ABSTRACT
Background: Poorly reversible airflow obstruction is a hallmark feature of chronic obstructive pulmonary disease (COPD). However, some COPD patients demonstrate significant bronchodilator reversibility (BDR). The pathologic features associated with the presence or absence of this phenomenon are not known. Methods: We analyzed 67 patients with advanced upper lobe predominant emphysema who underwent lung volume reduction surgery and divided them into 2 groups: the reversible group [BD(+)] had a >12% and >200 mL increase in FEV1 or FVC with bronchodilator; the irreversible group [BD(−)] had a ≤12% and ≤20 mL increase in FEV1 and FVC. We measured the epithelial height (EH) and areas of epithelium (EA), subepithelium (SEA), smooth muscle (SMWA), and total wall (TWA) of the small airways (<2 mm in internal diameter) in the resected specimens, and adjusted these measurements for basement membrane area (BMA) or perimeter (BMP). Results: Despite similar baseline characteristics, the BD(+) group had a smaller EH (0.036 mm vs. 0.042 mm, p = 0.005) and EH/BMP (0.012 vs. 0.014, p = 0.007), and a greater SMWA/BMA (0.491 vs. 0.430, p = 0.034) compared to the BD(−) group. In addition, EA trended to be smaller in the BD(+) group when compared to the BD(−) group (0.160 mm2 vs. 0.184 mm2, p = 0.06). In a subset of patients with consistent patterns of BDR on serial testing, the BD(+) group had greater SMWA/BMA (0.518 vs. 0.433, p = 0.049) and TWA/BMA (1.405 vs. 1.266, p = 0.036) compared to the BD(−) group. Conclusions: Small airway smooth muscle mass may play a role in determining BDR in severe emphysema.
INTRODUCTION
Chronic Obstructive Pulmonary Disease (COPD) is an inflammatory lung disease that is associated with progressive and poorly reversible airflow obstruction. This physiologic phenomenon has classically distinguished COPD from asthma, a disease characterized by reversible airflow obstruction. However, studies over the last decade have challenged this concept.
Although the definitions of reversibility have varied, multiple studies involving more than 8,000 total COPD patients have shown that 54–80% of the patients have bronchodilator reversibility (BDR) (Citation1–3). To complicate matters, this phenomenon is not a consistent one in all patients. Calverley et al. performed bronchodilator testing in patients with COPD and, using American Thoracic Society criteria, found that 52% changed their reversible/irreversible status between visits (Citation4). In the National Intermittent Positive Pressure Breathing trial, Anthonisen and Wright found that 65% of patients had at least 1 visit with 15% improvement in lung function with administration of isoproterenol over a period of 2 years (Citation5). As a result, it has been difficult to identify the mechanisms of BDR in COPD.
Why some COPD patients demonstrate BDR whereas others do not is unknown. Airflow obstruction in COPD often occurs as a result of emphysema and loss of airway elastic recoil. However, airway pathology, such as epithelial remodeling, airway wall fibrosis, mucous metaplasia and smooth muscle hypertrophy (Citation6,7), also plays a significant role in this process. Both aspects of this disease lead to airflow limitation to varying degrees; the relative contribution of each is variable, and both airway disease and emphysema often coexist in the same patient. It has been hypothesized that the phenomenon of BDR is primarily due to airway disease, mostly because of the high degree of airway disease associated with asthma. However, many patients with a predominantly emphysematous phenotype do demonstrate acutely reversible airflow obstruction. We hypothesize that emphysema patients with BDR have different airway pathology, with more smooth muscle mass, compared to those without BDR.
MATERIALS AND METHODS
Patient selection
We analyzed 67 patients with advanced upper lobe predominant emphysema who underwent lung volume reduction surgery (LVRS) between January 1996 and November 2006 at our institution. Criteria for LVRS are identical to those proposed in the National Emphysema Treatment Trial (Citation8). Patients were divided into 2 groups, based on their last set of pulmonary function tests (PFT) performed within 6 months prior to surgery: the reversible group [BD(+)] had a >12% and a >200 mL increase in either forced expiratory volume in one second (FEV1) or forced vital capacity (FVC) after short acting bronchodilator (BD), using the American Thoracic Society/European Respiratory Society criteria for BDR (Citation9); the irreversible group [BD(−)] had a ≤12% and ≤200 mL increase in FEV1 and FVC. We also reviewed the patients that had two or more available PFTs prior to LVRS, and analyzed the airway morphometry in a subset of patients (n = 35) with consistent patterns of BDR (i.e., consistently BD(+) or BD(−) in the majority of PFTs).
Pulmonary function testing
Patients were instructed to stop long acting BD 24 hours and short-acting BD 8 hours before testing. All tests were performed under standard conditions in our pulmonary function laboratory. Spirometry was measured before and after 180 mcg of albuterol was administered by metered dose inhaler (System 6200, Autobox DL; SensorMedics; Yorba Linda, CA) using American Thoracic Society guidelines (Citation10). Thoracic gas volumes were measured by plethysomography (Citation11). Diffusion capacity of carbon monoxide (DLCO) was measured by single breath technique (Citation12). The six-minute walk test was performed according to American Thoracic Society criteria (Citation13).
Airway selection
The lung specimens were inflated with 10% formalin, fixed for at least 12 hours, and then embedded in paraffin. Sections 4 μm thick were stained with hematoxylin and eosin. Digital photographs were taken of all airways <2 mm in greatest internal diameter at 100x. Airways were excluded from analysis if the entire airway could not be included in the photograph, the smooth muscle borders were not well defined, or the epithelial layer was disrupted.
Quantitative assessment
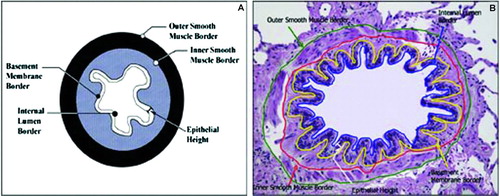
Small airway morphometry was performed as previously described by us (Citation14). Briefly, the measurements included the following: diameter of the internal lumen, both longest and shortest dimensions (DLONG and DSHORT); areas surrounded by and perimeters of the internal lumen (IA, IP), basement membrane (BMA, BMP), outer smooth muscle border (OSMA, OSMP), and inner smooth muscle border (ISMA, ISMP); epithelial height (EH), measured at 15 locations. depicts these measurements in a representative airway. The adventitia was excluded from the analysis because of significant disruption and irregularity of the adventitial borders. Ten airways per patient were measured. The investigator performing the measurements was blinded to the patients’ identity and group assignment. The following variables were calculated:
Epithelial area (EA) = BMA-IA
Subepithelial area (SEA) = ISMA-BMA
Smooth muscle wall area (SMWA) = OSMA-ISMA
Total wall area (TWA) = OSMA-IA
Figure 1. A. Illustration of the small airway depicting quantitative measurements performed. B. Representative airway with borders and compartments measured are outlined with different colors. From reference 14 Reprinted with permission of the American Thoracic Society. Copyright © American Thoracic Society. Victor Kim, Gerard J. Criner, Heba Y. Abdallah, John P. Gaughan, Satoshi Furukawa, and Charalambos C. Solomides. Airway Morphometry and Improvement in Pulmonary Function after Lung Volume Reduction Surgery. AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE, Jan 2005; 171: 40–47. OFFICIAL JOURNAL OF THE AMERICAN THORACIC SOCIETY DIANE GERN, Publisher
The EA, SEA, SMWA, and TWA were divided by the BMA of each airway, and the EH was divided by the BMP of each airway to adjust for differences in airway size. These adjusted variables were called EA/BMA, SEA/BMA, SMWA/BMA, and TWA/BMA, respectively.
Data analysis
Descriptive statistics are reported as mean ± SD. A p-value of <0.05 was considered statistically significant. Unpaired t-test, Chi Square test, and Fisher's exact test were used to compare baseline data between the two groups. Similar tests were performed to compare the subset characteristics to the entire cohort. A two-way ANOVA was used to analyze the multi-sample histopathologic measurements. The model used in the analysis was a linear mixed model modified to allow for modeling of the “within subject” covariance structure. Different covariance structures were attempted with the final choice dictated by the goodness of fit. PROC MIXED (SAS V.9, SAS Institute, Cary, NC) (Citation15) was used with the treatment group as the fixed effect and the multiple samples (within subject) as the random effect using a compound symmetry covariance structure. All data was transformed to normalized ranks (Citation16, 17) prior to analysis to accommodate non-normality of the data set.
RESULTS
Patient characteristics
Patient characteristics are summarized in . There were 33 males and 34 females, 54 Caucasians and 13 African-Americans. None of the patients had a history of asthma or allergic rhinitis. Age was 62 ± 8 years, FEV1 29 ± 8% predicted, FVC 72 ± 18% predicted, and FEV1/FVC was 0.31 ± 0.07. There were 29 patients in the BD(+) group and 38 in the BD(−) group. There were no significant differences between the two groups in demographics, pulmonary function, or exercise capacity. The BD(+) group had a 17 ± 12% and 105 ± 82 mL increase in FEV1 and a 23 ± 14% and 464 ± 262 mL increase in FVC after albuterol. The BD(−) group had a 5 ± 9% and 21 ± 57 mL change in FEV1 and a 4 ± 10% and 93 ± 25mL change in FVC after albuterol (see ). In the BD(+) group, 26 patients met criteria for BDR by FVC alone, 1 by FEV1 alone, and 2 by both FEV1 and FVC. Overall, 48% of the patients were on long term oxygen therapy; 37% were on oral steroids of various duration and dose within the 6 months preceding surgery; inhaled corticosteroids 68%; long acting beta agonists, 52%; short acting beta agonists and anticholinergics 100%; and long acting anticholinergics 19%. A greater percentage of patients in the BD(−) group were treated with theophylline compared to the BD(+) group (55% vs. 28%, p = 0.044).
Figure 2. Changes in lung function after acute administration of short acting bronchodilator. Data presented as mean ± SD. ABS = absolute; BD = bronchodilator. *p < 0.0001.
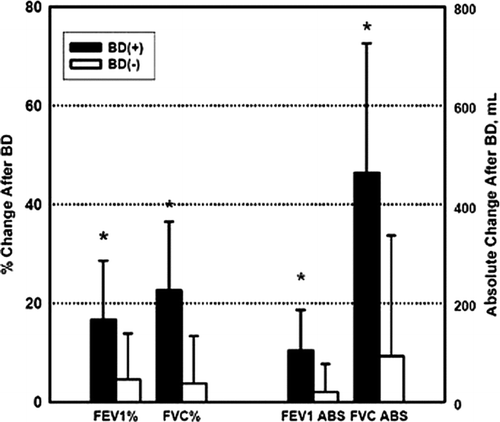
Table 1. Patient characteristics
Airway morphometry
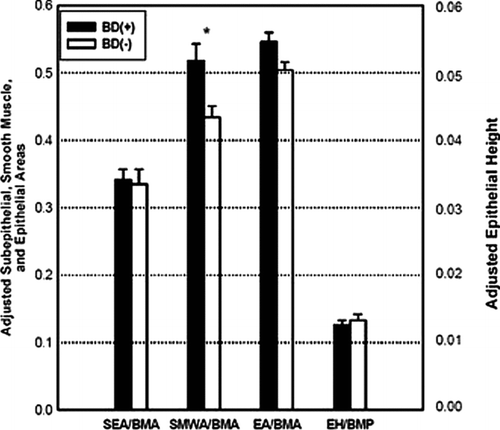
Morphometric data are summarized in . EA and EA/BMA were similar between groups but EH and EH/BMP were smaller in the BD(+) group compared to the BD(−) group (0.036 ± 0.017 mm vs. 0.042 ± 0.018 mm, p = 0.005 and 0.012 ± 0.007 vs. 0.014 ± 0.011, p = 0.007, respectively). There was no difference in SMWA, SEA, SEA/BMA, TWA, or TWA/BMA between groups. However, SMWA/BMA was greater in the BD(+) group compared to the BD(−) group (0.491 ± 0.274 vs. 0.430 ± 0.340, p = 0.034); see .
Figure 3. Adjusted airway morphometry in each group. SEA/BMA = Adjusted subepithelial area; SMWA/BMA = Adjusted smooth muscle wall area; EA/BMA = Adjusted epithelial area; EH/BMP = Adjusted epithelial height. Data presented as mean ± SE. *p = 0.007. †p = 0.034.
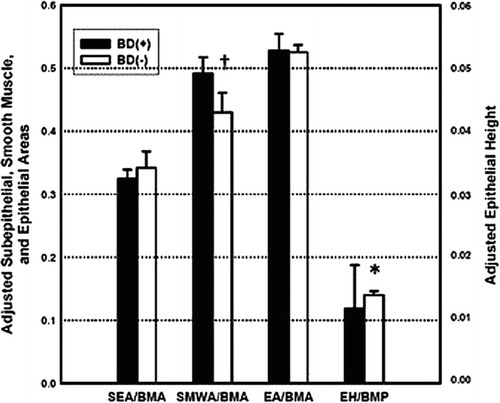
Table 2. Small airway morphometry
Morphometry in Patients with Consistent BDR
Of the entire cohort, there were 23 and 29 patients in the BD(+) and BD(−) groups, respectively, with two or more available PFTs prior to surgery. Of these, 16 in the BD(+) group consistently met BDR criteria, and 19 in the BD(−) group consistently did not meet criteria. No patient switched groups based on this analysis. All patients in the reversible group met BDR criteria by improvements in FVC. Patient characteristics are summarized in . Demographic data, pulmonary function testing, and exercise capacity were similar in each group and also in comparison to the entire cohort. Respiratory medication use was again similar compared to the entire cohort. Again seen was the greater use of theophylline in the BD(−) group compared to the BD(+) group (68% vs. 25%, p = 0.026).
Table 3. Characteristics of patients with consistent bronchodilator reversibility patterns
Table 4. Small airway morphometry in patients with consistent bronchodilator reversibility patterns
Morphometry in this cohort are summarized in . SMWA and TWA were similar between groups but SMWA/BMA and TWA/BMA were greater in the BD(+) group compared to the BD(−) group (0.518 ± 0.308 vs. 0.434 ± 0.231, p = 0.049, and 1.405 ± 0.533 vs. 1.266 ± 0.563, p = 0.036, respectively); see . There were no other significant differences in other measurements between groups.
DISCUSSION
It is accepted that airflow obstruction in many lung diseases is a result of airway pathology. In COPD, numerous studies have shown that inflammatory infiltration of the small airways worsens as disease severity increases (Citation18–20), which results in epithelial thickening, subepithelial fibrosis, and smooth muscle hypertrophy (Citation14, Citation21, Citation22). The degree of airway remodeling, however, is not a uniform process, and heterogeneity in airway disease has been found in those with the same degree of airflow obstruction.
In advanced emphysema patients who underwent LVRS, small airway epithelial thickness (Citation14), and mucus metaplasia (Citation23) varied significantly, and these differences in airway pathology predicted postoperative outcomes. In a similar vein, some studies have found increased airway smooth muscle mass in COPD(Citation24–26) whereas others have not (Citation20, Citation27, Citation28). Regardless, chronic airway inflammation from cigarette smoke causes constriction and hypertrophy of even normal airway smooth muscle (Citation21), and direct correlations between airway smooth muscle mass and airflow obstruction have been demonstrated in COPD (Citation22, Citation29). It is reasonable, therefore, to hypothesize that these differences in airway pathology are associated with the presence or absence of BDR in emphysema.
The overlap in airway pathology between asthma and COPD has been well described. Smooth muscle hypertrophy is a characteristic feature of asthma pathology (Citation30), and the increased smooth muscle mass combined with its interaction with surrounding structures and the local cytokine environment is responsible for bronchoconstriction and airway hyperresponsiveness (AHR) (Citation31, 32). AHR has also been described in COPD; it has been considered a risk factor for the development (Citation33, 34) and progression (Citation35, 36) of airflow limitation in COPD, and also has been correlated with small airway inflammation and mucous metaplasia (Citation37, 38). The pathology associated with BDR in COPD, however, has received little attention.
In contrast to prior studies that have related airway pathology to the degree of airflow obstruction, we compared two groups, one with BDR and the other without, both having severe airflow obstruction and emphysema. We showed that differences in airway smooth muscle mass and epithelial thickness were associated with BDR in those with advanced emphysema. Those with reversible airflow obstruction had more smooth muscle mass and thinner epithelial layers compared to those with fixed airflow obstruction. These data provide preliminary evidence of these pathologic differences may be responsible for BDR in COPD. To our knowledge, this is the first study that has quantified small airway morphometry and correlated it with BDR in advanced emphysema patients, a group classically not associated with airways disease, let alone reversible airflow obstruction.
Many patients changed bronchodilator reversible/irreversible status on serial lung function testing, which is consistent with prior studies (Citation4, 5). To account for this, we analyzed a subset of patients with consistent responses to bronchodilators, to minimize the confounding effects of this phenomenon. In this more “pure” subset, epithelial morphometry was not different between groups, suggesting that epithelial remodeling is not an important anatomic determinant of BDR in COPD. The differences in smooth muscle mass, however, were similar to the entire cohort, which is consistent with our hypothesis and provides further validation to our conclusions. The increased total wall area in the reversible group paralleled the increase in smooth muscle mass.
A unique contribution of our study is the detailed pathologic analysis of those with advanced emphysema, which was correlated with clinical characteristics and airway physiology. Similar to other clinical studies, forty-two percent of our patients demonstrated BDR (Citation1–3). This phenomenon was irrespective of the demographic characteristics, smoking history, lung function, or history of respiratory medications, with the exception of theophylline use. As this is a retrospective analysis, we cannot conclude that theophylline causes poorly reversible airflow obstruction. An alternative hypothesis is that the BD(−) group was more dyspneic and was more frequently prescribed theophylline to alleviate symptoms.
It should also be noted that the majority of the reversible COPD patients met BDR criteria by improvements in FVC, or a “volume response,” instead of a “flow response” (FEV1). This phenomenon has been described in advanced COPD, and more patients with severe airflow obstruction demonstrate a volume response compared to COPD patients with milder airflow obstruction (Citation39–41). This volume response may reflect greater decreases in the peripheral airways resistance, in comparison to a flow response which reflects changes in large airways resistance (Citation42).
There are a few limitations of this study. Quantitative emphysema data were not available, so we were unable to correlate BDR to degree and pattern of emphysema. It is also unclear if these pathologic differences exist in those with inconsistent BDR or in those with less severe emphysema, thereby making these results less applicable to the general COPD population. In addition, it is possible (although unlikely) that some of the differences found were false positive findings, given the multiple comparisons performed. Finally, as this analysis was performed on the resected specimens from patients with upper lobe predominant emphysema, it is unclear if the pathology seen in this part of the lung represents the remaining, less emphysematous lung. This remains an assumption that needs to be validated in other pathologic studies.
Regardless, analysis of small airway morphometry in the reversible group demonstrated increased smooth muscle mass compared to irreversible group, suggesting that smooth muscle hypertrophy contributes to airflow obstruction and BDR in advanced emphysema. The number of patients in each group is limited, particularly in the groups selected for their consistency in BDR, so the results are hypothesis-generating but should be interpreted with caution. The reasons why some develop smooth muscle mass than others are still not known and deserve further investigation. These pathologic differences may also provide insight into the pathogenesis of airway disease in COPD and may identify potential targets for drug development.
ACKNOWLEDGEMENT
This study was partially supported by the 2006 Richard L. Evans Foundation Faculty Development Research Award. VK has participated in clinical trials sponsored by Boehringer Ingelheim, Glaxo-Smith-Kline, and Roche pharmaceuticals. GJC has served on Advisory Committees for Ortho-Biotech, Schering-Plough, Boehringer Ingelheim, Actelion, Shire and Sepracor Pharmaceticals. All of these sums are less than $2,500. GJC has received research grants from: Schering-Plough, Boehringer Ingelheim, Actelion, Glaxo-Smith-Kline, Advanta, Daiichi Asubio, Pfizer, Roche and Sepracor Pharmaceticals, Emphasys Medical, Inc., and Aeris Therapeutics. All research grant monies are deposited and controlled by Temple University. RMP, MA, and JPG claim no financial relationships.
Declaration of interests
The authors have no conflicts of interest to disclose. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Dorinsky PM, Reisner C, Ferguson GT, Menjoge SS, Serby CW, Witek TJ, Jr. The combination of ipratropium and albuterol optimizes pulmonary function reversibility testing in patients with COPD. Chest 1999; 115:966–971.
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, Shah T. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166:1084–1091.
- Tashkin DP, Celli B, Decramer M, Liu D, Burkhart D, Cassino C, Kesten S. Bronchodilator responsiveness in patients with COPD. Eur Respir J 2008; 31:742–750.
- Calverley PM, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax 2003; 58:659–664.
- Anthonisen NR, Wright EC. Bronchodilator response in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986; 133:814–819.
- Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med 2001; 164:S28–38.
- Kim V, Rogers TJ, Criner GJ. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5:478–485.
- National Emphysema Treatment Trial Research Group. A randomized trial comparing lung volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348:2059–2073.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, Van Der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948–968.
- Miller MR, Hankinson J Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, Van Der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 2005; 26:319–338.
- Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, Van Der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. Eur Respir J 2005; 26:511–522.
- Macintyre N, Crapo RO, Viegi G, Johnson DC, Van Der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005; 26:720–735.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166:111–117.
- Kim V, Criner GJ, Abdallah HY, Gaughan JP, Furukawa S, Solomides CC. Small airway morphometry and improvement in pulmonary function after lung volume reduction surgery. Am J Respir Crit Care Med 2005; 171:40–47.
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. SAS Institute 1996.
- Conover WJ, Iman RL. Rank transformations as a bridge between parametric and nonparametric statistics. Am Statist 1981; 35:124–129.
- Harter HL. Expected values of normal order statistics. Biometrika 1961; 48:151–165.
- Saetta M, Baraldo S, Corbino L, Turato G, Braccioni F, Rea F, Cavallesco G, Tropeano G, Mapp CE, Maestrelli P, Ciaccia A, Fabbri LM. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160:711–717.
- Lams BE, Sousa AR, Rees PJ, Lee TH. Immunopathology of the small-airway submucosa in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 158:1518–1523.
- Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, Macklem PT. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med 1978; 298:1277–1281.
- Baraldo S, Saetta M, Cosio MG. Pathophysiology of the small airways. Semin Respir Crit Care Med 2003;24:465–472.
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:2645–2653.
- Hogg JC, Chu FS, Tan WC, Sin DD, Patel SA, Pare PD, Martinez FJ, Rogers RM, Make BJ, Criner GJ, Cherniack RM, Sharafkhaneh A, Luketich JD, Coxson HO, Elliott WM, Sciurba FC. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med 2007; 176:454–459.
- Bosken CH, Wiggs BR, Pare PD, Hogg JC. Small airway dimensions in smokers with obstruction to airflow. Am Rev Respir Dis 1990; 142:563–570.
- Nagai A, West WW, Thurlbeck WM. The National Institutes of Health Intermittent Positive-Pressure Breathing trial: pathology studies. II. Correlation between morphologic findings, clinical findings, and evidence of expiratory air-flow obstruction. Am Rev Respir Dis 1985; 132:946–953.
- Kuwano K, Bosken CH, Pare PD, Bai TR, Wiggs BR, Hogg JC. Small airways dimensions in asthma and in chronic obstructive pulmonary disease. Am Rev Respir Dis 1993; 148:1220–1225.
- Petty TL, Silvers GW, Stanford RE, Baird MD, Mitchell RS. Small airway pathology is related to increased closing capacity and abnormal slope of phase III in excised human lungs. Am Rev Respir Dis 1980; 121:449–456.
- Tiddens HA, Pare PD, Hogg JC, Hop WC, Lambert R, de Jongste JC. Cartilaginous airway dimensions and airflow obstruction in human lungs. Am J Respir Crit Care Med 1995; 152:260–266.
- Finkelstein R, Fraser RS, Ghezzo H, Cosio MG. Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med 1995; 152:1666–1672.
- James A, Carroll N. Airway smooth muscle in health and disease; methods of measurement and relation to function. Eur Respir J 2000; 15:782–789.
- Amrani Y, Panettieri RA, Jr. Cytokines induce airway smooth muscle cell hyperresponsiveness to contractile agonists. Thorax 1998; 53:713–716.
- Grootendorst DC, Rabe KF. Mechanisms of bronchial hyperreactivity in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 2004; 1:77–87.
- Xu X, Rijcken B, Schouten JP, Weiss ST. Airways responsiveness and development and remission of chronic respiratory symptoms in adults. Lancet 1997; 350:1431–1434.
- Laprise C, Boulet LP. Asymptomatic airway hyperresponsiveness: a three-year follow-up. Am J Respir Crit Care Med 1997; 156:403–409.
- Taylor RG, Joyce H, Gross E, Holland F, Pride NB. Bronchial reactivity to inhaled histamine and annual rate of decline in FEV1 in male smokers and ex-smokers. Thorax 1985; 40:9–16.
- Rijcken B, Schouten JP, Xu X, Rosner B, Weiss ST. Airway hyperresponsiveness to histamine associated with accelerated decline in FEV1. Am J Respir Crit Care Med 1995; 151:1377–1382.
- Finkelstein R, Ma HD, Ghezzo H, Whittaker K, Fraser RS, Cosio MG. Morphometry of small airways in smokers and its relationship to emphysema type and hyperresponsiveness. Am J Respir Crit Care Med 1995; 152:267–276.
- Nagai A, Thurlbeck WM, Konno K. Responsiveness and variability of airflow obstruction in chronic obstructive pulmonary disease. Clinicopathologic correlative studies. Am J Respir Crit Care Med 1995; 151:635–639.
- Cerveri I, Pellegrino R, Dore R, Corsico A, Fulgoni P, van de Woestijne KP, Brusasco V. Mechanisms for isolated volume response to a bronchodilator in patients with COPD. J Appl Physiol 2000; 88:1989–1995.
- Schermer T, Heijdra Y, Zadel S, Van Den Bemt L, Boonman-de Winter L, Dekhuijzen R, Smeele I. Flow and volume responses after routine salbutamol reversibility testing in mild to very severe COPD. Respir Med 2007; 101:1355–1362.
- Walker PP, Calverley PM. The volumetric response to bronchodilators in stable chronic obstructive pulmonary disease. COPD 2008; 5:147–152.
- Sciurba FC. Physiologic similarities and differences between COPD and asthma. Chest 2004; 126:117S–124S; discussion 159S–161S.