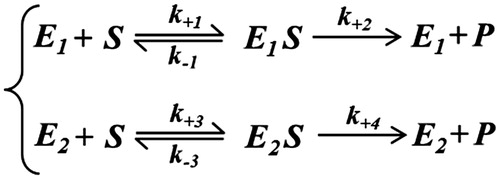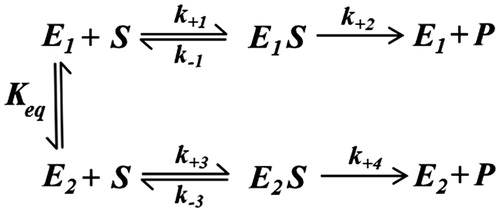Abstract
It is well known that a negative cooperative behavior displayed by a monomeric enzyme may be associated with the simultaneous presence of two enzymes acting on the same substrate. In this paper, emphasis is given to the effect exerted by a rapid equilibrium between the enzyme forms in leading to a hyperbolic behavior, thus masking the presence of multiple enzyme forms.
Introduction
In appreciating the extraordinary features that multimeric enzymes can exhibit when different sites interact (i.e. allosteric enzymes), it is important to be aware of the pitfalls that nonhyperbolic kinetics can generate, thus leading to a misinterpretation of the results.
The usual advice is that as a multimeric enzyme may not always give rise to cooperative behavior, a cooperative response does not always mean multisite interactions. Measurements done in nonequilibrium or quasiequilibrium conditions have been reported as the most frequent cause of the false-cooperative behavior of monomeric enzymesCitation1. Another strategy adopted by nature to tease researchers with false cooperativity, specifically negative cooperativity, is the presence in solution of two different monomeric enzymes that act on the same substrateCitation2. Such a frequently unsuspected situation may occur either when different isoenzymes are present in supposed pure enzyme preparationsCitation3, or when the enzyme undergoes conformational changes and/or covalent modification during the isolation processCitation4 or storageCitation5.
When these conditions occur, leading to enzyme forms with altered kinetic parameters compared to the native enzyme, the interpretation of the kinetic results, especially in terms of the kinetic model, can be puzzling. For example, the apparent negative cooperativity observed for aldose reductase acting on glucose was indeed explained for a while by the presence in the enzyme preparations of two enzyme formsCitation6–10. This interpretation was only revised when a partial inhibition of the enzyme exerted by the hemiacetal form of glucose was reportedCitation11.
This paper highlights how the occurrence of an equilibrium between different enzymes acting on the same substrate may mask the false-cooperative behavior, thus generating classical hyperbolic responses.
Methods
The present analysis assumes that classical Michaelis–Menten kinetics is obeyed. The two enzyme forms present are able to interact with and transform the same substrate into the same product, displaying different Michaelis constants (KM) and also different catalysis rate constants (kcat). Rate equations at zero time, derived for different conditions, are graphically represented by double reciprocal plotsCitation12.
Kinetic models and discussion
Adopting a classical kinetic approach, it is easy to be convinced that a false-negative cooperative response originates when two different enzymes, E1 and E2, despite obeying the Michaelis kinetic equation, act on the same substrate. The model describing this situation is reported (lower case constants refer to kinetic constants) and can be solved by a classical steady-state kinetic approach as shown in Scheme 1.
The general rate equation that applies to the model can be written as follows:
1
By considering the two enzyme–substrate complexes in a steady state condition it follows:
2
3
Different enzyme forms can be expressed in terms of [ E1 S] and [ E2 S]
4
5
where KM1 and KM2 represent
and
, respectively.
Taking into account, the mass balance for both enzymes:
It follows from EquationEquations 44 and Equation5
5 :
6
7
Substituting in the general rate equation (EquationEquation (1)1 ):
Rearranging by simple algebra steps, it follows:
and then
8
EquationEquation (8)8 describes the dependence of the reaction rate on the concentration of the substrate contended for by two enzymes and fits with an apparent negative cooperative behavior (i.e. downward curvature in the double reciprocal plots). On commenting on this kinetic model, it is conceivable that when there are two enzymes acting on the same substrate, the enzyme entering the game first at the lowest substrate concentration is the one with the higher affinity. The less efficient enzyme will significantly contribute only at relatively higher substrate concentrations. This, as predicted by EquationEquation (8)
8 , generates a false-negative cooperative behavior, making the occurrence of a false-positive cooperativity intuitively impossible. The graphical representation of EquationEquation (8)
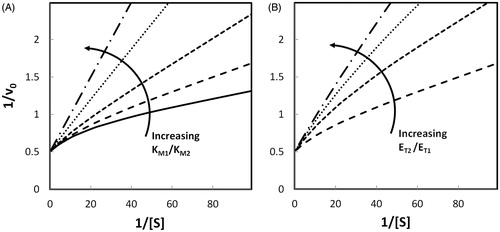
8 at different KM and different Vmax values has been previously reportedCitation2 with the emphasis on the evaluation of the kinetic parameters of the two enzymes. A similar simulation, reported here only for continuity purposes, is shown in . In this case, assuming for the sake of simplicity that k+ 2 = k+ 4 (i.e. the two enzyme forms differ only in their KM values), the effect of the difference in substrate affinity and in the ratio of the two enzymes on the apparent (i.e. false) cooperative behavior is reported. In particular (), the curves generated by EquationEquation (8)
8 , tend to linearity with the progressive increase in KM1, becoming a straight line when KM1 equals KM2, as expected for a mixture of two enzymes with identical kinetic parameters (i.e. the same kcat and the same KM). Similarly, the curves tend to linearity () when the mixture composition of the two enzymes is strongly unbalanced towards one of two components.
Figure 1. Simulation of the apparent negative cooperative behavior of two enzymes acting on the same substrate. Double reciprocal plots of reaction rates v0 versus substrate concentration were generated by computer-assisted simulation using Equation (8). Panel A: curves were plotted by fixing ET1 and ET2 at 1.0 μM, k + 2 and k + 4 at 1.0 s− 1, KM2 at 0.1 mM and varying KM1 from 0.005 mM to 0.1 mM. Ratios KM1/KM2 were 0.05 (solid line), 0.1 (long-dashed line), 0.2 (short-dashed line), 0.5 (dotted line), 1 (dashed dotted line). Panel B: curves were plotted by fixing k + 2 and k + 4 at 1.0 s− 1, KM1 and KM2 at 0.01 mM and 0.1 mM, respectively, and varying ET2/ET1 ratio at a fixed sum ET1 +ET2 = 2.0 μM. Ratios were 1 (long-dashed line), 3 (short-dashed line), 7 (dotted line) and 19 (dashed dotted line). The long-dashed line in Panel A corresponds to the long-dashed line in Panel B.
Besides the above limiting conditions, there is at least one further situation that masks the expected apparent cooperative behavior of a mixture of two monomeric enzymes that catalyze the transformation of the same substrate. This is the case of a rapid equilibrium between the two enzymes, in which a hyperbolic behavior is observed as a response to changes in substrate concentration. This situation can also be easily approached by a simple steady state analysis of the kinetic model reported in Scheme 2, in which the substrate transformation is catalyzed by two interconvertible enzymes, E1 and E2. In the scheme, lower case constants refer to kinetic constants, while Keq refers to the equilibrium constant of E2 formation, that is.:
9
In this case, EquationEquation (1)1 also represents the general rate equation which applies to the model.
By considering the two enzyme–substrate complexes in a steady-state condition and the enzyme interconversion at equilibrium, the analysis proceeds as above (EquationEquations (2–5)) and then taking into account EquationEquation (9)9 , it follows:
10
11
From EquationEquations (10)10 and Equation(11)
11
12
and then
13
Taking into account, the mass balance for the enzyme:
14
and normalizing the reaction rate v0 (EquationEquation (1))
1 for ET:
Simplifying for [E1 S], and [E2 S] and rearranging through simple algebra steps it follows:
and then:
15
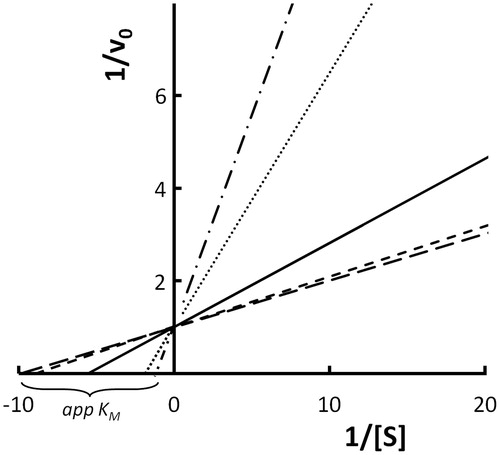
EquationEquation (15)15 represents the typical rectangular hyperbolic function that appears as a straight line in a double reciprocal plot. reports the computer simulation of EquationEquation (15)
15 as a double reciprocal plot at a fixed KM1/KM2 ratio and at different Keq values. Each Keq value determines a defined ratio between the concentration of the two enzyme forms and, having maintained the restrictions that k+ 2 = k+ 4 and that the total enzyme concentration ([ET1] + [ET2]) is constant, the straight lines related to different Keq converge on the ordinate axis, while an array of apparent KM values (appKM) are shown on the abscissa axis. The lower the formation constant for E2, which is assumed to be the less efficient enzyme, the lower the observed appKM is. EquationEquation (15)
15 highlights that limit values for appKM are KM1 or KM2. These limit values can be obtained only for rather low or rather high KM1/KM2 ratios, respectively, and for an equilibrium constant favoring the enzyme that binds the substrate more efficiently.
Figure 2. Simulation of the behavior of two enzymes linked by a rapid equilibrium acting on the same substrate. Double reciprocal plots of reaction rates v0 versus substrate concentration were generated by computer-assisted simulation using Equation (15). Curves were plotted by fixing ET at 1.0 μM, k + 2 and k + 4 at 1.0 s−1, KM1 at 0.1, KM2 at 1 mM and varying Keq from 0.01 to 100. Keq values were 0.01 (long-dashed line), 0.1 (short-dashed line), 1 (solid line), 10 (dotted line) and 100 (dashed dotted line).
EquationEquation (15)15 also reveals that the imposed restriction of k+ 2 = k+ 4 adopted in the computer simulation does not affect the hyperbolic nature of the kinetic model. In fact, when the restriction is removed, what changes for the straight lines related to different Keq is their convergence point, whose coordinates are as follows:
The quadrant of the convergence point will depend on the relative values of the four constants k + 2, k + 4, KM1 and KM2. In any case, we are assuming that the less effective enzyme will move toward the more active form through the substrate interaction equilibrium. This is the same kind of triggering that the substrate exerts to generate positive cooperativity in the concerted model (MWC) of allosteric enzymesCitation13,Citation14. Obviously, although the model in Scheme 2 possesses two-thirds of the restrictions defining the MCW model, the impossibility of a net increase in active catalytic centers (the present model considers a monomeric enzyme) rules out any (even false) positive cooperative behavior.
At this point, it should be underlined that the kinetic efficiency of the thermodynamic link imposed between the two enzymes (i.e. the equilibrium condition) is not irrelevant. Indeed, slow equilibration steps between enzyme forms have been proposed as a model to explain the apparent cooperative behavior of monomeric enzymesCitation15,Citation16. In our case, the lower the kinetic constants, which govern the equilibrium, the more evident the transition is from a hyperbolic to a false-cooperative behavior of the enzyme mixture. It is sufficient to consider the limit case (Scheme 1) of an equilibrium that is so slow as to be irrelevant while the reaction is taking place. Indeed, the conditions considered here were already included in a general analysis and the conclusions could be then drawn, although with difficulty, from more general equations. However, the lack of emphasis in this regard needs highlighting.
Actually, attempting to reveal a situation such as that described in Scheme 2 within a classical steady-state kinetic analysis at zero time of a monomeric enzyme is not easy. It would require carefully scrutinizing the enzyme preparations, which itself is not stimulated by the kinetic results, unless other information was available. In fact, a negative biphasic behavior for a monomeric enzyme is an unexpected signal requiring further analysis for explanation (i.e. two enzymatic components or kinetic models of non or quasi-equilibrium conditions). Conversely, the hyperbolic behavior observed for a monomeric enzyme, which is assumed to be present alone, is rarely suspected of being a more complex situation. In fact, nobody would even think of investigating the presence of two enzyme forms for a monomeric enzyme when the observed kinetics is of a hyperbolic type.
However, whenever the presence of two or more enzyme forms acting on the same substrate is ascertained, we should be aware that the lack of negative cooperativity behavior expected for this system must not necessarily be interpreted as an indistinguishable kinetic behavior of the two enzyme forms, but may arise from a rapid equilibrium between them.
Although as yet we cannot offer authentic examples in supporting the model under discussion, this might be possible. This especially applies considering cases of dynamic redox conditions, such as those occurring, for instance, in the thiols-redox modifications of enzymes analyzed in thiol/disulfide buffering systems, or for enzymes susceptible to covalent cyclic interconversion if analyzed in the (known or unknown) presence of the modifying/demodifying system, or simply for enzymes undergoing conformational changes in which the interconversion rate is not a limiting step.
To conclude, this analysis underlies the importance of the adopted model in the interpretation of experimental data, and it may be regarded as a simple but clear warning signal in enzymological studies of the possibility of being caught out by the subtle tricks played by nature in interpreting kinetics data in terms of the mechanism involved.
Declaration of interest
This work was supported by the University of Pisa.
References
- Porter CM, Miller BG. Cooperativity in monomeric enzymes with single ligand binding sites. Bioorg Chem 2012;43:44–50.
- Dixon M, Webb EC. Enzymes. 3rd ed. London: Longman; 1979:72–5
- Thompson WJ, Appleman MM. Characterization of cyclic nucleotide phosphodiesterases of rat tissues. J Biol Chem 1971;246:3145–50.
- Cloosa PAC, Christgaua S. Non-enzymatic covalent modifications of proteins: mechanisms, physiological consequences and clinical applications. Matrix Biol 2002;21:2002–39.
- Iyer PV, Ananthanarayan L. Enzyme stability and stabilization-aqueous and non-aqueous environment. Process Biochem 2008;43:1919–32.
- Grimshaw CE, Shahbaz M, Jahangiri G, et al. Kinetic and structural effects of activation of bovine kidney aldose reductase. Biochemistry 1989;28:5343–53.
- Daly AK, Mantle TJ. Purification and characterization of the multiple forms of aldehyde reductase in ox kidney. Biochem J 1982;205:373–80.
- Grimshaw CE, Lai CJ. Oxidized aldose reductase: in vivo factor not in vitro artifact. Arch Biochem Biophys 1996;327:89–97.
- Del Corso A, Barsacchi D, Giannessi M, et al. Change in stereospecificity of bovine lens aldose reductase modified by oxidative stress. J Biol Chem 1989;264:17653–5.
- Srivastava SK, Hair GA, Das B. Activated and unactivated forms of human erythrocyte aldose reductase. Proc Natl Acad Sci USA 1985;82:7222–6.
- Balestri F, Cappiello M, Moschini R, et al. Modulation of aldose reductase activity by aldose hemiacetals. Biochim Biophys Acta 2015;1850:2329–39.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–66.
- Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. J Mol Biol 1965;12:88–118.
- Cornish-Bowden A. Fundamentals of enzyme kinetics. 4th ed. Weinheim: Wiley-Blackwell; 2014
- Ainslie GR, Shill JP, Neet KE. Transients and cooperativity. A slow transition model for relating transients and cooperative kinetics of enzymes. J Biol Chem 1972;247:7088–96.
- Cárdenas ML. Understanding mechanisms of enzyme co-operativity: the importance of not being at equilibrium. Perspect Sci 2015;4:10–16.