Abstract
Objective:
The purpose of this study was to determine the cost-effectiveness of enzymatic debridement using collagenase relative to autolytic debridement with a hydrogel dressing for the treatment of pressure ulcers.
Methods:
A 3-stage Markov model was used to determine the expected costs and outcomes of wound care for collagenase and hydrogel dressings. Outcome data used in the analysis were taken from a randomized clinical trial that directly compared collagenase and hydrogel dressings. The primary outcome in the clinical trial was the proportion of patients achieving a closed epithelialized wound. Transition probabilities for the Markov states were estimated from the clinical trial. A 1-year time horizon was used to determine the expected number of closed wound days and the expected costs for the two alternative debridement therapies. Resource utilization was based on the wound care treatment regimen used in the clinical trial. Resource costs were derived from standard cost references and medical supply wholesalers. The economic perspective taken was that of the long-term care facility. No cost discounting was performed due to the short time horizon of the analysis. A deterministic sensitivity analysis was conducted to analyze economic uncertainty.
Results:
The number of expected wound days for the collagenase and hydrogel cohorts are estimated at 48 and 147, respectively. The expected direct cost per patient for pressure ulcer care was $2003 for collagenase and $5480 for hydrogel debridement. The number of closed wound days was 1.5-times higher for collagenase (317 vs 218 days) than with the hydrogel. The estimated cost/closed wound day was 4-times higher for the hydrogel ($25) vs collagenase ($6).
Conclusions:
In this Markov model based on a randomized trial of pressure ulcer care in a long-term care setting collagenase debridement was economically dominant over autolytic debridement, yielding better outcomes at a lower total cost. Since it was a single institution study with a small sample size, the results should be interpreted with caution. Specifically, the findings may not necessarily be generalized to other hydrogel dressings, healthcare settings, age groups, or to wounds of other etiologies.
Introduction
Pressure ulcers (also known as pressure sores, bed sores, or decubitus ulcers) pose significant clinical and economic challenges to health systems, providers, patients, and society. The prevalence of pressure ulcers in the US is as high as 28% in long-term care settingsCitation1,Citation2. A successful multi-disciplinary treatment process for management of chronic, indolent, or non-healing pressure ulcers incorporates good wound bed preparationCitation3. A seminal component of wound bed preparation is debridement, which may include autolytic, surgical, biologic, mechanical, and enzymatic techniquesCitation4. The recommended debridement approach varies with the wound, potential for infection, the patient’s clinical and demographic characteristics, time, as well as available medical expertise, knowledge, and resources within the care settingCitation5,Citation6. Moreover, it is increasingly important to also consider the cost-effectiveness of treatment options in the management of indolent pressure ulcersCitation7.
Normal wound healing is a dynamic process with complex interactions between the extracellular matrix, controlled angiogenesis, epidermal and dermal cells, and soluble mediatorsCitation8. This dynamic process of wound healing involves three overlapping phases—inflammation, proliferation, and remodeling. Unencumbered, these phases of wound healing follow a specific time sequence in a chronological order yielding a sustained restoration of anatomic and functional integrityCitation9,Citation10. Conversely, chronic wound healing lacks the orderly and timely process and fails to achieve anatomic and functional integrity. Thus, the healing process of chronic wounds is prolonged and incomplete, proceeds in an uncoordinated manner, and results in a poor outcomeCitation8–11. These wounds are characterized by persistent inflammatory stimuli such as repeat trauma, relative ischemia, and bacterial contaminationCitation12, and require long-term care.
Within the long-term care or nursing home setting, the use of autolytic and enzymatic wound debridement have long been accepted as effective strategies for the management of pressure ulcers and both methods are considered simple, safe, and practicalCitation5. These wound debridement approaches are more efficacious than non-selective, mechanical debridement using wet-to-dry dressingsCitation13–15, more cost-effectiveCitation16, and are less painful for the patientCitation17. Compared with bedside sharp debridement, autolytic and enzymatic methods do not require a physician’s technical skills, a significant advantage since physician availability may be limited within the long-term care environmentCitation17–19.
Autolytic debridement using hydrogels, which are comprised of hydrophilic polymers within a dimensional matrix, create a moist wound bed environment facilitating the body’s intrinsic enzymes to break down necrotic or non-viable tissueCitation20. Enzymatic debridement with a selective debriding agent such as collagenase ointment (derived from Clostridium histolyticum) has been used for removal of necrotic tissue from chronic cutaneous wounds and burnsCitation4.
Milne et al.Citation5,Citation21 recently reported a prospective, randomized two-phased study assessing debridement and closure rates of pressure ulcers in 27 nursing home residents. The debridement rates were evaluated at 42 days and the wound closure rates were assessed at 84 days. The study demonstrated statistically significant differences in both the debridement rates and the wound closure rates between the two treatment groups. Given the acquisition cost difference between collagenase ointment and hydrogel dressings, there is a need to assess the relative cost-effectiveness of the two alternatives for the management of pressure ulcers.
To our knowledge, only a few studies have evaluated the cost of collagenase vs hydrogel treatmentCitation14,Citation22,Citation23. These studies found that collagenase was more cost-effective than hydrogel therapy. However, the study conclusions are of limited use because the cost data are 10 or more years old, or they were conducted in countries with notably different healthcare systems than the USCitation22,Citation23, or efficacy data were derived by expert panels rather than clinical trialsCitation14. Consequently, there is a need to address the cost-effectiveness of these therapies using randomized clinical trial data and current cost estimates. In this economic analysis a Markov decision process model was developed to assess the cost-effectiveness of collagenase enzymatic debridement relative to hydrogel autolytic debridement for the treatment of pressure ulcers in a long-term care setting.
Patients and methods
Study and model design
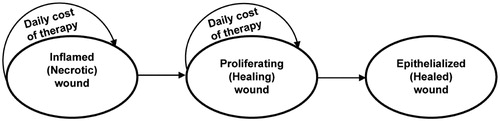
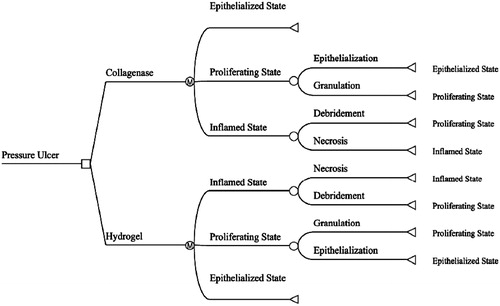
Decision analysis is a well-accepted methodology for comparing costs and benefits based on the probability of clinical outcomesCitation24. A three-state Markov state-transition model with a cycle length of 1 day () was developed using TreeAge Pro (TreeAge Pro version 2012, TreeAge Software Inc., Williamstown, MA) to compare the cost and outcomes of collagenase ointment (Santyl Ointment, Healthpoint, Ltd, Fort Worth, TX) with hydrogel autolysis (SoloSite Gel, Smith & Nephew, Largo, FL). Markov models are particularly well-suited to aid decision-making in clinical situations when events and costs transition over timeCitation25.
The clinical and resource utilization data for this analysis were derived from a US-based, prospective, randomized, 12-week trial, described in detail by Milne et al.Citation5,Citation21. The two-phase clinical study was conducted in a single long-term care center consisting of 27 institutionalized patients with stage 3 and 4 pressure ulcers that had ≥85% necrotic non-viable tissue. Patients were randomly assigned to receive daily dressing changes with either hydrogel (n = 13) or collagenase (n = 14) covered with a standard semi-occlusive dressing. Consistent with typical clinical practice within the long-term care setting, neither sharp debridement nor cross-hatching of the wound bed was performed for either therapeutic agent. Dressing changes (i.e., normal saline irrigation, study drug application, semi-occlusive dressing) were performed daily, or more frequently as needed, by investigator-trained nursing staff.
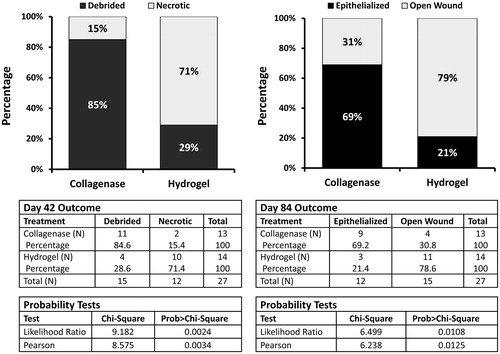
The primary clinical outcome for Phase I of the clinical study was the proportion of patients completely debrided at 42 days. Wound assessment was conducted by two blinded primary study investigators using calibrated, non-invasive wound measurement planimetry software (PictZar®, BioVisual Technologies, Elmwood Park, NJ). Digital planimetry results in more accurate wound measurements compared with standard manual measurements, which may over-estimate wound area by ∼40%Citation26. The primary clinical outcome for Phase II of the clinical study was the proportion of patients achieving complete wound closure by day 84. Two blinded investigators evaluated photographs of wound healing parameters using the same method as described above in Phase I of the study. Results from both phases of the clinical trial were statistically significant. In Phase I of the clinical trial ∼85% of the collagenase arm achieved complete debridement at 42 days compared to 29% in the hydrogel arm (p < 0.03)Citation5. In Phase II of the study, ∼69% of the collagenase arm achieved complete epithelialization (wound closure) by day 84 compared to 21% of the hydrogel arm (p = 0.0213)Citation21. The results from Phases I and II of the prospective pressure ulcer debridement study are presented in .
Figure 2. Clinical results from the prospective randomized Phase I and II pressure ulcer debridement study evaluating the efficacy of enzymatic (collagenase) debridements vs hydrogel autolytic debridements.
A Markov model was developed using the debridement and wound closure rates from the clinical study to estimate the number of healed wound days and the expected pressure ulcer cost per patient. Outputs from the Markov model were then used to derive a cost-effectiveness ratio for each product, defined as the expected cost per epithelialized wound day. Using this approach we were able to estimate the number of closed wound days in the 365 day period as well as the average cost to achieve wound closure. This method provides an estimate of the cost-effectiveness of the two alternative debridement therapies.
Model inputs
Time horizon
A 1-year time horizon was chosen to allow sufficient time for wound closure in both groups. Costs associated with occlusive wound dressings (i.e., supplies, nursing time) continued to accrue until the epithelialized state was reached (wound closure).
Three state Markov model
A three state Markov model with a cycle length of 1 day was chosen to emulate the inflammatory, proliferative, and epithelialized stages of a full thickness necrotic pressure ulcer healing by secondary intention (). State 1, the inflammatory phase, represents a necrotic non-healing pressure ulcer. State 2, the proliferative phase, represents the initiation of healing via angiogenesis and the formation of granulation tissue. State 3, the epithelialized phase, represents a closed wound requiring no further dressing and, consequently, incurring no further costs ().
Wound state transition probabilities
The removal of the necrotic tissue through debridement allows the wound to transition from the inflammatory phase to the proliferative phase where angiogenesis occurs, granulation tissue forms, and epithelialization occurs. The transition probabilities from inflammation to proliferation for the hydrogel and collagenase groups were derived from the prospective, randomized, 12-week trial, reported by Milne et al.Citation5,Citation21. In Phase I one of the clinical trial, 11 of the 13 collagenase patients (85%) achieved a fully debrided wound by day 42 as compared to four of the 14 hydrogel patients (29%)Citation5.
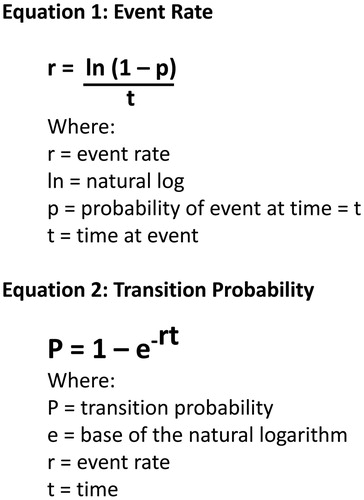
The transition probabilities from the proliferative phase to epithelialized phase were based on wound closure rates in Phase II of the randomized, 12-week trial, reported by Milne et al.Citation21. By day 84 of the trial, nine out of 11 debrided collagenase patients’ (82%) wounds had closed compared to three out of four hydrogel patients (75%). The proportions for debridement and epithelialization at days 42 and 84, respectively, were converted into daily transition probabilities using equations () described by Briggs and co-workersCitation27–29. These daily transition probabilities were used to populate the Markov model and to determine the clinical and economic outcomes ().
Table 1. Event rates and transition probabilities.
Clinical outcomes definition
The clinical benefit for the cost-effectiveness analysis was defined as ‘epithelialized days’, and represents the expected number of days that the wound was closed over the 1 year time horizon. Additionally the mathematical compliment of epithelialized days will be presented as wound days to effectively demonstrate the differences in the wound healing trajectories between the two methods of debridement. Wound days represent the expected time, in days, that wounds remained open in either the inflammatory or proliferative phases. Re-stated, wound days will represent the average amount of time that wounds are expected to remain open in the two comparative cohorts given their respective transition probabilities from the necrotic inflammatory state to the proliferating (healing) state to the absorbing epithelialized (healed) state.
Economic outcome definition
The perspective of the analysis was that of the healthcare system, specifically long-term care. Only the direct medical costs of care to the long-term care facility were considered in the economic analysis. Resource utilization included the following: nursing time, collagenase ointment, hydrogel dressing, secondary semi-occlusive dressings, wound irrigation, and wound care kits. Resource use estimates were derived from the clinical trial or, in the absence of detailed data, from information provided by the lead clinical study investigatorCitation5 or from the literatureCitation22. For this analysis, it was assumed that wounds were cleaned and dressed daily as they were in the clinical trial. Associated costs for the resources used were based on standard costs references (). All costs were reported in 2012 US dollars, and no discounting of costs was utilized because the duration of the model was 1 year (i.e., 365 days). A cost-effectiveness analysis was performed assessing costs per epithelialized day on a per-patient basis.
Table 2. Resource use and cost estimates.
Results
As patients progressed thorough the course of treatment the probability of their pressure ulcer remaining in the necrotic state fell while the probability of transitioning to the proliferative and epithelialized states increased. contrasts the time-course of wound state probability for the two debridement methods. As time progresses patients transition out of one wound state to the next.
Figure 5. Comparison of Markov state transition rates between collagenase ointment and hydrogel autolysis ulcer debridement approaches.
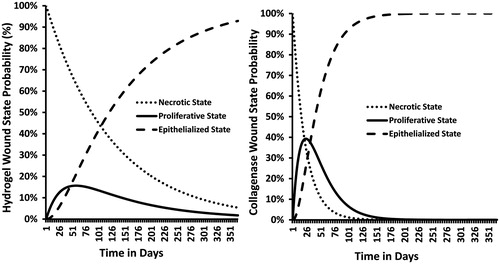
Note the difference in the wound progression trajectories between the two debridement methods. The higher debridement and wound closure rates reported for the collagenase patients at days 42 and 84, respectively, directly translates into faster rates of proliferation and epithelialization. This is clearly evident in the steeper slopes shown in the collagenase cohort compared to the hydrogel cohort. For example by day 80 the probabilities of being in either the necrotic, proliferative, or epithelialized states in the hydrogel group are ∼52%, 15%, and 32%, respectively. This is in stark contrast to the collagenase cohort which is ∼3%, 12%, and 85% for the necrotic, proliferative, or epithelialized states, respectively (). The differences in the state transition rates directly influence the outcomes and costs of debridement therapy.
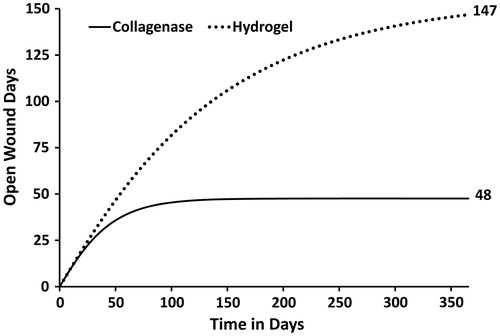
Although three states are used in the Markov model, the clinical results can essentially be dichotomized into two clinical states: the wound is either in the inflammatory or proliferative state (open) or the wound is in the epithelialized state (closed). Using the wound state transition rates taken from the prospective clinical trial it is possible to calculate the expected amount of time that the wounds will remain open, thus requiring care and bandaging. The term ‘expected’, when used in the context of a health economic analysis, represents a weighted average based on the probability of occurrence. The term is analogous to a sample mean calculated on the basis of patient level data. illustrates the number of expected wound days for the collagenase and hydrogel debridement therapies. Based on the pressure ulcer debridement and closure rates taken from the prospective clinical trial the faster inflammatory and proliferative transition rates observed in collagenase therapy cohort directly translate into an improved wound healing trajectory. Consequently, the number of expected wound days for the collagenase and hydrogel cohorts are estimated at 48 and 147, respectively.
Figure 6. Expected cumulative (open) wound days by treatment. The number of expected wound days for the collagenase and hydrogel cohorts are estimated at 48 and 147, respectively.
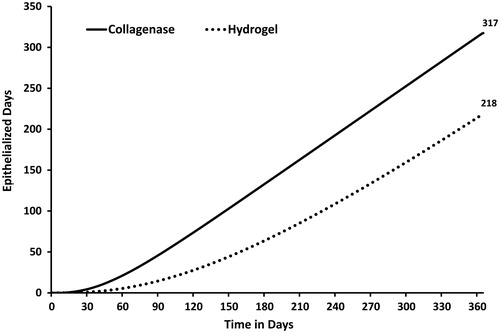
The clinical compliment to open wound days are epithelialized, or closed wound days. The number of epithelialized wound days represents the primary outcome measure when discussing the economic results. Based on the inflammatory and proliferative state transition rates of the prospective clinical trial, the expected number of epithelialized days accumulated over the 1 year time horizon was 1.5-times greater in the collagenase cohort as compared to the hydrogel cohort (317 vs 218 days, ). The model predicted that the expected number of epithelialized days accumulated at the end of the study (i.e., at day 84) were 41 and 12 for the collagenase and hydrogel cohorts, respectively. Once again this difference in outcomes can be attributed to the differences in the wound progression trajectories. The faster a wound debrides the sooner cells can proliferate to close the wound.
Figure 7. Expected clinical benefit among collagenase and hydrogel cohorts. Epithelialized (closed) wound days by treatment. The expected number of epithelialized days accumulated 1 year time horizon was 1.5-times greater in the collagenase cohort as compared to the hydrogel cohort (317 vs 218).
The 365 day time span was chosen to adequately collect the total costs across the entire episode of care for both therapeutic cohorts. The differences in the epithelialization rates between the two debridement therapies lead to substantial cost differences between the two debridement alternatives. Once the pressure ulcer epithelializes, wound care therapy is essentially complete and no further costs accrue. Given the difference in the wound closure trajectories between the two debridement approaches the expected cost (US dollars) per pressure ulcer over the 1-year time horizon was $2003 in the collagenase group and $5480 for the hydrogel group, a difference of $3477 (). The analysis indicated that the expected costs (US dollars) per pressure ulcer at the end of the study (i.e., at day 84) were $1845 and $2685 for the collagenase and hydrogel cohorts, respectively.
Figure 8. Expected cost of pressure ulcer debridement by treatment. The expected costs per pressure ulcer for the collagenase and hydrogel groups were $2003 and $5480 US dollars, respectively.
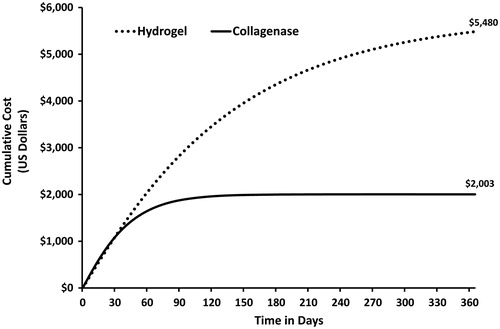
The difference in the cost profiles is due to the progression rates of the two therapeutic debridement alternatives. A faster debridement rate improves wound progression trajectory leading to earlier epithelialization. Once epithelialization occurs wound care costs cease to accrue. At 120 days of therapy the likelihood of closure in the collagenase group is ∼96%. Consequently, the likelihood of accruing additional wound care costs is only 4% as evident by the flat collagenase cumulative cost curve at 120 days. Alternatively, at 120 days the probability of an epithelialized pressure ulcer in the hydrogel groups is ∼50%, indicating that additional costs are likely to accrue. Another way to consider this information is in terms of patient cohorts. If there were 10 patients in each treatment cohort at 120 days the hydrogel cohort would still have five patients requiring wound care, while the collagenase group cohort for all practical purposes would be completely epithelialized, requiring no further wound care therapy.
Cost-effectiveness
When the total costs of pressure ulcer care were estimated over the course of 1 year, collagenase debridement therapy economically dominated hydrogel autolysis. Economic dominance means that debridement with collagenase is both more effective and less costly than autolysis with a hydrogel. A cost-effectiveness ratio was also derived for each product based on the expected total costs per patient and the clinical benefit conferred, based on the number of epithelialized days occurring across a 1-year time horizon (). Patients treated with a hydrogel dressing incurred total treatment costs that were ∼2.7-times higher than those treated with collagenase. The clinical benefit of collagenase was ∼1.5-times greater when compared to the hydrogel. The estimated cost (US dollars) per ulcer-free day was more than 4-times higher for hydrogel ($25/closed wound day) than for collagenase ($6/closed wound day).
Table 3. Cost-effectiveness results comparing collagenase with hydrogel over a 1-year time horizon.
Sensitivity analysis
One-way sensitivity analyses were performed on the Markov model parameters to determine the effects of uncertainty on the model assumptions. All model parameters were varied by ±20%, with the exception of the frequency of dressing changes which were varied from twice daily to every 3 days. Since the recommended administration schedule for collagenase is daily its administration frequency was only varied in the proliferative state after complete debridement had occurred. The one-way deterministic sensitivity analyses revealed no thresholds where collagenase lost economic dominance. The variable that exerted the most influence on the results was the frequency of dressing changes. The tornado diagram in shows the influence of the sensitivity analysis on the expected cost of collagenase therapy.
Figure 9. Tornado diagram demonstrating the influence of parameter uncertainty on the expected cost of collagenase wound care therapy. ARP, Average retail price; WAC, Wholesale acquisition costs.
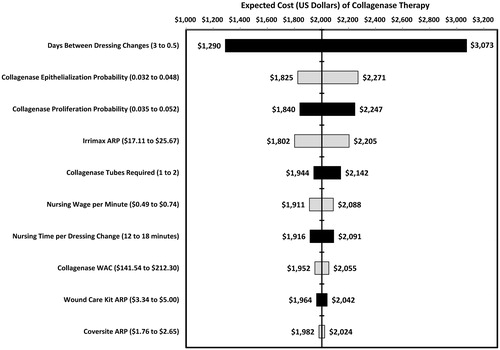
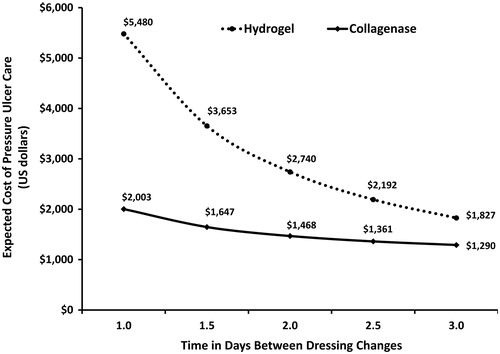
Since one of the often cited advantages of hydrogel autolysis is the reduced need for dressing changes, a one-way sensitivity analysis was performed to examine the influence on the expected cost of pressure ulcer care. The assumption of this one-way sensitivity analysis is that the clinical outcomes remain the same and only the costs will differ. The dressing change frequency was varied from daily to every 3 days. The frequency of dressing changes for the collagenase cohort in the inflammatory state remained fixed daily due to the labeled dosing and administration requirements of collagenase. Regardless of the frequency of dressing changes the total cost of collagenase therapy remained lower than the total cost of the hydrogel therapy. At a 3-day dressing change frequency the cost of hydrogel autolytic therapy was estimated at $1827 compared to $1290 for collagenase therapy. illustrates the relationship between the frequency of dressing changes and the expected costs for the collagenase and hydrogel debridement therapies.
Discussion
The results of this Markov analysis demonstrate that, although collagenase ointment has a higher acquisition cost compared to the hydrogel dressing, enzymatic debridement with collagenase can accelerate the wound healing trajectory, thus reducing the overall cost of pressure ulcer care. Specifically, the acquisition cost for a 30 g tube of collagenase ointment was 10-times the cost of a 90 g tube of the hydrogel dressing. However, the therapeutic effect of collagenase was ∼1.5-times greater than the hydrogel dressing when assessing the number of closed wound bed days across the entire episode of care. The collagenase cohort had an expected mean of 313 closed wound days compared with the 218 days in the hydrogel cohort. When one considers the total cost of pressure ulcer care the difference in expected costs are quite substantial. The expected total cost in the collagenase cohort was estimated at $2003 vs $5480 in the hydrogel cohort, yielding a cost saving of $3477 (US dollars) favouring enzymatic debridement with collagenase ointment. These results establish a compelling argument for the economic value of collagenase ointment for the treatment of necrotic pressure ulcers in a long-term care setting.
There are a small number of economic analyses comparing collagenase with hydrogel dressingCitation14,Citation22,Citation23, and the results are consistent with the current study’s clinical and cost-effectiveness findings. Mosher et al.Citation14 found that collagenase therapy resulted in a higher likelihood of achieving a clean wound bed and a lower cost of treatment over 1 month than autolysis ($611 vs $921 US dollars, respectively) among Medicare patients with pressure ulcers. Specifically, collagenase was estimated to be 1.3-times more cost-effective than hydrogel autolysis. It is important to note the effectiveness outcomes for this study were derived from expert panels, although the results were consistently favorable for collagenase. Another study by Muller et al.Citation22 found that wound healing of grade IV pressure sores located on the heel was achieved 4 weeks earlier with collagenase than with hydrogel (10 weeks vs 14 weeks, respectively), while average costs were 54% higher with hydrocolloid dressing, leading to a better cost-effectiveness ratio for collagenase (cost savings of 899 Dutch guilders [1998 value] per successfully-treated patient). The analysis was based on 24 hospitalized patients in The Netherlands, and data were derived from a clinical trial. A study conducted in Germany compared five treatments for pressure and venous leg ulcers using Monte Carlo simulation, and found that a hydroactive wound dressing plus an enzymatic debridement ointment was more cost-effective than the alternatives (gauze, impregnated cause, or calcium alginate), due to a reduction in duration of treatment and personnel costsCitation23.
The current study builds upon earlier analysesCitation14,Citation22,Citation23 using currently available products and costs, with clinical inputs derived from a randomized controlled clinical trial, and also reflects an important perspective of the nursing home population. However, results from decision modeling are only as robust as the data inputs and, thus, should be interpreted in light of certain limitations.
The clinical data used in this analysis were derived from a clinical trialCitation5,Citation21 conducted in a single center with a relatively small patient sample and, thus, the results cannot necessarily be generalized to other hydrogel dressings, healthcare settings, age groups, or to wounds of other etiologies. However, because the difference in clinical benefit was substantial for collagenase compared with hydrogel, and it was this clinical benefit that was the primary driver of the expected cost savings, it is reasonable to conclude that larger enrollment results would not produce markedly different results from an economic value perspective.
Conclusion
In this economic analysis based on a prospective, randomized, clinical trial of necrotic pressure ulcer therapy occurring in a long-term care setting collagenase ointment demonstrated its cost-effectiveness relative to autolysis with a considerably less expensive hydrogel dressing. Based on the clinical trial a larger proportion of collagenase patients achieved complete wound bed debridement and wound closure compared to the hydrogel group over the episode of care. Faster debridement associated with collagenase therapy translates into better outcomes and a substantial total cost savings relative to autolytic debridement with the hydrogel dressing. Despite the higher acquisition cost of collagenase ointment, its clinical benefit offsets its initial cost difference. In a long-term care setting treatment of necrotic pressure ulcers with collagenase ointment can result in a substantial cost savings compared to autolysis with a hydrogel dressing.
Transparency
Declaration of funding
This was an investigator initiated study funded by Healthpoint Biotherapeutics, Fort Worth, TX, USA.
Declaration of financial/other interests
Catherine T. Milne has disclosed that she is on the speaker’s bureau for Healthpoint Biotherapeutics and served as the Principal Investigator for the clinical trial discussed in this manuscript. Curtis Waycaster has disclosed that he is a full-time employee of Healthpoint Biotherapeutics. JME Peer Reviewers on this manuscript have no relevant financial relationships to disclose.
Acknowledgments
The authors thank G. Kesava Reddy, PhD, MHA of Creative Medical Communications, LLC, for providing medical writing support that was funded by Healthpoint Biotherapeutics.
Previous presentation
These data have previously been presented at the American Professional Wound Care Association's (APWCA) National Clinical Conference held in Orlando, Florida, USA on March 31–April 3, 2012.
References
- Horn SD, Bender SA, Bergstrom N, et al. Description of the National Pressure Ulcer Long-Term Care Study. J Am Geriatr Soc 2002;50:1816-25
- Cereda E, Gini A, Pedrolli C, et al. Disease-specific, versus standard, nutritional support for the treatment of pressure ulcers in institutionalized older adults: a randomized controlled trial. J Am Geriatr Soc 2009;57:1395-402
- Schultz G, Mozingo D, Romanelli M, et al. Wound healing and TIME; new concepts and scientific applications. Wound Repair Regen 2005;13(4 Suppl):S1-11
- Ramundo J, Gray M. Collagenase for enzymatic debridement: a systematic review. J Wound Ostomy Continence Nurs 2009;36:S4-11
- Milne CT, Ciccarelli AO, Lassy M. A comparison of collagenase to hydrogel dressings in wound debridement. Wounds 2010;22:270-4
- Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11(1 Suppl):S1-28
- Carter MJ. Cost-effectiveness research in wound care: definitions, approaches, and limitations. Ostomy Wound Manage 2010;56:48-59
- Robson MC, Steed DL, Franz MG. Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg 2001;38:72-140
- Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994;130:489-93
- Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen 1994;2:165-70
- Nwomeh BC, Yager DR, Cohen IK. Physiology of the chronic wound. Clin Plast Surg 1998;25:341-56
- Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen 1996;4:411-20
- Moore J, Jensen P. Assessing the role and impact of enzymatic debridement. Podiatry Today 2004;17:54-6.
- Mosher BA, Cuddigan J, Thomas DR, et al. Outcomes of 4 methods of debridement using a decision analysis methodology. Adv Wound Care 1999;12:81-8
- Thomas S, Jones H. Clinical experiences with a new hydrogel dressing. J Wound Care 1996;5:132-3
- Mulder GD. Cost-effective managed care: gel versus wet-to-dry for debridement. Ostomy Wound Manage 1995;41:68-70
- Ayello EA, Cuddigan J, Kerstein MD. Skip the knife: debriding wounds without surgery. Nursing 2002;32:58-63; quiz 64
- Vowden KR, Vowden P. Wound debridement, part 2: sharp techniques. J Wound Care 1999;8:291-4
- Vowden KR, Vowden P. Wound debridement, part 1: non-sharp techniques. J Wound Care 1999;8:237-40
- Turner T. The development of wound management products. In: Krasner DL, Rodeheaver GT, Sibbald RG, eds. Chronic wound care: a clinical source book for healthcare professionals. 3rd edn. Wayne, PA: HMP Communications, 2001
- Milne CT, Ciccarelli AO, Lassy M. A comparison of collagenase to hydrogel dressings in maintenance debridement and wound closure. Wounds 2012;24:317-22
- Muller E, van Leen MW, Bergemann R. Economic evaluation of collagenase-containing ointment and hydrocolloid dressing in the treatment of pressure ulcers. Pharmacoeconomics 2001;19:1209-16
- Bergemann R, Lauterbach KW, Vanscheidt W, et al. Economic evaluation of the treatment of chronic wounds: hydroactive wound dressings in combination with enzymatic ointment versus gauze dressings in patients with pressure ulcer and venous leg ulcer in Germany. Pharmacoeconomics 1999;16:367-77
- Richardson WS, Detsky AS. Users' guides to the medical literature. VII. How to use a clinical decision analysis. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA 1995;273:1292-5
- Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993;13:322-38
- Rogers LC, Bevilacqua NJ, Armstrong DG, et al. Digital planimetry results in more accurate wound measurements: a comparison to standard ruler measurements. J Diabetes Sci Technol 2010;4:799-802
- Briggs AH, Goeree R, Blackhouse G, et al. Probabilistic analysis of cost-effectiveness models: choosing between treatment strategies for gastroesophageal reflux disease. Med Decis Making 2002;22:290-308
- Briggs AH, Ades AE, Price MJ. Probabilistic sensitivity analysis for decision trees with multiple branches: use of the Dirichlet distribution in a Bayesian framework. Med Decis Making 2003;23:341-50
- Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics 2000;17:479-500