison with celecoxib (affinity in kcal/mol −6.5330).
Introduction
Traditional non-steroidal anti-inflammatory drugs (NSAIDs) reduce the pain and inflammation through inhibition of both cyclooxygenase-1 and -2 (COX-1 and COX-2)Citation1. While COX-1 enzyme is constitutively expressed and plays an important role as a housekeeping enzyme such as protection of gastric mucosa, vascular homeostasis and platelet aggregation, COX-2 enzyme is expressed and significantly upregulated during acute and chronic inflammation, pain and oncogenesisCitation2. Non-selective NSAIDs such as aspirin, ibuprofen and indomethacin inhibit the activities of both enzymes and in turn inhibit the production of gastro protective prostaglandins (PGs) synthesized through COX-1 pathway causing gastro intestinal side effects like ulcers, perforation and bleedingCitation3. For this reason, selective COX-2 inhibitor drugs (coxibs) such as celecoxib (1), rofecoxib (2) and valdecoxib (3) () which have the same anti-inflammatory efficacy as traditional NSAIDs with minimized unwanted gastrointestinal side effects take much consideration in the last two decadesCitation4. Unfortunately, highly selective COX-2 inhibitors caused cardiovascular side effects such as increased systemic blood pressure and myocardial infarction that ultimately prompted the withdrawal of rofecoxib and valdecoxibCitation5. At present, celecoxib (1) is the only and highly marketed coxib, so, there is still a need for synthesis of COX-2 inhibitor with no adverse effects (). We have previously reportedCitation6–11 some derivatives of celecoxib (4–8) which showed considerable anti-inflammatory activity.
Figure 1. Chemical structures of the selective cyclooxygenase-2 (COX-2) inhibitors celecoxib (1), rofecoxib (2) and valdecoxib (3), and celecoxib analogs (4–8).
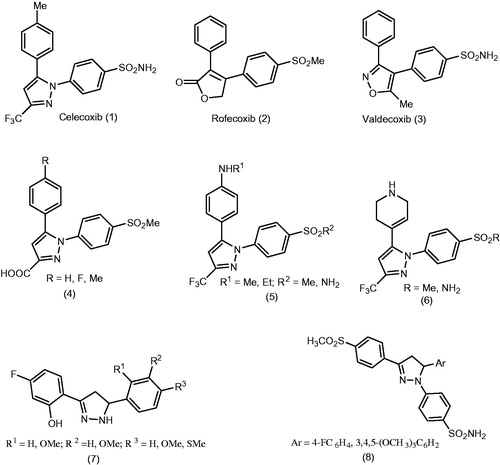
Figure 2. Chemical structures of the selective cyclooxygenase-2 (COX-2) inhibitor celecoxib (1) and the designed 1,3,5-triaryldihydropyrazoles 13a–p.
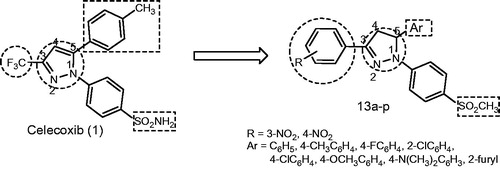
In continuation of our previous work for development of safe anti-inflammatory derivatives, we now describe the synthesis, in vitro evaluation as COX-1/COX-2 inhibitors, in vivo anti-inflammatory (AI) activity and ulcerogenic liability for a new series of 1,3,5-triaryl-4,5-dihydro-1H-pyrazoles 13a–p as celecoxib derivatives in which; (i) pyrazole ring of celecoxib was replaced with 4,5-dihydropyrazole nucleus, (ii) trifluoromethyl moiety was replaced with substituted aryl moiety; it was reported that the substituent at pyrazole C-3 has little steric restrictions for COX-2, and observed that COX-2 inhibition should be remainedCitation12, (iii) tolyl group was maintained or replaced with different substituted aryl or heteroaryl moieties and (iv) the COX-2 pharmacophore (sulphamoyl, SO2NH2) group on the N1-phenyl ring was replaced with another COX-2 pharmacophoric moiety (methanesulfonyl, SO2CH3).
Experimental
Chemistry
Melting points were determined on a Griffin apparatus and are uncorrected. Infrared (IR) spectra were recorded on a Shimadzu 435 spectrometer using KBr discs. 1H NMR and 13C NMR spectra were measured on a Bruker 400 MHz spectrometer (Faculty of Pharmacy, Beni-Suef University, Beni-Suef, Egypt) in D2O, DMSO-d6 with TMS as the internal standard, where J (coupling constant) values were estimated in hertz (Hz). Mass spectra were run on Hewlett Packard 5988 spectrometer. Microanalysis was performed for C, H, N at the Micro Analytical Center, Cairo University, Egypt and was within ±0.4% of theoretical values. All other reagents, purchased from the Acros Chemical Company (Milwaukee, WI), were used without further purification. Chalcones 11aCitation13, 11b–dCitation14, 11eCitation15, 11fCitation14 11 gCitation16, 11 hCitation17, 11i–jCitation18, 11kCitation19, 11lCitation20, 11mCitation21, 11nCitation18, 11oCitation22, 11pCitation23 and 4-methanesulfonylphenylhydrazine hydrochloride (12)Citation24 were prepared according to reported procedures.
Spectral data (14) for chalcones 11b, 11c, 11d and 11f are listed below
3-(4-Chlorophenyl)-1-(4-nitrophenyl)prop-2-en-1-one (11b)
IR (KBr disk) 3097(CH), 1662 (C=O), 1606 (Ar–C=C); 1H NMR (DMSO-d6) δ 7.57–7.54 (m, 2H, Ar–H), 7.83–7.77 (d, 1H, CH=CH, J = 15.5 Hz), 8.02–7.95 (m, 3H, Ar–H and CH=CH), 8.38 (s, 4H, Ar–H).
3-(4-Fluorophenyl)-1-(4-nitrophenyl)prop-2-en-1-one (11c)
IR (KBr disk) 1609 (Ar–C=C), 1H NMR (DMSO-d6) 7.36–7.30 (m, 2H, Ar–H), 7.83 (s, 1H, CH=CH), 7.90 (s, 1H, CH=CH), 8.03–8.00 (m, 2H, Ar–H), 8.40–8.33 (m, 4H, Ar–H).
3-(4-(Dimethylamino) phenyl)-1-(4-nitrophenyl)prop-2-en-1-one (11d)
IR (KBr disk) 3047 (C–H), 2909 (C–H), 1653 (C=O), 1598 (Ar–C=C), 3.02 (s, 6H, N(CH3)2, 6.77–6.74 (d, 2H, Ar–H, J = 8.7 Hz), 7.64–7.60 (d, 1H, CH=CH, J = 11.7 Hz), 7.75–7.71 (m, 3H, Ar–H and CH=CH), 8.36–8.28 (m, 4H, Ar–H).
3-(4-Methoxyphenyl)-1-(4-nitrophenyl)prop-2-en-1-one (11f)
IR (KBr disk) 1655 (C=O), 1595 (Ar–C=C); 1H NMR (DMSO-d6) δ 3.83 (s, 3H, OCH3), 7.03 (d, 2H, Ar–H, J = 6 Hz),7.77 (d, 2H, Ar–H, J = 12 Hz), 7.86 (d, 2H, CH=CH, J = 16.5 Hz), 8.38–8.29 (m, 4H, Ar–H).
General procedure for preparation of 1,3,5-triaryl-4,5-dihydro-1H-pyrazoles (13a–p)
To a solution of the appropriate chalcone 11a–p (2.0 mmol) in ethanol (20 mL), 4-methanesulfonylphenylhydrazine hydrochloride (12, 3.0 mmol, 0.666 g) was added and the reaction mixture was heated under reflux for 12–18 h. The reaction was monitored every 60 min interval on TLC plates using chloroform/methanol (9.5:0.5 V/V). After completion of the reaction, the mixture was poured into ice cold water. The obtained solid was filtered, washed with water, dried and crystallized from ethyl acetate to give the respective triarylpyrazoles 13a–p. Physical and spectral data for 13a–p are listed below.
1-(4-Methanesulfonylphenyl)-3-(4-nitrophenyl)-5-phenyl-4,5-dihydro-1H-pyrazole (13a)
Yield: 58%; orange powder; m.p. 118–120 °C; IR (KBr disk) 2923 (C–H aliphatic), 1322, 1136 (SO2); 1H NMR (DMSO-d6) δ 3.09 (s, 3H, SO2CH3), 3.29 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 4.06 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.79 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 7.22 (d, J = 8.8 Hz, 2H, phenyl H-3, H-5), 7.27–7.30 (m, 3H, phenyl H-2, H-6, H-4), 7.37 (d, J = 7.2 Hz, 2H, methanesulphonylphenyl H-2, H-6), 7.75 (d, J = 8.8 Hz, 2H, methanesulphonylphenyl H-3, H-5), 7.96 (d, J = 8.8 Hz, 2H, nitrophenyl H-2, H-6), 8.30 (d, J = 9.2 Hz, 2H, nitrophenyl H-3, H-5); 13C NMR (DMSO-d6) δ 43.15 (pyrazole C-4), 44.88 (SO2CH3), 64.12 (pyrazole C-5), 113.28, 124.08, 125.51, 126.52, 128.44, 128.97, 129.74, 130.34, 137.97, 140.39, 147.08, 147.19, 147.75; MS (m/z): 421 (M+., 5.10%); Anal. Calcd. for C22H19N3O4S: C, 62.69; H, 4.54; N, 9.97. Found: C, 62.81; H, 4.60; N, 10.12.
5-(4-Chlorophenyl)-1-(4-methanesulfonylphenyl)-3-(4-nitrophenyl)-4,5-dihydro-1H-pyrazole (13b)
Yield: 63%; orange powder; m.p. 264–266 °C; IR (KBr disk) 2924 (C–H aliphatic), 1331, 1137 (SO2); 1H NMR (DMSO-d6) δ 3.10 (s, 3H, SO2CH3), 3.30 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 4.05 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.81 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 7.21 (d, J = 8.8 Hz, 2H, chlorophenyl H-2, H-6), 7.32 (d, J = 8.4 Hz, 2H, chlorophenyl H-3, H-5), 7.44 (d, J = 8.4 Hz, 2H, methanesulphonylphenyl H-2, H-6), 7.72 (d, J = 8.4 Hz, 2H, methanesulphonylphenyl H-3, H-5), 8.03 (d, J = 8.4 Hz, 2H, nitrophenyl H-2, H-6), 8.30 (d, J = 8.4 Hz, 2H, nitrophenyl H-3, H-5); 13C NMR (DMSO-d6) δ 42.86 (pyrazole C-4), 44.51 (SO2CH3), 62.69 (pyrazole C-5), 113.31, 124.46, 127.54, 128.26, 129.15, 129.71, 130.90, 132.89, 138.26, 140.49, 146.77, 147.72, 149.04; MS (m/z): 455 (M+., 77.15%); Anal. Calcd. for C22H18N3O4SCl: C, 57.96; H, 3.98; N, 9.22. Found: C, 57.9; H, 3.93; N, 9.35.
5-(4-Flourophenyl)-1-(4-methanesulfonylphenyl)-3-(4-nitrophenyl)-4,5-dihydro-1H-pyrazole (13c)
Yield: 71%; orange powder; m.p. 226–228 °C; IR (KBr disk) 2927 (C–H aliphatic), 1336, 1140 (SO2); 1H NMR (DMSO-d6) δ 3.10 (s, 3H, SO2CH3), 3.29 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 4.03 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.82 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 7.17–7.21 (m, 2H, flourophenyl H-2, H-6), 7.21 (d, J = 6.8 Hz, 2H, flourophenyl H-3, H-5), 7.33 (d, J = 5.2 Hz, 2H, methanesulphonylphenyl H-2, H-6), 7.72 (d, J = 8.8 Hz, 2H, methanesulphonylphenyl H-3, H-5), 8.03 (d, J = 8.8 Hz, 2H, nitrophenyl H-2, H-6), 8.29 (d, J = 8.8 Hz, 2H, nitrophenyl H-3, H-5); 13C NMR (DMSO-d6) δ 43.18 (pyrazole C-4), 44.86 (SO2CH3), 63.48 (pyrazole C-5), 113.3, 116.75, 124.10, 126.54, 127.35, 129.00, 130.66, 136.19, 137.82, 146.96, 147.15, 147.86, 162.62 (d, J = 247 Hz, C-F); MS (m/z): 439 (M+., 99.21%); Anal. Calcd. for C22H18N3O4SF: C, 60.13; H, 4.13; N, 9.56. Found: C, 60.41; H, 4.17; N, 9.64.
5-(4-Dimethylaminophenyl)-1-(4-methanesulfonylphenyl)-3-(4-nitrophenyl)-4,5-dihydro-1H-pyrazole (13d)
Yield: 60%; red crystals; m.p. 162–165 °C; IR (KBr disk) 2918 (C–H aliphatic), 1334, 1136 (SO2); 1H NMR (DMSO-d6) δ 2.96 (s, 6H, N(CH3)2), 3.10 (s, 3H, SO2CH3), 3.26 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 4.04 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.77 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 7.11 (d, J = 8.4 Hz, 2H, dimethylaminophenyl H-3, H-5), 7.23 (d, J = 8.8 Hz, 2H, dimethylaminophenyl H-2, H-6), 7.72 (d, J = 8.8 Hz, 2H, methanesulphonylphenyl H-2, H-6), 7.81 (d, J = 8.8 Hz, 2H, methanesulphonylphenyl H-3, H-5), 8.04 (d, J = 8.8 Hz, 2H, nitrophenyl H-2, H-6), 8.30 (d, J = 8.8 Hz, 2H, nitrophenyl H-3, H-5); 13C NMR (DMSO-d6) δ 43.13 (pyrazole C-4), 44.89 (SO2CH3), 50.88 (N(CH3)2), 63.8 (pyrazole C-5), 113.30, 124.06, 124.57, 126.44, 126.62, 128.90, 129.03, 129.29, 129.73, 130.04, 138.20, 147.22, 147.65; MS (m/z): 464 (M+., 42.01%); Anal. Calcd. for C24H24N4O4S: C, 62.05; H, 5.21; N, 12.06. Found: C, 62.17; H, 5.29; N, 12.31.
1-(4-Methanesulfonylphenyl)-5-(4-methylphenyl)-3-(4-nitrophenyl)-4,5-dihydro-1H-pyrazole (13e)
Yield: 45%; orange powder; m.p. 214–216 °C; IR (KBr disk) 2976 (C–H aliphatic), 1328, 1135 (SO2); 1H NMR (DMSO-d6) δ 2.25 (s, 3H, CH3), 3.09 (s, 3H, SO2CH3), 3.25 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 4.03 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.74 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 7.15 (s, 4H, methylphenyl H-2, H-6, H-3, H-5), 7.22 (d, J = 8.4 Hz, 2H, methanesulphonylphenyl H-2, H-6), 7.71 (d, J = 8.8 Hz, 2H, methanesulphonylphenyl H-3, H-5), 8.04 (d, J = 8.8 Hz, 2H, nitrophenyl H-2, H-6), 8.30 (d, J = 8.8 Hz, 2H, nitrophenyl H-3, H-5); 13C NMR (DMSO-d6) δ 21.10 (CH3), 43.03 (pyrazole C-4), 44.50 (SO2CH3), 63.18 (pyrazole C-5), 113.29, 124.48, 126.14, 127.46, 129.06, 130.26, 130.61, 137.63, 138.40, 138.60, 146.89, 147.62, 148.93; MS (m/z): 435 (M+., 72.10%); Anal. Calcd. for C23H21N3O4S: C, 63.43; H, 4.86; N, 9.65. Found: C, 63.60; H, 4.94; N, 9.78.
1-(4-Methanesulfonylphenyl)-5-(4-methoxyphenyl)-3-(4-nitrophenyl)-4,5-dihydro-1H-pyrazole (13f)
Yield: 55%; orange powder; m.p. 212–214 °C; IR (KBr disk) 2921 (C–H aliphatic), 1332, 1138 (SO2); 1H NMR (DMSO-d6) δ 3.00 (s, 3H, SO2CH3), 3.79 (s, 3H, OCH3), 3.23 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 3.93 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.46 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 6.89 (d, J = 8.0 Hz, 2H, methoxyphenyl H-2, H-6), 7.18 (d, J = 7.6 Hz, 4H, methoxyphenyl H-3, H-5 and methanesulphonylphenyl H-2, H-6), 7.71 (d, J = 8.4 Hz, 2H, methanesulphonylphenyl H-3, H-5), 7.88 (d, J = 8.4 Hz, 2H, nitrophenyl H-2, H-6), 8.24 (d, J = 8.4 Hz, 2H, nitrophenyl H-3, H-5); 13C NMR (DMSO-d6) δ 43.19 (pyrazole C-4), 44.89 (SO2CH3), 55.34 (OCH3), 63.69 (pyrazole C-5), 113.31, 114.92, 124.07, 126.49, 126.80, 128.91, 130.26, 132.39, 138.09, 147.13, 147.22, 147.71, 159.55; MS (m/z): 451 (M+., 100%); Anal. Calcd. for C23H21N3O5S: C, 61.18; H, 4.69; N, 9.31. Found: C, 61.42; H, 4.78; N, 9.47.
5-(2-Chlorophenyl)-1-(4-methanesulfonyl-phenyl)-3-(4-nitrophenyl)-4,5-dihydro-1H-pyrazole (13g)
Yield: 71%; orange powder; m.p. 220–222 °C; IR (KBr disk) 2918 (C–H aliphatic), 1339, 1139 (SO2); 1H NMR (DMSO-d6) δ 3.11 (s, 3H, SO2CH3), 3.28 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 4.14 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.94 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 7.01–7.13 (m, 3H, chlorophenyl H-4, H-5, H-6), 7.37 (d, J = 6.8 Hz, 2H, methanesulphonylphenyl H-2, H-6), 7.60 (s, 1H, chlorophenyl H-3), 7.75 (d, J = 9.2 Hz, 2H, methanesulphonylphenyl H-3, H-5), 8.04 (d, J = 8.8 Hz, 2H, nitrophenyl H-2, H-6), 8.29 (d, J = 8.8 Hz, 2H, nitrophenyl H-3, H-5); 13C NMR (DMSO-d6) δ 41.77 (pyrazole C-4), 44.87 (SO2CH3), 61.06 (pyrazole C-5), 113.18, 124.08, 126.59, 127.93, 129.11, 129.66, 130.44, 130.67, 131.78, 137.05, 137.80, 146.81, 147.69, 147.86; MS (m/z): 455 (M+., 24.27%); Anal. Calcd. for C22H18N3O4SCl: C, 57.96; H, 3.98; N, 9.22. Found: C, 57.92; H, 4.05; N, 9.40.
5-(2-Furyl)-1-(4-methanesulfonyl-phenyl)-3-(4-nitrophenyl)-4,5-dihydro-1H-pyrazole (13h)
Yield: 62%; grey powder; m.p. 152–154 °C; IR (KBr disk) 2924 (C–H aliphatic), 1334, 1137 (SO2); 1H NMR (DMSO-d6) δ 3.12 (s, 3H, SO2CH3), 3.52 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 3.91 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.93 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 6.41 (s, 1H, furyl H-5), 6.58 (s, 1H, furyl H-4), 7.37 (d, J = 8.8 Hz, 2H, methanesulphonylphenyl H-2, H-6), 7.58 (s, 1H, furyl H-3), 7.75 (d, J = 8.8 Hz, 2H, methanesulphonylphenyl H-3, H-5), 8.05 (d, J = 8.8 Hz, 2H, nitrophenyl H-2, H-6), 8.31 (d, J = 8.8 Hz, 2H, nitrophenyl H-3, H-5); 13C NMR (DMSO-d6) δ 39.72 (pyrazole C-4), 44.88 (SO2CH3), 57.52 (pyrazole C-5), 110.64, 113.14, 120.80, 124.08, 126.55, 128.96, 129.76, 130.35, 131.54, 133.60, 147.40, 148.64, 151.53; MS (m/z): 411 (M+., 73.78%); Anal. Calcd. for C20H17N3O5S: C, 58.38; H, 4.16; N, 10.21. Found: C, 58.52; H, 4.23; N, 10.37.
1-(4-Methanesulfonyl-phenyl)-3-(3-nitrophenyl)-5-phenyl-4,5-dihydro-1H-pyrazole (13i)
Yield: 65%; yellow powder; m.p. 194–196 °C; IR (KBr disk) 2922 (C–H aliphatic), 1349, 1132 (SO2); 1H NMR (DMSO-d6) δ 3.09 (s, 3H, SO2CH3), 3.30 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 4.08 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.76 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 7.20 (d, J = 8.8 Hz, 2H, phenyl H-3, H-5), 7.25–7.34 (m, 3H, phenyl H-2, H-6, H-4), 7.37 (d, J =6.8 Hz, 2H, methanesulphonylphenyl H-2, H-6), 7.70 (d, J = 8.8 Hz, 2H, methanesulphonylphenyl H-3, H-5), 7.75 (t, J = 7.6 Hz, 1H, nitrophenyl H-5), 8.21 (d, J = 7.6 Hz, 1H, nitrophenyl H-6), 8.26 (d, J = 6 Hz, 1H, nitrophenyl H-4), 8.55 (s, 1H, nitrophenyl H-2); 13C NMR (DMSO-d6) δ 43.35 (pyrazole C-4), 44.91 (SO2CH3), 63.93 (pyrazole C-5), 113.08, 120.78, 123.70, 125.51, 128.39, 128.98, 129.60, 129.74, 130.34, 131.51, 133.73, 140.39, 147.26; MS (m/z): 421 (M+., 100%); Anal. Calcd. for C22H19N3O4S: C, 62.69; H, 4.54; N, 9.97. Found: C, 62.88; H, 4.58; N; 10.04.
5-(4-Chlorophenyl)-1-(4-methanesulfonyl-phenyl)-3-(3-nitrophenyl)-4,5-dihydro-1H-pyrazole (13j)
Yield: 45%; yellow powder; m.p. 222–224 °C; IR (KBr disk) 2922 (C–H aliphatic), 1347, 1132 (SO2); 1H NMR (DMSO-d6) δ 3.09 (s, 3H, SO2CH3), 3.37 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 4.06 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.79 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 7.19 (d, J = 8.8 Hz, 2H, chlorophenyl H-2, H-6), 7.32 (d, J = 8.8 Hz, 2H, chlorophenyl H-3, H-5), 7.44 (d, J = 8.4 Hz, 2H, methanesulphonylphenyl H-2, H-6), 7.72 (d, J = 9.2 Hz, 2H, methanesulphonylphenyl H-3, H-5), 7.77 (t, J =8.0 1H, nitrophenyl H-5), 8.20 (d, J = 8.0 Hz, 1H, nitrophenyl H-6), 8.27 (d, J = 8 Hz, 1H, nitrophenyl H-4), 8.54 (s, 1H, nitrophenyl H-2); 13C NMR (DMSO-d6) δ 43.03 (pyrazole C-4), 44.56 (SO2CH3), 62.47 (pyrazole C-5), 113.07, 120.72, 124.17, 128.28, 129.15, 129.66, 130.49, 130.91, 132.83, 133.69, 140.56, 147.01, 148.65, 149.25; MS (m/z): 455 (M+., 24.53%); Anal. Calcd. for C22H18N3O4SCl: C, 57.96; H, 3.98; N, 9.22. Found: C, 57.85; H, 3.86; N, 9.22.
5-(4-Flourophenyl)-1-(4-methanesulfonyl-phenyl)-3-(3-nitrophenyl)-4,5-dihydro-1H-pyrazole (13k)
Yield: 65%; yellow powder; m.p. 192–194 °C; IR (KBr disk) 2923 (C–H aliphatic), 1347, 1136 (SO2); 1H NMR (DMSO-d6) δ 3.09 (s, 3H, SO2CH3), 3.29 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 4.06 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.78 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 7.16–7.20 (m, 2H, flourophenyl H-2, H-6), 7.20 (d, J = 3.2 Hz, 2H, flourophenyl H-3, H-5), 7.33 (d, J = 3.2 Hz, 2H, methanesulphonylphenyl H-2, H-6), 7.71 (d, J = 9.2 Hz, 2H, methanesulphonylphenyl H-3, H-5), 7.77 (t, J = 8 Hz, 1H, nitrophenyl H-5), 8.20 (d, J = 8 Hz, 1H, nitrophenyl H-6), 8.26 (d, J = 6.4 Hz, 1H, nitrophenyl H-4), 8.54 (s, 1H, nitrophenyl H-2); 13C NMR (DMSO-d6) δ 43.36 (pyrazole C-4), 44.89 (SO2CH3), 63.25 (pyrazole C-5), 113.09, 116.70, 120.78, 123.79, 127.35, 128.99, 129.79, 130.23, 131.52, 133.60, 136.27, 147.26, 148.63, 162.47 (d, J = 247 Hz, C-F); MS (m/z): 439 (M+., 100%); Anal. Calcd. for C22H18N3O4SF: C, 60.13; H, 4.13; N, 9.56. Found: C, 59.92; H, 3.89; N, 9.66.
5-(4-Dimethylaminophenyl)-1-(4-methanesulfonyl-phenyl)-3-(3-nitrophenyl)-4,5-dihydro-1H-pyrazole (13l)
Yield: 47%; green powder; m.p. 192–194 °C; IR (KBr disk) 2917 (C–H aliphatic), 1349, 1141 (SO2); 1H NMR (DMSO-d6) δ 2.84 (s, 6H, N(CH3)2), 3.08 (s, 3H, SO2CH3), 3.25 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 4.00 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.62 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 6.68 (d, J = 8.8 Hz, 2H, dimethylaminophenyl H-3, H-5), 7.10 (d, J = 8.8 Hz, 2H, dimethylaminophenyl H-2, H-6), 7.22 (d, J = 9.2 Hz, 2H, methanesulphonylphenyl H-2, H-6), 7.69 (d, J = 8.8 Hz, 2H, methanesulphonylphenyl H-3, H-5), 7.77 (t, J = 8.0 Hz, 1H, nitrophenyl H-5), 8.19 (d, J = 7.6 Hz, 1H, nitrophenyl H-6), 8.25 (d, J = 6.0 Hz, 1H, nitrophenyl H-4), 8.55 (s, 1H, nitrophenyl H-2); 13C NMR (DMSO-d6) δ 43.61 (pyrazole C-4), 44.92 (SO2CH3), 50.88 (N(CH3)2), 63.57 (pyrazole C-5), 113.09, 113.19, 120.71, 123.55, 126.64, 128.90, 129.02, 129.71, 131.47, 133.93, 147.33, 147.43, 148.61; MS (m/z): 464 (M+., 36.40%); Anal. Calcd. for C24H24N4O4S: C, 62.05; H, 5.21; N, 12.06. Found: C, 62.31; H, 5.32; N, 12.17.
1-(4-Methanesulfonylphenyl)-5-(4-methylphenyl)-3-(3-nitrophenyl)-4,5-dihydro-1H-pyrazole (13m)
Yield: 48%; yellow powder; m.p. 172–174 °C; IR (KBr disk) 2926 (C–H aliphatic), 1348, 1137 (SO2); 1H NMR (DMSO-d6) δ 2.25 (s, 3H, CH3), 3.07 (s, 3H, SO2CH3), 3.25 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 4.03 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.68 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 7.15 (s, 4H, methylphenyl H-2, H-6, H-3, H-5), 7.22 (d, J = 8.4 Hz, 2H, methanesulphonylphenyl H-2, H-6), 7.68 (d, J = 8.4 Hz, 2H, methanesulphonylphenyl H-3, H-5), 7.75 (t, J = 8.0 Hz, 1H, nitrophenyl H-5), 8.17 (d, J = 8.0 Hz, 1H, nitrophenyl H-6), 8.24 (d, J = 8.0 Hz, 1H, nitrophenyl H-4), 8.52 (s, 1H, nitrophenyl H-2); 13C NMR (DMSO-d6) δ 21.08 (CH3), 43.19 (pyrazole C-4), 44.52 (SO2CH3), 62.96 (pyrazole C-5), 113.04, 120.56, 124.06, 126.14, 129.04, 130.10, 130.22, 130.92, 132.73, 133.76, 137.59, 138.62, 147.14, 148.61, 149.11; MS (m/z): 435 (M+., 66.81%); Anal. Calcd. for C23H21N3O4S: C, 63.43; H, 4.86; N, 9.65. Found: C, 63.3; H, 4.43; N, 9.68.
1-(4-Methanesulfonylphenyl)-5-(4-methoxyphenyl)-3-(3-nitrophenyl)-4,5-dihydro-1H-pyrazole (13n)
Yield: 84%; yellow powder; m.p. 184–186 °C; IR (KBr disk) 2920 (C–H aliphatic), 1348, 1136 (SO2); 1H NMR (DMSO-d6) δ 3.08 (s, 3H, SO2CH3), 3.28 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 3.70 (s, 3H, OCH3), 4.03 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.69 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 6.92 (d, J = 8.8 Hz, 2H, methoxyphenyl H-2, H-6), 7.20 (d, J = 6.8 Hz, 2H, methoxyphenyl H-3, H-5), 7.22 (d, J = 6.0 Hz, 2H, methanesulphonylphenyl H-2, H-6), 7.70 (d, J = 8.8 Hz, 2H, methanesulphonylphenyl H-3, H-5), 7.77 (t, J = 8 Hz, 1H, nitrophenyl H-5), 8.20 (d, J = 8 Hz, 1H, nitrophenyl H-6), 8.27 (d, J = 8 Hz, 1H, nitrophenyl H-4), 8.54 (s, 1H, nitrophenyl H-2); 13C NMR (DMSO-d6) δ 43.25 (pyrazole C-4), 44.56 (SO2CH3), 55.53(OCH3), 62.73 (pyrazole C-5), 113.08, 115.00, 120.61, 124.06, 127.54, 129.04, 130.21, 130.91, 132.74, 133.53, 133.86, 147.17, 148.66, 149.12, 159.21; MS (m/z): 451 (M+., 100%); Anal. Calcd. for C23H21N3O5S: C, 61.18; H, 4.69; N, 9.31. Found: C, 61.44; H, 4.81; N, 9.44.
5-(2-Chlorophenyl)-1-(4-methanesulfonyl-phenyl)-3-(3-nitrophenyl)-4,5-dihydro-1H-pyrazole (13o)
Yield: 75%; yellow powder; m.p. 198–200 °C; IR (KBr disk) 2926 (C–H aliphatic), 1349, 1139 (SO2); 1H NMR (DMSO-d6) δ 3.10 (s, 3H, SO2CH3), 3.30 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 4.17 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.93 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 7.01–7.13 (m, 3H, chlorophenyl H-4, H-5, H-6), 7.37 (d, J =6.8 Hz, 2H, methanesulphonylphenyl H-2, H-6), 7.60 (s, 1H, chlorophenyl H-3), 7.7 (d, J = 8.8 Hz, 2H, methanesulphonylphenyl H-3, H-5), 7.75 (s, 1H, nitrophenyl H-5), 8.21 (d, J = 7.6 Hz, 1H, nitrophenyl H-4), 8.26 (d, J = 6.8 Hz, 1H, nitrophenyl H-6), 8.56 (s, 1H, nitrophenyl H-2); 13C NMR (DMSO-d6) δ 41.94 (pyrazole C-4), 44.89 (SO2CH3), 61.06 (pyrazole C-5), 112.99, 120.82, 123.83, 126.64, 127.90, 129.12, 129.62, 129.77, 130.32, 130.43, 131.55, 131.81, 133.58, 137.11, 147.03, 147.78, 148.62; MS (m/z): 455 (M+., 90.64%); Anal. Calcd. for C22H18N3O4SCl: C, 57.96; H, 3.98; N, 9.22. Found: C, 57.7; H, 3.80; N, 9.17.
5-(2-Furyl)-1-(4-methanesulfonylphenyl)-3-(3-nitrophenyl)-4,5-dihydro-1H-pyrazole (13p)
Yield: 73%; green powder; m.p. 186–188 °C; IR (KBr disk) 2923 (C–H aliphatic), 1348, 1131 (SO2); 1H NMR (DMSO-d6) δ 3.11 (s, 3H, SO2CH3), 3.56 (dd, J = 17.6, 5.6 Hz, 1H, pyrazole H-4), 3.93 (dd, J = 17.6, 12.0 Hz, 1H, pyrazole H′-4), 5.91 (dd, J = 12.0, 5.6 Hz, 1H, pyrazole H-5), 6.41 (s, 1H, furfural H-5), 6.57 (s, 1H, furfural H-4), 7.37 (d, J = 8.8 Hz, 2H, methanesulphonylphenyl H-2, H-6), 7.58 (s, 1H, furfural H-3), 7.74 (d, J = 8.8 Hz, 2H, methanesulphonylphenyl H-3, H-5), 7.79 (d, J = 8 Hz, 1H, nitrophenyl H-5), 8.24 (d, J = 8 Hz, 1H, nitrophenyl H-6), 8.28 (d, J = 8 Hz, 1H, nitrophenyl H-4), 8.56 (s, 1H, nitrophenyl H-2); 13C NMR (DMSO-d6) δ 39.92 (pyrazole C-4), 44.92 (SO2CH3), 57.36 (pyrazole C-5), 107.90, 110.64, 113.14, 120.80, 123.79, 128.96, 129.76, 130.35, 131.54, 133.60, 142.96, 147.40, 148.64, 151.53; MS (m/z): 411 (M+., 79.48%); Anal. Calcd. for C20H17N3O5S: C, 58.38; H, 4.16; N, 10.21. Found: C, 58.49; H, 4.21; N, 10.42.
Biological evaluation
COX-1/COX-2 inhibition colorimetric assay
The in vitro inhibition of ovine COX-1/COX-2 was measured using an enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions and as reported beforeCitation25.
In vivo anti-inflammatory activity
Animals
Male Wister albino rats with average body weight of 150 g were used in the experiments. The animals were kept under controlled environment of humidity 30–60%, light period of 12 h/day and temperature 27 ± 2 °C with access to food and water. The experimental procedures were carried out in compliance with the Institutional Animal Ethics Committee regulations. All experiments were performed in the morning according to the guidelines for the care of laboratory animals.
Carrageenan induced rat paw edema
Rats were divided into 18 groups of five animals each. The first group was administered vehicle; the second one was administrated celecoxib (50 mg/kg) while the remaining groups were administrated test compounds (13a–p, 50 mg/kg, and one group per one compound). Immediately thereafter and under light anesthesia, the animals received 100 μL of vehicle or carrageenan (1% in saline) S.C. on the plantar surface of the left hind paw as reported beforeCitation26. The development of paw edema was assessed by measuring paw-volume changes at 1, 3 and 5 h after carrageenan injection using a pair of dial thickness gauge calipers accurate to 0.001 cm. The right hind paw served as a reference of non-inflamed paw for comparison. Results are expressed as paw-volume change (mL).
Additionally, the ED50 values for the most potent derivatives (13d, 13f, 13k and 13o) were calculated using three different doses and paw thickness of each rat was measured after 3 h of carrageenan injection according to the reported procedureCitation26.
Ulcerogenic liability study
Ulcerogenic liability for the most biologically active synthesized compounds (13d, 13f, 13k and 13o), celecoxib and aspirin was evaluated using the previously reported proceduresCitation27. Adult male albino rats weighing between 120 and 150 g were used in this study and divided into seven groups each of five animals. The animals were fasted for 20 h before drug administration. The first group was given 1% aqueous solution of tween 80 and was used as the control group. The second and third groups received celecoxib and aspirin, respectively, were used as reference drugs in a dose 100 mg/kg suspended in 1% tween. The remaining groups received the tested compounds (13d, 13f, 13k and 13o) in a dose 50 mg/kg suspended in 1% tween. Treatment was continued once daily for three successive days in all groups. Two hours after the last dose, rats were sacrificed under general anesthesia and the stomach was removed, opened along the greater curvature and rinsed with saline. The gastric mucosa was examined with a magnifying lens (10×) for the presence of lesions in the form of hemorrhages or linear breaks and erosions. The expression of the degree of ulcerogenic effect was in terms of dividing the percentage incidence of ulcers in each group of animal by 10, the average number of ulcers per stomach and the visual observation of the ulcer score (average severity of ulcer). The ulcer scores are divided into 0 ulcer score which indicates no ulcer, 1 ulcer score which indicates only mucosal erythema, 2 ulcer score which indicates mild mucosal edema, slight bleeding or slight erosions, 3 ulcer score which indicates moderate edema, bleeding ulcers or erosions and 4 ulcer index which indicates severe ulceration, erosions, edema and tissue necrosis. The sum of all above values indicates the ulcer index.
Statistical analysis
Significant difference among groups was assessed using two way ANOVA followed by post hoc Tukey test. The results were expressed as mean ± standard error (SE). Differences were considered significant at *p < 0.01.
Molecular modeling
The crystal structures of celecoxib bound at the COX-2 (PDB ID: 2AW1) active sitesCitation28 (obtained from protein data bank at Research Collaboration for Structural Bioinformatics (RSCB) protein database [PDB]). Docking of the co-crystallized ligand should be carried out to study the scoring energy (s), root mean standard deviation (rmsd) and amino acid interactions. Root mean square deviation (rmsd) which is the measure of superposing was 0.50 Å for the lead compounds. Docking was performed using London dG force and refinement of the results was achieved using force field energy. Preparation of the s compounds for docking was achieved via their 3D structure built by Molecular Operating Environment (MOE, Version 2005.06, Chemical Computing Group Inc., Montreal, Quebec, Canada). Certain procedures were taken before docking which include: 3D protonation of the structures, running conformational analysis using systemic search, selecting the least energetic conformer and applying the same docking protocol used with ligands. Docking for the most active biologically compounds (13d, 13f, 13k and 13o) was applied. Binding interactions between the amino acid residues and functional groups of the compounds are summarized in .
Results and discussion
Chemistry
A group of 3-(3/4-nitrophenyl)-5-(substituted-aryl)-1-(4-methanesulphonylphenyl)-4,5-dihydro-1H-pyrazoles (13a–p) were synthesized using the reaction sequence illustrated in Scheme 1. Accordingly, the reaction of 3-nitro and 4-nitroacetophenone (9a, b) with different aldehydes 10a–h provided the corresponding chalcones 11a–p which upon cyclization with 4-methanesulfonylphenylhydrazine hydrochloride (12) in ethanol under reflux conditions gave the target triarylpyrazole derivatives 13a–p in good yields (45–84%). All the newly synthesized compounds have been characterized by IR, 1H NMR, 13C NMR, mass spectra and elemental analyses.
The IR spectra of dihydropyrazole compounds 13a–p showed two sharp peaks at 1141–1131 cm−1 and 1349–1322 cm−1 corresponding to SO2. The 1H NMR spectra of dihydropyrazole compounds 13a–p showed three signals as doublet of doublet (dd), each of one proton intensity, one at δ 3.23–3.56, second at δ 3.91–4.17, and the third at δ 5.46–5.94 with three different J values (17.6, 12.0, 5.6 Hz) corresponding to three protons of the dihydropyrazole ring. The highest J value was due to geminal coupling of two protons at position 4 while the other two J values due to coupling of two geminal protons with proton at position 5. Additionally, 13C NMR spectra of 13a–p confirmed the presence of dihydropyrazole ring through presence of three peaks at δ 39.72–43.61, 61.06–64.12 and 147.26–149.04 corresponding to dihydropyrazole C-4, C-5 and C-3, respectively.
Biological evaluation
In vitro COX inhibition assay
In vitro structure–activity relationships acquired for this group of triarylpyrazole derivatives (13a–p) showed that they exhibit a broad range (moderately potent to weekly potent) of COX-1 (IC50 = 3.4–13.4 μM), and COX-2 (IC50 = 0.74–5.4 μM range, see data in ), inhibitory activities. All compounds were more potent inhibitors for COX-2 isozyme than COX-1 isozyme. Compounds having 4-CH3C6H4 (13e, 13m), 4-OCH3C6H4 (13f, 13n) and 2-furyl (13h, 13p) moieties were generally more potent inhibitors of COX-2 and in turn more COX-2 selective (COX-2 S.I.=3.61–5.23) than compounds having the other moieties. The 4-methoxy derivative (13f) was the most potent COX-2 inhibitor (IC50 = 0.74 μM) while the 4-tolyl derivative (13m) was the most COX-2 selective (S.I.=5.23) in comparison with the reference drug celecoxib (COX-2 IC50 = 1.3 μM, S.I.=6.07).
Table 1. In vitro COX-1 and COX-2 inhibition of triarylpyrazoles 13a–p and celecoxib.
In vivo anti-inflammatory activity
The anti-inflammatory activity of the all prepared triarylpyrazole derivatives (13a–p) were evaluated using carrageenan-induced rat paw edema assay. Each compound was administered orally (100 mg/kg) immediately prior to induction of inflammation by carrageenan subcutaneous injection. The anti-inflammatory activity was then calculated based on paw-volume changes at 1, 3 and 5 h after carrageenan injection as presented in .
Table 2. Anti-inflammatory activities for triarylpyrazoles 13a–p and celecoxib.
A comparable study of the anti-inflammatory activity of the test compounds relative to celecoxib as a reference drug at different time intervals indicated that after 1 h, the triarylpyrazole derivatives (13a–p) showed edema inhibition percentage activities 37.58–93.43% and the 4-dimethylamino derivative (13d) was the most potent one (93.43% edema inhibition) while after 3 h, 13a–p showed higher edema inhibition percentage activities 70.42–97.18% and both 4-dimethylamino derivative 13d and 2-chloro derivative 13o were the most potent derivatives (97.18% edema inhibition for both) (). After 5 h, all compounds showed higher edema inhibition percentage activities of 91.09–99.10 and the 4-dimethylamino derivative (13d) was still the highest potent derivative (99.10% edema inhibition). At all time intervals (1, 3 and 5 h), 4-dimethylamino derivative 13d, 4-methoxy derivative 13f, 4-fluoro derivative 13k and 2-chloro derivative 13o displayed good anti-inflammatory activities (93.43–99.34, 66.1–98.61, 69.14–99.10 and 74.24–99.10%, respectively) comparable to that of celecoxib (92.77–99.51%) and were the most potent derivatives among the 16 triarylpyrazole compounds (13a–p).
Table 3. The anti-inflammatory activity (ED50, μmol/kg) of most potent triarylpyrazoles 13d, 13f, 13k, 13o and celecoxib.
Moreover, the ED50 values for the most potent derivatives (13d, 13f, 13k, 13o) were calculated in comparison with celecoxib (). The four derivatives showed good anti-inflammatory activities (ED50 = 66.5–79.8 μmol/kg), while 4-dimethylamino derivative 13d (ED50 = 66.5 μmol/kg) was more potent than celecoxib (ED50 = 68.1 μmol/kg), 4-methoxy derivative 13f, 4-fluoro derivative 13k and 2-chloro derivative 13o were less potent but with acceptable ED50 values (ED50 = 73.4, 79.8 and 70.5 μmol/kg, respectively).
Ulcerogenic liability
The most potent anti-inflammatory compounds (13d, 13f, 13k and 13o), low ulcerogenic reference drug (celecoxib) and high ulcerogenic reference drug (aspirin) were subjected to ulcerogenic liability at 100 mg/kg dose and the results are recorded in . It was clear that the tested compounds caused ulceration effect comparable to that of celecoxib (the relative ulcerogenicity of the tested compounds to celecoxib = 1.16–1.48). While the 4-dimethylamino derivative 13d (ulcer index = 3.89) was the least ulcerogenic derivative in comparison with celecoxib (ulcer index = 3.35), the other three derivatives (13f, 13k, 13o) showed ulcerogenic potential (ulcer index = 4.86, 4.96 and 3.92, respectively) slightly higher than celecoxib (ulcer index = 3.35). In comparison with the traditional NSAID aspirin (ulcer index = 22.75), all the tested compounds were about 4–6 folds less ulcerogenic with relative ulcerogenicity range of 0.17–0.22.
Table 4. Ulcerogenic effect of triarylopyrazoles 13d, 13f, 13k, 13o, aspirin and celecoxib.
Molecular modeling
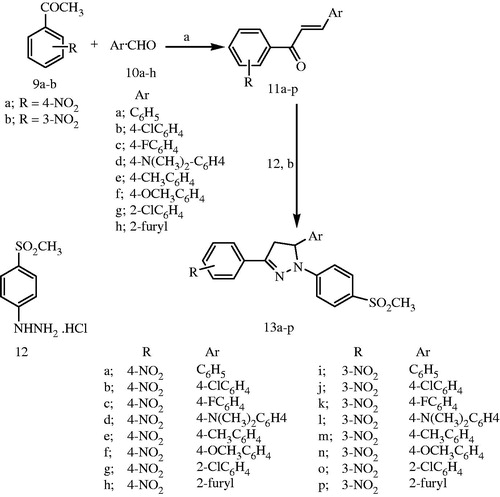
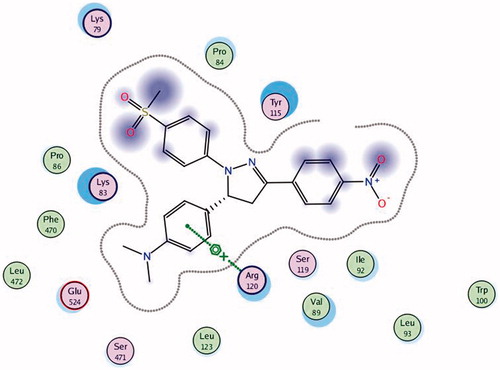
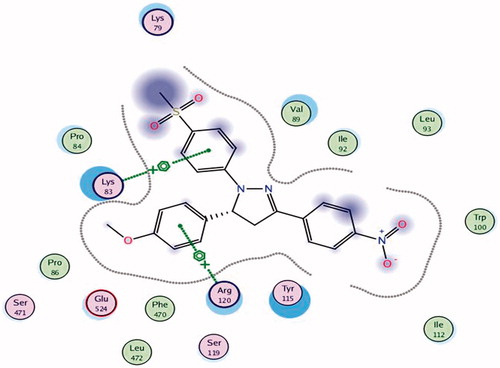
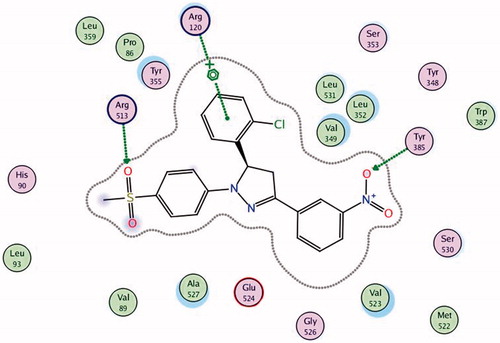
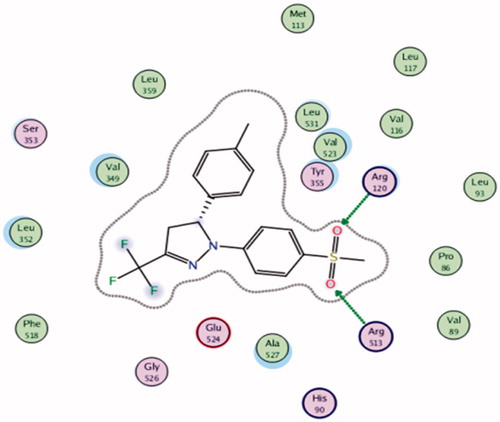
To know the plausible mode of interactions of the most potent anti-inflammatory compounds (13d, 13f, 13k and 13o), molecular docking experiments were performed using X-ray crystal structure data for COX-2 obtained from the protein data bank using the COX-2 selective reference drug (celecoxib, 1) as a ligand (). The docking results including the energy associated with intermolecular interactions (affinity in kcal/mol) obtained upon computational docking for all compounds (13d, 13f, 13k, 13o and celecoxib) within COX-2 active sites and the binding interactions between the amino acid residues and functional groups of the compounds are summarized in . While compounds 13d and 13f showed appreciable binding interactions (affinity in kcal/mol −6.9498 and −6.7965, respectively), compounds 13k and 13o showed binding interactions (affinity in kcal/mol −2.5760 and −2.1774, respectively) in comparison with celecoxib (affinity in kcal/mol −6.5330).
Table 5. Molecular modeling data for compounds 13d, 13f, 13k, 13o and celecoxib during docking in COX-2 (PDB ID: 1CX2) active site.
Conclusion
A new series of 1,3,5-triaryl-4,5-dihydro-1H-pyrazole derivatives 13a–p were synthesized for evaluation as COX inhibitors, anti-inflammatory agents and ulcerogenic liability. Structure–activity data acquired and biological studies showed that (i) all compounds were more potent COX-2 inhibitors than COX-1, (ii) most of the evaluated compounds showed good anti-inflammatory activity at different time intervals especially compounds 13d, 13f, 13k and 13o, (iii) while compound 13d was more potent than celecoxib compounds, 13f, 13k and 13o were less potent but with acceptable ED50 values and (iv) the four compounds (13d, 13f, 13k and 13o) were 4–6 folds less ulcerogenic than aspirin and showed ulceration effect comparable to that of celecoxib.
Supplementary material available online
Declaration of interest
The authors have declared no conflict of interest.
References
- Nonato FR, Nogueira TMO, Barros TAA, et al. Antinociceptive and anti-inflammatory activities of Adiantum latifolium lam.: evidence for a role of IL-1β inhibition. J Ethnopharm 2011;136:518–24
- Al-Hourani BJ, Sharma SK, Mane JY, et al. Synthesis and evaluation of 1,5-diaryl-substituted tetrazoles as novel selective cyclooxygenase-2 (COX-2) inhibitors. Bioorg Med Chem Lett 2011;21:1823–6
- Ghodsi R, Zarghi A, Daraei B, Hedayati M. Design, synthesis and biological evaluation of new 2,3-diarylquinoline derivatives as selective cyclooxygenase-2 inhibitors. Bioorg Med Chem Lett 2010;18:1029–33
- Rathish IG, Javed K, Ahmad S, et al. Synthesis and antiinflammatory activity of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide. Bioorg Med Chem Lett 2009;19:255–8
- Dogné JM, Supuran CT, Pratico D. Adverse cardiovascular effects of the coxibs. J Med Chem 2005;48:2251–7
- Abdellatif KRA, Chowdhury MA, Knaus EE. Synthesis of new 1-[4-methane (amino) sulfonylphenyl)]-5-[4-(aminophenyl)]-3-trifluoromethyl-1H-pyrazoles. J Het Chem 2008;45:1707–10
- Abdellatif KRA, Chowdhury MA, Dong Y, Knaus EE. Diazen-1-ium-1,2-diolated nitric oxide donor ester prodrugs of 1-(4-methanesulfonylphenyl)-5-aryl-1H-pyrazol-3-carboxylic acids: synthesis, nitric oxide release studies and anti-inflammatory activities. Bioorg Med Chem 2008;16:6528–34
- Abdellatif KRA, Chowdhury MA, Dong Y, et al. Diazen-1-ium-1,2-diolated nitric oxide donor ester prodrugs of 5-(4-hydroxymethylphenyl)-1-(4-aminosulfonylphenyl)-3-trifluoromethyl-1H-pyrazole and its methanesulfonyl analog: synthesis, biological evaluation and nitric oxide release studies. Bioorg Med Chem 2008;16:9694–8
- Chowdhury MA, Abdellatif KRA, Dong Y, Knaus EE. Synthesis of new 4-[2-(4-methyl(amino)sulfonylphenyl)-5-trifluoromethyl-2H-pyrazol-3-yl]-1,2,3,6-tetrahydropyridines: a search for novel nitric oxide donor anti-inflammatory agents. Bioorg Med Chem 2008;16:8882–8
- Abdellatif KRA, Elshemy HAH, Salama. SA, Omar HA. Synthesis, characterization and biological evaluation of novel 4′-fluoro-2′-hydroxy-chalcones derivatives as antioxidant, anti-inflammatory and analgesic agents. J Enz Inhib Med Chem 2015;30:484–91
- Abdellatif KRA, Abdelwahab MA, Labib M, Zidan TH. Synthesis, cyclooxygenase inhibition, anti-inflammatory evaluation and ulcerogenic liability of novel triarylpyrazoline derivatives as selective COX-2 inhibitors. Bioorg Med Chem Lett 2015;25:5787–91
- Ahlström MM, Redderström M, Zamora I, Luthman K. CYP2C9 structure – metabolism relationships: optimizing the metabolic stability of COX-2 inhibitors. J Med Chem 2007;50:4444–52
- Romagnoli R, Baraldi PG, Carrion MD, et al. Hybrid alpha-bromoacryloylamido chalcones. Design, synthesis and biological evaluation. Bioorg Med Chem Lett 2009;19:2022–8
- Gupta RA, Kaskhedikar SG. Synthesis, antitubercular activity, and QSAR analysis of substituted nitroaryl analogs: chalcone, pyrazole, isoxazole, and pyrimidines. Med Chem Res 2013;22:3863–80
- Fun HK, Chantrapromma S, Patil PS, et al. (E)-3-(4-methyl-phen-yl)-1-(4-nitro-phenyl)prop-2-en-1-one. Acta Crystallograph E Struct Reprts Online 2008;64:o954–5
- Indorkar D, Chourasia OP, Limaye SN. Synthesis and characterization of some new synthesis of 1-acetyl-3-(4-nitrophenyl)-5-(substituted phenyl) pyrazoline derivative and antimicrobial activity. Int J Curr Microbial APP Sci 2015;4:670–8
- Zheng CJ, Jiang SM, Chen ZH, et al. Synthesis and anti-bacterial activity of some heterocyclic chalcone derivatives bearing thiofuran, furan, and quinoline moieties. Archiv Pharm 2011;344:689–95
- Mishra SK, Sahoo SP, Panda K, et al. Synthesis and antimicrobial activity of some 1-(2,4-dinitro-phenyl)-3-(3-nitrophenyl)-5-(4-substituted phenyl)-4-bromo-2-pyrazolines and 1-(2,4-dinitrophenyl)-3-(3-nitrophenyl)-5-(4-substituted phenyl)-2-pyrazoline-4-ones. Acta Pol Pharmaceut Drug Res 2007;64:359–64
- Qiu X, Li S, Shi A. Synthesis and biological activities of chalcones derived from nitroacetophenone. Adv Mater Res 2012;518:255–60
- Mageed IY, Jassim WK, Ahmed A, Jaasim IK. Synthesis and characterization of some new compounds including heterocyclic units. Aust J Basic Appl Sci 2014;8:404–11
- Balaji PN, Sai Sreevani M, Harini P, et al. Antimicrobial activity of some novel synthesized heterocyclic compounds from substituted chalcones. J Chem Pharm Res 2010;2:754–8
- Azam MA, Suresh B. Synthesis and biological evaluation of some novel 2-mercaptobenzothiazoles carrying 2-pyrazoline. J Sci Ind Res 2012;71:113–19
- Mamaghani M, Mahmoodi NO, Moghisseh AA, Pourmohamad L. Synthesis and kinetic resolution of furyl substituted secondary carbinols by porcine pancreatic lipase under solvent free conditions. J Iran Chem Soc 2008;5:238–43
- Abdellatif KRA, Chowdhury MA, Velázquez C, et al. Celecoxib prodrugs possessing a diazen-1-ium-1,2-diolate nitric oxide donor moiety: synthesis, biological evaluation and nitric oxide release studies. Bioorg Med Chem Lett 2010;20:4544–9
- Roschek BJ, Fink RC, Li D, et al. Pro-inflammatory enzymes, cyclooxygenase 1, cyclooxygenase 2, and 5-lipooxygenase, inhibited by stabilized rice bran extracts. J Med Food 2009;12:615–23
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol 1962;111:544
- Hassan GS, Abou-Seri SM, Kamel G, Ali MM. Celecoxib analogs bearing benzofuran moiety as cyclooxygenase-2 inhibitors: design, synthesis and evaluation as potential anti-inflammatory agents. Eur J Med Chem 2014;76:482–93
- Wang JL, Limburg D, Graneto MJ, et al. The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: The second clinical candidate having a shorter and favorable human half-life. Bioorg Med Chem Lett 2010;20:7159–63