Abstract
Drug addiction or substance-use disorder is a chronically relapsing disorder that progresses through binge/intoxication, withdrawal/negative affect and preoccupation/anticipation stages. These stages represent diverse neurobiological mechanisms that are differentially involved in the transition from recreational to compulsive drug use and from positive to negative reinforcement. The progression from recreational to compulsive substance use is associated with downregulation of the brain reward systems and upregulation of the brain stress systems. Individual differences in the neurobiological systems that underlie the processing of reward, incentive salience, habits, stress, pain, and executive function may explain (i) the vulnerability to substance-use disorder; (ii) the diversity of emotional, motivational, and cognitive profiles of individuals with substance-use disorders; and (iii) heterogeneous responses to cognitive and pharmacological treatments. Characterization of the neuropsychological mechanisms that underlie individual differences in addiction-like behaviors is the key to understanding the mechanisms of addiction and development of personalized pharmacotherapy.
La adicción a drogas o el trastorno por uso de sustancias es un trastorno crónico con recaídas que progresa a través de las etapas de compulsión / intoxicación, abstinencia/afecto negativo y preocupación/anticipación. Estas etapas representan diversos mecanismos neurobiológicos que participan diferenciadamente en la transición desde el uso recreacional al uso compulsivo de la droga y desde un refuerzo positivo a uno negativo. La progresión, desde un uso recreacional de la sustancia a uno compulsivo, está asociada con una regulación negativa de los sistemas cerebrales de recompensa y una regulación positiva de los sistemas cerebrales del estrés. Las diferencias individuales en los sistemas neurobiológicos que están a la base del procesamiento de la recompensa, del aumento del incentivo, de los hábitos, del estrés, del dolor, y de la función ejecutiva pueden explicar: 1) la vulnerabilidad al trastorno por uso de sustancias, 2) la diversidad de los perfiles emocionales, motivacionales y cognitivos de los sujetos con trastornos por uso de sustancias y 3) las respuestas heterogéneas a los tratamientos cognitivos y farmacológicos. La clave para comprender los mecanismos de la adicción y el desarrollo de una farmacoterapia personalizada es la caracterización de los mecanismos neuropsicológicos que subyacen a las diferencias individuales en las conductas adictivas.
L'addiction aux drogues ou le trouble de l'usage d'une substance est une maladie à rechutes chroniques qui évolue par des étapes de compulsion/intoxication, sevrage/effet négatif et préoccupation/anticipation. Ces étapes représentent des mécanismes neurobiologiques variés différemment impliqués dans la transition allant de l'usage récréatif à l'usage compulsif d'une drogue et du renforcement positif au renforcement negatif. Le passage de l'usage récréatif à l'usage compulsif d'une substance est associé à une régulation négative des systèmes cérébraux de récompense et à une régulation positive des systèmes cérébraux de stress. Des différences individuelles dans les systèmes neurobiologiques sous-tendant le processus de récompense, de saillance incitative, d'habitudes, de stress, de douleur et de fonction exécutive peuvent expliquer 1) la vulnérabilité aux troubles liés à l'usage de substances ; 2) la diversité des profits émotionnels, motivationnels et cognitifs des individus souffrant de troubles liés à l'usage de substances et 3) les réponses hétérogènes aux traitements cognitifs et pharmacologiques. La clé de la compréhension des mécanismes d'addiction et du développement de traitements pharmacologiques personnalisés est la caractérisation des mécanismes neuropsychologiques sous-tendant les différences individuelles dans les comportements addictifs.
Keywords:
Psychopathological framework
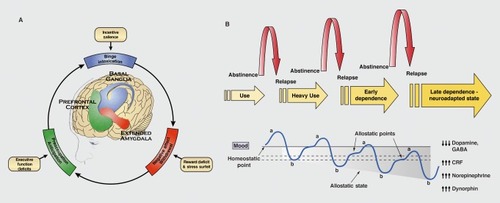
Three stages of the addiction cycle: binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation
Drug addiction is a chronically relapsing disorder that is characterized by compulsion to seek and take the drug, loss of control in limiting drug intake, and emergence of a negative emotional state, reflecting a motivational withdrawal syndrome, when access to the drug is prevented.Citation1 Drug addiction includes three stages: preoccupation/anticipation, binge/intoxication, and withdrawal/negative affect. These three stages feed into each other to produce an addiction cycle. Each stage becomes more intense after each cycle, leading to the pathological state of addiction. These three stages reflect incentive salience/pathological habits, reward deficits/stress surfeit, and executive function deficits, respectively, which provide a powerful impetus for compulsive drug-seeking behavior that is associated with drug addiction. These domains of dysfunction correspond to neuroadaptations that reflect allostatic changes in three key neurocircuits that mediate compulsive drug seeking: basal ganglia, extended amygdala, and prefrontal cortex, respectively ().Citation2. Allostasis in the context of addiction is the process by which the body responds to challenges to maintain apparent homeostasis through changes in brain reward and stress mechanisms.Citation3 The allostatic state represents a chronic deviation of reward set point that is mostly observed during abstinence and not observed when the individual is actively taking drug. Thus, the allostatic view extends counteradaptive theory by stating that not only does the b-process get larger with chronic drug use but the reward set point also progressively shifts downward, thus creating an allostatic state (Figure 1 B).Citation3,Citation4 This model has been proposed to explain the persistent changes in motivation in drug-dependent individuals. We propose that the relative contribution of each of these three stages to drug use and drug addiction varies both between and within individuals across time.
From positive to negative reinforcement
Another level of complexity that is added to these three stages is the fact that drug addiction includes a transition from impulsive to compulsive behaviors and from positive to negative reinforcement (). Citation5,Citation6 Impulsivity is defined as “a predisposition toward rapid, unplanned reactions to internal or external stimuli without regard for the negative consequences of these reactions to [themselves] or others.”Citation7 Compulsivity is defined as “perseverative, repetitive actions that are excessive and inappropriate.”Citation8 Positive reinforcement is defined as the process by which presentation of a stimulus increases the probability of a response. Negative reinforcement is defined as the process by which removal of an aversive stimulus (or aversive state in the case of addiction) increases the probability of a response.Citation9 Impulsivity often dominates at the early stages of drug addiction through repeated binge/intoxication and positive reinforcement. Individuals seek and take the drug for its initial pleasurable and reinforcing effects without regard for the potential future negative consequences of using drugs. Compulsivity dominates at later stages of drug addiction through the emergence of negative emotional states in the withdrawal/negative affect stage and anticipation of obtaining the drug in the preoccupation/anticipation stage. Such compulsivity leads to the escalation of drug intake and perseverative drug use despite adverse consequences. The transition from positive to negative reinforcement reflects a change in the underlying psychological and neurobiological mechanisms of motivation (Figure 2). Motivation can be defined as a “tendency of the whole animal to produce organized activity.”Citation10 The neural substrates for the two sources of reinforcement that play a key role in allostatic neuroadaptations derive from two key motivational systems that are required for survival: brain reward system and brain stress system.
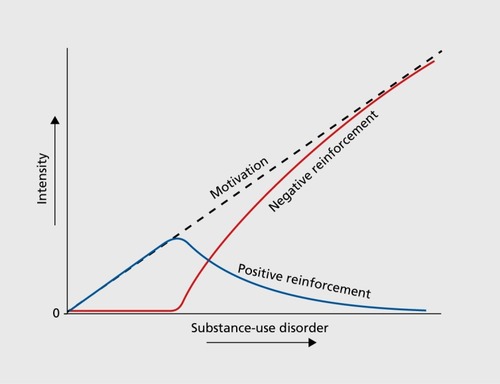
Within the addiction process, the concept of motivation is linked to hedonic, affective, and emotional states in the context of temporal dynamics that are elaborated by Solomon's opponent process theory of motivation.Citation11 Hedonic, affective, or emotional states, once initiated, are modulated by the central nervous system with mechanisms that reduce the intensity of hedonic feelings. This theory postulates that any motivational stimulus activates two opposing motivational processes. The a-process consists of positive or negative hedonic responses, has a fast onset and offset, correlates with the intensity, quality, and duration of the stimulus, and shows tolerance. The b-process appears after the a-process has terminated, is opposite in direction, is sluggish in onset, is slow to build up and decay, and gets larger with repeated exposure. The initial acute effect of a drug of abuse (ie, the a-process or positive hedonic response) was hypothesized to be opposed or counteracted by the b-process as homeostatic changes in brain systems. With repeated exposure to drugs, the b-process sensitizes, appears earlier after the unconditioned stimulus, lasts longer, and masks the a-process, leading to apparent tolerance.Citation12 Two types of biological processes have been proposed to describe the mechanisms that underlie the neuroadaptations that are associated with generation of the opponent process in drug addiction: within-system adaptation and between-system adaptation.Citation13 In the within-system process, the drug elicits an opposing, neutralizing reaction within the same system in which the drug elicits its primary and unconditioned reinforcing actions. In the between-system process, neurobiological systems are recruited that are different from the ones that were initially activated by the drug.
Neurobiological mechanisms of drug addiction
Binge/intoxication stage
Intense research efforts over the past three decades have been dedicated to revealing the neurochemical elements and neuronal networks that are responsible for the binge/intoxication stage ().Citation14 Converging evidence suggests that three main contributors to the binge/intoxication stage in the early stages of drug addiction are (i) the acute positive hedonic value of drugs,Citation15 (ii) sensitization of incentive salience,Citation16 and (iii) inherent poor cognitive insight.Citation17 The later stages of drug addiction also exhibit a binge/intoxication stage that also includes tolerance and is fueled by the negative emotional states that are an important driving force to the maintenance of chronic and heavy drug use.
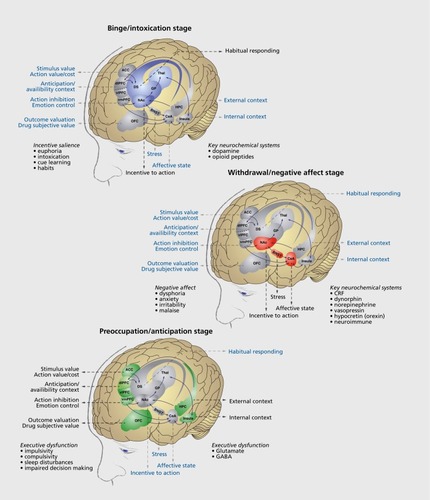
Intoxicating doses of drugs, including alcohol, produce the release of dopamine and endogenous opioids in the ventral striatum that correlate with the subjective effects of drugs, including feelings of being “high.”Citation18,Citation19 Earlier preclinical work showed that all drugs of abuse increase dopamine release in the ventral striatum, leading to theories that suggested that this increase may be related to the hedonic value of drugs of abuse.Citation15 Moreover, dopamine plays a key role in psychostimulant reward, but dopamine -independent reward has also been demonstrated for opioids and alcohol.Citation20-Citation22 Further work established a key common role for dopamine in addiction. The dopaminergic system is important within a subcomponent of motivational systems that allows the attribution of incentive salience. Incentive salience (anchored within the construct of conditioned reinforcement) is a phenomenon by which a previously neutral stimulus acquires incentive value through pairing with a drug of abuse.Citation16 Robust evidence indicates that dopamine plays a minor role, if any, in reward processing per se and may represent instead a reward prediction error signal.Citation23
The theory of sensitization of incentive salience has its origins in early work on conditioned reinforcement.Citation24 Prominent work has shown that dopamine neurons are crucial for mediating such conditioned reinforcement. Dopamine neurons in the ventral tegmental area and substantia nigra have been shown to exhibit phasic responding to a nonpredicted reward. After repeated exposure, the same neurons stop responding to a predictable reward and instead start responding to the earliest cue that predicts the future reward.Citation25,Citation26 This process allows neutral cues to acquire incentive salience and thus elicit behavioral approach. One hypothesis is that the progressive sensitization of this phasic responding to cues that is associated with drug reward may contribute to maladaptive craving that is observed in individuals with substance-use disorders,Citation27,Citation28 although clinical evidence for such a phenomenon is sparse.Citation29 Preclinical evidence indicates that drugs of abuse can produce a shift in the excitatory balance of dopamine neurons after acute administration,Citation30-Citation38 suggesting that neuroadaptations in the dopamine incentive salience system can occur early in the addiction process. Moreover, a recent study in humans showed that the phenomenon of conditioned responding of dopamine neurons to drug-predictive cues occurred in recreational cocaine users who did not meet the criteria for cocaine-use disorders.Citation39 These results suggest that the phenomenon of sensitization of incentive salience may be important early in the addiction process but may not be a key mechanism in later stages of addiction. In contrast, there is converging evidence in the preclinical literature that the later stages of addiction may instead involve a transition from goal-directed behavior that is mediated by the ventral striatum to habit behavior that is under the control of the dorsal striatum and that is facilitated by chronic exposure to the drug.Citation40-Citation49
Preclinical work has shown that the activation of dopamine D1 but not D2 receptors,Citation50,Citation51, μ-opioid receptors (MORs),Citation52 nociceptin opioid (NOP) receptors,Citation53 and α4-, β2-, and α6-containing nicotinic acetylcholine receptors (nAChRs) are required for the acute rewarding and reinforcing effects of drugs. However, these requirements are usually specific to the particular drug of abuse (D1 for cocaine, MOR for opioids, and nAChRs for nicotine). The only exception may be NOP receptors, which have recently been shown to affect cocaine, heroin, and alcohol self-administration and drug-induced conditioned place preference.Citation53 Other neurotransmitter systems, including the serotonin 5-hydroxytryptamine (5-HT),Citation54,Citation55 γ-aminobutyric acid (GABA),Citation55 acetylcholinergic (ACh),Citation55 and endocannabinoidCitation35,Citation56-Citation59 systems, are believed to contribute to the binge/intoxication stage by modulating dopamine, opioid, and nicotinic systems, although a more central role for the GABA system has been identified in the mediation of the intoxicating and reinforcing effects of alcohol.Citation60
Withdrawal/negative affect stage
Our understanding of the neurobiology of the withdrawal/negative affect stage has dramatically increased in the past decade. This stage includes different sources of motivation to take drugs, including chronic irritability, emotional pain, malaise, dysphoria, alexithymia (inability to identify/express emotions), states of stress, and the loss of motivation for natural rewards. For example, chronic administration of all major drugs of abuse leads to stress and anxiety-like responses during acute and protracted abstinence.Citation61
One explanation for the blunted function of the reward system during abstinence involves within-system neuroadaptations, in which the primary target of the drug rapidly adapts to neutralize the effect of the drug. Long-lasting within-system adaptations can then lead to a decrease in brain reward function when the drug is removed.Citation13 For example, cocaine acutely produces dopamine and serotonin release, but decreases in dopaminergic and serotonergic transmission have been observed in the ventral striatum during cocaine withdrawal in rats.Citation62 Even more compelling are studies in humans that reported lower self-reported rewarding effects of drugs and a lower striatal dopamine response after amphetamine/methylphenidate challenges in active and detoxified abusers than in controls.Citation63-Citation66 Similar neuroadaptations are hypothesized to occur for other classes of drugs, including increases in MOR responsivity during opioid withdrawal,Citation67,Citation68 decreases in GABAergic transmission in the ventral striatum, and increases in N-methyl-D-aspartate glutamatergic transmission in the ventral striatum.Citation69,Citation70 Complex regional changes in function in key brain regions, including the ventral tegmental area, ventral striatum, interpeduncular nucleus (IPN), amygdala, and habenula, have been reported for nicotine and alcohol addiction, among other addictions.Citation71,Citation72 Such within-system neuroadaptations may contribute to the withdrawal/negative affect stage by decreasing brain reward function during abstinence but may also be involved in the preoccupation/anticipation stage by providing a greater hedonic driving force to resume drug use.
Another explanation for the lower function of the reward system during abstinence involves betweensystem neuroadaptations, in which systems other than those that are involved in the positive rewarding effects of drugs are recruited or dysregulated by chronic drug use to oppose the rewarding effects of drugs of abuse.Citation13 A central component of this between-system neuroadaptation is activation of the stress pathways, including the hypothalamic-pituitary-adrenal (HPA) axis and extrahypothalamic brain stress systems that are mediated by corticotropin-releasing factor (CRF), norepinephrine, and dynorphin.Citation73-Citation75 Withdrawal from drugs of abuse acutely increases adrenocorticotropic hormone, corticosterone, and extended amygdala CRF and dynorphin during withdrawal.Citation76-Citation83
Two main brain circuits probably contribute to these opponent-like processes that lower brain reward function and increase brain stress system function. Both of these circuits are heavy influenced by CRF. One circuit involves the extended amygdala, which encompasses the central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST), and part of the nucleus accumbens (Figure 3). The extended amygdala integrates brain arousal-stress and reward system informationCitation84 to produce the between-system opponent process that is elaborated above. The CRF system in the extended amygdala is activated during acute withdrawal from cocaine, alcohol, opioids, ΔCitation9-tetrahydrocannabinol, and nicotine.Citation78,Citation85,Citation86 Similar effects have been observed with alcohol in the lateral BNSTCitation81 Cocaine withdrawal produces anxiety-like responses that can be reversed by a CRF-receptor antagonist.Citation87,Citation88 Similar results have been observed with nicotine,Citation86,Citation89,Citation90 alcohol,Citation79,Citation91 and opioids.Citation88,Citation92,Citation93 Moreover, the ability of CRF receptor antagonists to block the anxiogenic-like and aversive-like motivational effects of drug withdrawal predicts the efficacy in reducing compulsive-like self-administration of cocaine,Citation94 nicotine,Citation86 and heroinCitation95 in rats. Although very promising, the clinical development of CRF1-receptor antagonists for the treatment of drugand alcohol-use disorders has mostly failed.Citation96,Citation97 However, these failures should be considered cautiously because the compounds that have been used have less than ideal pharmacokinetics/pharmacodynamics and physicochemical properties, and several other shortcomings in these studies may explain their negative outcomes (for details, see Spierling and ZorrillaCitation98).
The excessive release of dopamine and opioid peptides produces subsequent activation of the dynorphin system in the basal ganglia and extended amygdala, which has been hypothesized to feed back to decrease dopamine release and contribute to the dysphoric syndrome that is associated with cocaine dependence.Citation99 Dynorphins produce aversive dysphoric-like effects in animals and humans and have been hypothesized to mediate negative emotional statesCitation100,Citation101 and depressionlike, aversive responses to stress and dysphoric-like responses during withdrawal from drugs of abuse. Recent evidence suggests that the dynorphin/κ-opioid system in the extended amygdala also mediates compulsive-like responding for methamphetamine, heroin, and alcohol with extended access and dependence.Citation101
Another system is the habenula-to-IPN pathway. The habenula plays a key role in encoding aversive states,Citation102,Citation103 in part by decreasing dopamine neuron firing in the ventral tegmental area after failure to receive an expected reward.Citation102,Citation103 This hypothesis is consistent with the finding that nAChRs in the habenula-IPN appear to modulate aversive responses to nicotineCitation104 and nicotine withdrawal.Citation105,Citation106 We recently reported that the habenula-IPN pathway is also under the influence of the CRF system.Citation90,Citation106 Activation of this pathway during nicotine withdrawal was potentiated by CRF-producing neurons in the ventral tegmental area that project to the IPN. The downregulation of CRF messenger RNA in the ventral tegmental area and CRF1-receptor blockade in the IPN prevented emergence of the negative emotional states associated with withdrawal and reduced excessive nicotine intake after abstinence.Citation90,Citation106
In addition to these subcortical circuits that involve the brain reward and stress systems, the insular cortex is an important cortical region for emotional aspects of the withdrawal/negative affect stage. Cravings for food, cocaine, and nicotine have been shown to activate the insular cortex,Citation107-Citation109 and tobacco smokers with damage to the insular cortex were able to stop smoking easily with little, if any, withdrawal symptoms, craving, or relapse.Citation110 The insula is hypothesized to integrate autonomic, visceral, and emotional informationCitation111 during withdrawal and abstinence to produce the motivation to obtain the drug within a negative reinforcement framework (ie, obtain relief from negative emotional states associated with withdrawal). Supporting this hypothesis, imaging studies have reported differential activation of the insula during craving, possibly reflecting interoceptive cues. Such activation during craving also could be driven by the activation of cortical CRF systems when considering the substantial level of CRF neurons and CKF1 receptors in the insula.Citation112,Citation113 Finally, reactivity of the insular cortex has been suggested to serve as a biomarker to help predict relapse.Citation114
Preoccupation/anticipation stage
Intoxicating doses of drugs, particularly alcohol, marijuana, and opioids, and high doses of psychostimulants are associated with cognitive impairments, including poor working memory, inattention, impulsivity, and delay discountingCitation115 (for review, see Oscar-Berman and IlutnerCitation116). Such cognitive impairment significantly contributes to relapse and the escalation of drug intake and results from drug-induced dysfunction of the dorsolateral, ventrolateral, and lateral prefrontal cortex and orbitofrontal cortex (Figure 3).,Citation115,Citation117-Citation120 For example, working-memory impairments have been associated with higher levels of alcohol, methamphetamine, and cocaine use in both humans and rats.Citation121-Citation125
Craving is a key part of the preoccupation/anticipation stage. Large interindividual variability has been observed in the intensity of craving and the source of craving. In humans, cue-induced craving activates the dorsolateral prefrontal cortex, anterior cingulate gyrus, and medial orbitofrontal cortex.Citation126-Citation130 Cues that are associated with cocaine craving also increase doparnine release in the ventral striatum, prefrontal cortex, and amygdala and endogenous opioid peptide release in the frontal cortex and anterior cingulate.Citation131-Citation134 Such activation of the reward/salience systems during acute craving episodes is further potentiated because of a decrease in the inhibitory function of the prefrontal cortex (orbitofrontal cortex, ventromedial cortex, and anterior cingulate cortex) in humans with substance-use disorders.Citation19,Citation135,Citation136 Indeed, substance-use disorder is associated with chronic executive dysfunction, including impairments in decision making, self-regulation, inhibitor control, attention, and working memory,Citation117 that may be caused by increases in GABAergic and CRF activity in the prefrontal cortex.Citation123,Citation137 Another key neurotransmitter system that is associated with impairments in behavioral inhibition is the dopaminergic system. Brain imaging has consistently shown lower dopamine D2 receptor availability in the striatum and prefrontal cortex after protracted abstinence in humans, nonhuman primates, and rodents.Citation138 Preclinical models have shown that lower D2 receptor availability is associated with greater motivation for cocaineCitation139 and cognitive deficits.Citation140 Decreases in striatal dopamine, combined with increases in GABA and CRF signaling in the prefrontal cortex, may lead to an overactive ”Go“ system that drives craving and habits and a hypoactive ”Stop“ system that normally inhibits impulsive behavior and negative emotional states through the activation of specific corticostriatal loops.Citation141-Citation144
Implications for personalized medicine
Our thesis is that there are considerable individual differences in the patterns of drug use and the psychological mechanisms that drive drug use. Drug use may be driven by the binge/intoxication stage for some individuals and by the withdrawal/negative affect stage for others. With psychostimulants and even alcohol, binge-like patterns can predominate in some individuals. Such individuals may escalate their drug intake in a binge-like pattern for various reasons, including peer pressure, sensation seeking, externalizing disorders, and drug-induced cognitive impairment (eg, decision making, monitoring, renegade attention, and transcendence failure) with little, if any, initial negative emotional symptoms. Other individuals may quickly develop a pattern of chronic and heavy use that is caused by either conscious or unconscious attempts to self-medicate existing negative emotional states. Such individuals often have preexisting conditions that generate powerful negative emotional states, such as posttraumatic stress disorders, sexual abuse, major depressive disorder, or anxiety disorder, and will use drugs to obtain relief from these negative emotional states. However, chronic high-dose binge-like patterns of drug intake can cause the development of negative emotional states and ultimately drive self-medication of a state that is created by the drug itself. Ultimately, both the binge/intoxication stage and withdrawal/negative affect stage will contribute to a pathological state of compulsive drug seeking and taking. One intriguing area of research is the identification of genetic, biological, and psychological subpopulations of humans with substance-use disorder within the framework of the three stages of addiction to better understand the drug addiction process and potentially predict treatment efficacy. Our thesis is that addiction treatments may benefit from the development of medications that specifically target each phase of the addiction process to personalize treatment and obtain better treatment outcome and compliance. In the past decade, notable advances have been made, and there is clear clinical evidence in humans that some treatments (eg, naltrexone) may be better suited for the treatment of the binge/intoxication stage, whereas others (eg, acamprosate) may be more appropriate for the preoccupation/anticipation stage. However, to date, these findings have had little impact in real life for the treatment of substance-use disorders because of the limited number of available medications and limited number of patients who receive appropriate treatment.
There are individual differences in executive function, prefrontal cortex function, brain stress system function, and dopamine reward signaling, and the genetics of negative emotional states may help identify subgroups of patients with substance-use disorder that may help predict treatment outcome. Attempts are being made to identify genetic markers, including single-nucleotide polymorphisms (SNPs), that may predict the vulnerability to substance-use disorders and responsiveness to treatment. Several research groups have identified gene variants in the metabolic enzymes and receptors that are directly modulated by drugs of abuse, such as MOR, nAChRs, cytochrome p450, and alcohol dehydrogenase. Such findings are very encouraging and suggest that some of these gene variants may predict the response to specific treatments.Citation145-Citation149 Several SNPs that are associated with the CRF system have also been associated with excessive alcohol use. An association was found between SNPs that are related to the CRF1 receptor gene (Crhr1) and binge drinking in adolescents and alcohol-dependent adults.Citation150-Citation152 Another important genetic association has been found between alcohol dependence and SNPs that are related to the gene that encodes neuropeptide Y (NPY). NPY is an anxiolytic peptide that is involved in emotional regulation and stress coping and is known to antagonize the effects of CRF on addiction-like behaviors. Studies have linked SNPs of the Y2, receptor gene (NPY2R) and alcohol dependence, alcohol withdrawal symptoms, comorbid alcohol and cocaine dependence, and cocaine dependence.Citation153 The G1258A polymorphism of the NPY gene has been linked to alcohol dependence.Citation154 The rsl6147 SNP of the NPY promoter gene was linked to tobacco addiction.Citation155 Should a medication become available that modulates CRF or NPY, such genetic analysis may reveal that subpopulations of subjects who carry specific SNPs might be more responsive than others.
Conclusions
Drug addiction is a chronically relapsing disorder that is associated with compulsive drug seeking and taking that progress through the binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation stages.
These three stages have diverse neurobiological mechanisms that are involved in the transition from recreational to compulsive drug use. We hypothesize that individual differences in the neurobiological systems that underlie the processing of reward, incentive salience, stress, pain, habits, and executive function may explain (i) the vulnerability to developing a substance-use disorder; (ii) the diversity of emotional, motivational, and cognitive profiles of individuals with substance-use disorders; and (iii) the heterogeneous responses to cognitive and pharmacological treatments. We propose that characterization of the neuropsychological mechanisms that underlie individual differences in addiction-like behaviors is a key to understanding the mechanisms of addiction and development of personalized medicine through genomic medicine and personalized pharmacotherapy.
The authors thank Michael Arends for assistance with manuscript preparation and proofreading. Preparation of this manuscript was financially supported by grants from the National Institutes of Health (AA006420, AA020608, AA022977, DA043799, and DA036691) and the Pearson Center for Alcoholism and Addiction Research. The authors have no conflicts of interest to disclose.
REFERENCES
- KoobGF.Le MoalM.Drug abuse: heclonic homeostaticdysregulation.Science.1997278533552589311926
- KoobGF.VolkowND.Neurobiology of addiction: a neurocircuitry analysis.Lancet Psychiatry.20163876077327475769
- KoobGF.Le MoalM.Drug addiction, dysregulation of reward, and allostasis.Neuropsychopharmacology.20012429712911120394
- HeiligM.KoobGF.A key role for corticotropin-releasing factor in alcohol dependence.Trends Neurosci.200730839940617629579
- KoobGF.Le MoalM.Addiction and the brain antireward system.Annu Rev Psychol.200859295318154498
- KoobGF.Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder.Curr Top Behav Neurosci.2013330
- MoellerFG.BarrattES.DoughertyDM.SchmitzJM.SwannAC.Psychiatric aspects of impulsivity.Am J Psychiatry.2001158111783179311691682
- BerlinGS.HollanderE.Compulsivity, impulsivity, and the DSM-5 process.CNS Spectr.2014191626824229702
- SkinnerBF.The Behavior of Organisms: An Experimental Analysis. New York, NY: Apple-Century-Crofts. 1938
- HebbDO.Textbook of Psychology, 3rd edition. Philadelphia, PA: WB Saunders.1972
- SolomonRL.CorbitJD.An opponent-process theory of motivation: 1 . Temporal dynamics of affect.Psychol Rev.19748121191454817611
- LaulinJP.CelerierE.LarcherA.Le Moal M Simonnet G Opiate tolerance to daily heroin administration: an apparent phenomenon associated with enhanced pain sensitivity.Neuroscience.199989363163610199599
- KoobGF.BloomFE.Cellular and molecular mechanisms of drug dependence.Science.198824248797157232903550
- KoobGF.EverittBJ.RobbinsTW.Reward, motivation, and addiction. In: Squire LG, Berg D, Bloom FE, Du Lac S, Ghosh A, Spitzer N, eds.Fundamental Neuroscience. 3rd edition. Amsterdam, the Netherlands: Academic Press.20089871016
- WiseRA.The role of reward pathways in the development of drug dependence.Pharmacol Then.1987351-2227263
- RobinsonTE.BerridgeKC.The neural basis of drug craving: an incentive-sensitization theory of addiction.Brain Res Rev.19931832472918401595
- GoldsteinRZ.CraigAD.BecharaA.et alThe neurocircuitry of impaired insight in drug addiction.Trends Cogn Sci.200913937238019716751
- MitchellJM.O'NeilJP.JanabiM.MarksSM.JagustWJ.Fields HL Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbensSci Transl Med.20124116116ra116
- VolkowND.FowlerJS.WangGJ.The addicted human brain: insights from imaging studies.J Clin Invest.2003111101444145112750391
- EttenbergA.PettitHO.BloomFE.KoobGF.Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems.Psychopharmacology (Berl).19827832042096296898
- PettitHO.EttenbergA.BloomFE.KoobGF.Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats.Psychopharmacology (Berl).19848421671736438676
- RassnickS.StinusL.KoobGF.The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat.Brain Res.1993623116248221085
- SchultzW.StaufferWR.LakA.The phasic dopamine signal maturing: from reward via behavioural activation to formal economic utility.Curr Opin Neurobiol.20174313914828390863
- RobbinsTW.Relationship between reward-enhancing and stereotypical effects of psychomotor stimulant drugs.Nature.19762645581575912471
- SchultzW.DayanP.MontaguePR.A neural substrate of prediction and reward.Science.19972755306159315999054347
- SchultzW.Dopamine reward prediction error coding.Dialogues Clin Neurosci.2016181233227069377
- RobinsonMJ.FischerAM.AhujaA.LesserEN.ManiatesH.Roles of “wanting” and “liking” in motivating behavior: gambling, food, and drug addictions.Curr Top Behav Neurosci.20162710513626407959
- BerridgeKC.RobinsonTE.Liking, wanting, and the incentive-sensitization theory of addiction.Am Psychol.201671867067927977239
- BoileauI.DagherA.LeytonM.et alModeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men.Arch Gen Psychiatry.200663121386139517146013
- UnglessMA.WhistlerJL.MalenkaRC.BonciA.Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons.Nature.2001411683758358711385572
- HausknechtK.ShenYL.WangRX.Haj-DahmaneS.ShenRY.Prenatal ethanol exposure persistently alters endocannabinoid signaling and endocannabinoid-mediated excitatory synaptic plasticity in ventral tegmental area dopamine neurons.J Neurosci.201737245798580828476947
- BocklischC.PascoliV.WongJC.et alCocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area.Science.201334161531521152524072923
- MaoD.GallagherK.McGeheeDS.Nicotine potentiation of excitatory inputs to ventral tegmental area dopamine neurons.J Neurosci.201131186710672021543600
- ChenBT.BowersMS.MartinM.et alCocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA.Neuron.200859228829718667156
- PanB.HillardCJ.LiuQS.Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons.J Neurosci.20082861385139718256258
- SartiF.BorglandSL.KharaziaVN.BonciA.Acute cocaine exposure alters spine density and long-term potentiation in the ventral tegmental area.Eur J Neurosci.200726374975617686047
- MelisM.CatnariniR.UnglessMA.BonciA.Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure.J Neurosci.20022262074208211896147
- MansvelderHD.McGeheeDS.Long-term potentiation of excitatory inputs to brain reward areas by nicotine.Neuron.20002723493S710985354
- CoxSM.YauY.LarcherK.et alCocaine cue-induced dopamine release in recreational cocaine users.Sci Rep. 2017;7:46665. doi:10.1038/ srep46665.
- FurlongTM.CorbitLH.BrownRA.BalleineBW.Methamphetamine promotes habitual action and alters the density of striatal glutamate receptor and vesicular proteins in dorsal striatum.Addict Biol. 2017 Jul 14. Epub ahead of print. doi:10.1 1 1 1/adb.1 2534.
- CorbitLH.NieH.JanakPH.Habitual responding for alcohol depends upon both AMPA and D2 receptor signaling in the dorsolateral striatum.Front Behav Neurosci.2014830125228865
- PalmS.NylanderI.Dopamine release dynamics change during adolescence and after voluntary alcohol intake.PLoS One.201495e9633724788731
- FanelliRR.KleinJT.ReeseRM.RobinsonDL.Dorsomedial and dorsolateral striatum exhibit distinct phasic neuronal activity during alcohol self-administration in rats.Eur J Neurosci.20133842637264823763702
- CorbitLH.NieH.JanakPH.Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum.Biol Psychiatry.201272538939522440617
- BelinD.EverittBJ.Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum.Neuron.200857343244118255035
- VanderschurenLJ.Di CianoP.EverittBJ.Involvement of the dorsal striatum in cue-controlled cocaine seeking.J Neurosci.200525388665867016177034
- ItoR.DalleyJW.RobbinsTW.EverittBJ.Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue.J Neurosci.200222146247625312122083
- WilluhnI.BurgenoLM.GroblewskiPA.PhillipsPE.Excessive cocaine use results from decreased phasic dopamine signaling in the striatum.Nat Neurosci.201417570470924705184
- WilluhnI.BurgenoLM.EverittBJ.PhillipsPE.Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use.Proc Natl Acad Sci U S A.201210950207032070823184975
- CaineSB.ThomsenM.GabrielKl.et alLack of self-administration of cocaine in dopamine D1 receptor knock-out mice.J Neurosci.20072748131401315018045908
- CaineSB.NegusSS.MelloNK.et alRole of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists.J Neurosci.20022272977298811923462
- RobertsAJ.McDonaldJS.HeyserCJ.et alμ-Opioid receptor knockout mice do not self-administer alcohol.J Pharmacol Exp Ther.200029331002100810869404
- KallupiM.ScuppaG.de GuglielmoG.et alGenetic deletion of the nociceptin/orphanin FQ receptor in the rat confers resilience to the development of drug addiction.Neuropsychopharmacology.201742369570627562376
- CunninghamKA.AnastasioNC.Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction.Neuropharmacology.201476pt B46047823850573
- VashchinkinaE.PanhelainenA.Aitta-ahoT.KorpiER.GABAa receptor drugs and neuronal plasticity in reward and aversion: focus on the ventral tegmental area.Front Pharmacol.2014525625625505414
- BuczynskiMW.HermanMA.HsuKL.et alDiacylglycerol lipase disinhibits VTA dopamine neurons during chronic nicotine exposure.Proc Natl Acad Sci U S A.201611341086109126755579
- MelisM.SaghedduC.De FeliceM.et alEnhanced endocannabinoid-mediated modulation of rostromedial tegmental nucleus drive onto dopamine neurons in Sardinian alcohol-preferring rats.J Neurosci.20143438127161272425232109
- Rashidy-PourA.PahlevaniP.VaziriA.et alInvolvement of CB1 receptors in the ventral tegmental area in the potentiation of morphine rewarding properties in acquisition but not expression in the conditioned place preference model.Behav Brain Res.201324725926723511249
- MalinenH.HyytiaP.Ethanol self-administration is regulated by CB1 receptors in the nucleus accumbens and ventral tegmental area in alcohol-preferring AA rats.Alcohol Clin Exp Res.200832111976198318782338
- HyytiaP.KoobGF.GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats.Eur J Pharmacol.19952831-31511597498304
- KoobGF.Le Moal M Plasticity of reward neurocircuitry and the “dark side” of drug addiction.Nat Neurosci.20058111442144416251985
- WeissF.MarkouA.LorangMT.KoobGF.Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration.Brain Res.199259323143181450939
- MartinezD.NarendranR.FoltinRW.et alAmphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine.Am J Psychiatry.2007164462262917403976
- VolkowND.WangGJ.TelangF.et alProfound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement.J Neurosci.20072746127001270618003850
- VolkowND.TomasiD.WangGJ.et alStimulant-induced dopamine increases are markedly blunted in active cocaine abusers.Mol Psychiatry.20141991037104324912491
- VolkowND.WangGJ.FowlerJS.et alDecreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects.Nature.199738666278308339126741
- StinusL.KoobGF.Le MoalM.Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal.Neuroscience.19903737677732247222
- MinkowskiCP.EpsteinD.FrostJJ.GorelickDA.Differential response to IV carfentanil in chronic cocaine users and healthy controls.Addict Biol.201217114915521054687
- DavidsonM.ShanleyB.WilceP.Increased NMDA-induced excitability during ethanol withdrawal: a behavioural and histological study.Brain Res.1995674191967773699
- DahchourA.de WitteP.BoloN.et alCentral effects of acamprosate: Part 1. Acamprosate blocks the glutamate increase in the nucleus accumbens microdialysate in ethanol withdrawn rats.Psychiatry Res.19988221071149754453
- DaniJA.HeinemannS.Molecular and cellular aspects of incotine abuse.Neuron.19961659059088630247
- ToluS.EddineR.MartiF.et alCo-activation of VTA DA and GABA neurons mediates nicotine reinforcement.Mol Psychiatry.201318338239322751493
- WalkerBM.KoobGF.Pharmacological evidence for a motivational role of k-opioid systems in ethanol dependence.Neuropsychopharmacology.200833364365217473837
- SchlosburgJE.Whitfield TWJr.ParkPE.et alLong-term antagonism of opioid receptors prevents escalation of and increased motivation for heroin intake.J Neurosci.20133349193841939224305833
- Whitfield TWJr.SchlosburgJ.WeeS.et alOpioid receptors in the nucleus accumbens shell mediate escalation of methamphetamine intake.J Neurosci.201535104296430525762676
- KoobGF.BuckCL.CohenA.et alAddiction as a stress surfeit disorder.Neuropharmacology.201476pt B37038223747571
- RivierC.BruhnT.ValeW.Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF).J Pharmacol Exp Then.19842291127131
- Merlo-PichE.LorangM.YeganehM.et alIncrease of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis.J Neurosci.1995158543954477643193
- KoobGF.HeinrichsSC.MenzaghiF.PichEM.BrittonKT.Corticotropin releasing factor, stress and behavior.Semin Neurosci.199464221229
- RasmussenDD.BoldtBM.BryantCA.MittonDR.LarsenSA.WilkinsonCW.Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypotha lamo-pituitary-adrena I axis.Alcohol Clin Exp Res.200024121836184911141043
- OliveMF.KoenigHN.NanniniMA.HodgeCW.Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake.Pharmacol Biochem Behav.199360512532
- DelfsJM.ZhuY.DruhanJP.Aston-JonesG.Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion.Nature.2000403676843043410667795
- KoobGF.Brain stress systems in the amygdala and addiction.Brain Res.20091293617519332030
- HeimerL.AlheidG.Piecing together the puzzle of basal forebrain anatomy. In: Napier TC, Kalivas PW, Hanin I, eds.The Basal Forebrain: Anatomy to Function. New York, NY: Plenum Press. Advances in Experimental Medicine and Biology; vol 295.1991142
- RichterRM.WeissF.In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats.Synapse.199932425426110332801
- GeorgeO.GhozlandS.AzarMR.et alCRF-CRF, system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats.Proc Natl Acad Sci USA.200710443171981720317921249
- SarnyaiZ.BiroE.GardiJ.VecsernyesM.JuleszJ.TelegdyG.Brain corticotropin-releasing factor mediates “anxiety-like” behavior induced by cocaine withdrawal in rats.Brain Res.19956751-289977796157
- BassoAM.SpinaM.RivierJ.ValeW.KoobGF.Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats.Psychopharmacology (Berl).19991451213010445369
- CohenA.TreweekJ.EdwardsS.et alExtended access to nicotine leads to a CRF, receptor dependent increase in anxiety-like behavior and hyperalgesia in rats.Addict Biol.2015201566823869743
- GriederTE.HermanMA.ContetC.et alVTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation.Nat Neurosci.201417121751175825402857
- RassnickS.HeinrichsSC.BrittonKT.KoobGF.Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal.Brain Res.1993605125328467387
- HeinrichsSC.MenzaghiF.SchulteisG.KoobGF.StinusL.Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal.Behav Pharmacol.199561748011224314
- SchulteisG.MarkouA.GoldLH.StinusL.KoobGF.Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis.J Pharmacol Exp Ther.19942713139113987996451
- GoedersNE.GuerinGF.Effects of the CRH receptor antagonist CP154,526 on intravenous cocaine self-administration in rats.Neuropsychopharmacology.200023557758611027923
- GreenwellTN.FunkCK.CottoneP.et alCorticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long-, but not short-access rats.Addict Biol.200914213014319291009
- KwakoLE.SpagnoloPA.SchwandtML.et alThe corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study.Neuropsychopharmacology.20154051053106325409596
- SchwandtML.CortesCR.KwakoLE.et alThe CRF1 antagonist verucerfont in anxious alcohol-dependent women: translation of neuroendocrine, but not of anti-craving effects.Neuropsychopharmacology.201641122818282927109623
- SpierlingSR.ZorrillaEP.Don't stress about CRF: assessing the translational failures of CRF, antagonists.Psychopharmacology (Berl).20172349101467148128265716
- Carlezon WAJr.NestlerEJ.NeveRL.Herpes simplex virus-mediated gene transfer as a tool for neuropsychiatric research.Crit Rev Neurobiol.2000141476711253955
- KoobGF.The dark side of emotion: the addiction perspective.Eur J Pharmacol.2015753738725583178
- ChavkinC.KoobGF.Dynorphin, dysphoria and dependence: the stress of addiction.Neuropsychopharmacology.2016411373374
- HikosakaO.The habenula: from stress evasion to value-based decision-making.Nat Rev Neurosci.201011750351320559337
- MatsumotoM.HikosakaO.Lateral habenula as a source of negative reward signals in dopamine neurons.Nature.200744771481111111517522629
- FowlerCD.LuQ.JohnsonPM.MarksMJ.KennyPJ.Habenular 5 nicotinic receptor subunit signalling controls nicotine intake.Nature.2011471734059760121278726
- SalasR.SturmR.BoulterJ.De BiasiM.Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice.J Neurosci.200929103014301819279237
- Zhao-SheaR.DeGrootSR.LiuL.et alIncreased CRF signalling in a ventral tegmental area-interpeduncular nucleus-medial habenula circuit induces anxiety during nicotine withdrawal.Nat Commun.201566770
- BonsonKR.GrantSJ.ContoreggiCS.et alNeural systems and cue-induced cocaine craving.Neuropsychopharmacology.200226337638611850152
- PelchatML.JohnsonA.ChanR.ValdezJ.RaglandJD.Images of desire: food-craving activation during fMRI.Neuroimage.20042341486149315589112
- WangZ.FaithM.PattersonF.et alNeural substrates of abstinence-induced cigarette cravings in chronic smokers.J Neurosci.20072751140351404018094242
- NaqviNH.RudraufD.DamasioH.BecharaA.Damage to the insula disrupts addiction to cigarette smoking.Science.2007315581153153417255515
- NaqviNH.BecharaA.The hidden island of addiction: the insula.Trends Neurosci.2009321566718986715
- SanchezMM.YoungLJ.PlotskyPM.InselTR.Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain.J Comp Neurol.1999408336537710340512
- GoudriaanAE.De RuiterMB.van den BrinkW.OosterlaanJ.VeltmanDJ.Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: an fMRI study.Addict Biol.201015449150320840335
- JanesAC.PizzagalliDA.RichardtS.et alBrain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence.Biol Psychiatry.201067872272920172508
- JentschJD.TaylorJR.Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli.Psychopharmacology.1999146437339010550488
- Oscar-BermanM.HutnerN.Frontal lobe changes after chronic alcohol ingestion. In: Hunt WA, Nixon SJ, eds. Alcohol-Induced Brain Damage. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 1993:121-156. NIAAA Research Monograph; vol 22.
- VolkowND.WangGJ.FowlerJS.TomasiD.TelangF.Addiction: beyond dopamine reward circuitry.Proc Natl Acad Sci USA.201110837150371504221402948
- BaldacchinoA.BalfourDJ.PassettiF.HumphrisG.MatthewsK.Neuropsychological consequences of chronic opioid use: a quantitative review and meta-analysis.Neurosci Biobehav Rev.20123692056206822771335
- LondonED.KohnoM.MoralesAM.BallardME.Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging.Brain Res.20151628pt A17418525451127
- RobbinsTW.ErscheKD.EverittBJ.Drug addiction and the memory systems of the brain.Ann N Y Acad Sci.2008114112118991949
- GeorgeO.KoobGF.Individual differences in prefrontal cortex function and the transition from drug use to drug dependence.Neurosci Biobehav Rev.201035223224720493211
- GeorgeO.MandyamCD.WeeS.KoobGF.Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working-memory impairments.Neuropsychopharmacology.200833102474248218033234
- GeorgeO.SandersC.FreilingJ.et alRecruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking.Proc Natl Acad Sci USA.201210944181561816123071333
- RecintoP.SamantAR.ChavezG.et alLevels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration.Neuropsychopharmacology.20123751275128722205547
- BriandLA.FlagelSB.SeemanP.RobinsonTE.Cocaine self-administration produces a persistent increase in dopamine D22 receptors.Eur Neuropsychopharmacol.2008 188 55155618284941
- KoberH.LacadieCM.WexlerBE.MalisonRT.SinhaR.PotenzaMN.Brain activity during cocaine craving and gambling urges: an fMRI study.Neuropsychopharmacology.201641262863726119472
- LeeJH.LimY.WiederholdBK.GrahamSJ.A functional magnetic resonance imaging (fMRI) study of cue-induced smoking craving in virtual environments.Appl Psychophysiol Biofeedback.20053019520416167185
- RisingerRC.SalmeronBJ.RossTJ.et alNeural correlates of high and craving during cocaine self-administration using BOLD fMRI.Neuroimage.20052631097110815886020
- VolkowND.WangGJ.MaY.et alActivation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction.J Neurosci.200525153932393915829645
- JasinskaAJ.SteinEA.KaiserJ.NaumerMJ.YalachkovY.Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies.Neurosci Biobehav Rev.20143811624211373
- FotrosA.CaseyKF.LarcherK.et alCocaine cue-induced dopamine release in the amygdala and hippocampus: a high-resolution PET [18F]fallypride study in cocaine dependent participants.Neuropsychopharmacology.20133891780178823546387
- VolkowND.WangGJ.TelangF.et alCocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction.J Neurosci.200626246583658816775146
- MilellaMS.FotrosA.GravelP.et alCocaine cue-induced dopamine release in the human prefrontal cortex.J Psychiatry Neurosci.201641532233026900792
- KoobGF.VolkowND.Neurocircuitry of addiction.Neuropsychopharmacology.20103521723819710631
- VolkowND.WangGJ.FowlerJS.et alAssociation of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction.Am J Psychiatry.1999156119269892293
- GoldsteinRZ.VolkowND.Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications.Nat Rev Neurosci.2011121165266922011681
- BryceCA.FlorescoSB.Perturbations in effort-related decision-making driven by acute stress and corticotropin-releasing factor.Neuropsychopharmacology.20164182147215926830960
- VolkowND.FowlerJS.WangGJ.BalerR.TelangF.Imaging dopamine's role in drug abuse and addiction.Neuropharmacology.200956suppl 13818617195
- ThanosPK.MichaelidesM.UmegakiH.VolkowND.D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats.Synapse.200862748148618418874
- GromanSM.LeeB.SeuE.et alDysregulation of D2-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure.J Neurosci.201232175843585222539846
- LoboMK.NestlerEJ.The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons.Front Neuroanat.201154121811439
- VolkowND.MoralesM.The brain on drugs: from reward to addiction.Cell.2015162471272526276628
- BecharaA.DamasioH.DamasioAR.LeeGP.Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making.J Neurosci.199919135473548110377356
- JohnstoneT.van ReekumCM.UrryHL.KalinNH.DavidsonRJ.Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression.J Neurosci.200727338877888417699669
- ClarkeTK.CristRC.KampmanKM.et alLow frequency genetic variants in the μ-opioid receptor (OPRM1) affect risk for addiction to heroin and cocaine.Neurosci Lett.2013542717523454283
- WachmanEM.HayesMJ.BrownMS.et alAssociation of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome.JAMA.2013309171821182723632726
- ThorgeirssonTE.GudbjartssonDF.SurakkaI.ENGAGEConsortium.et alSequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior.Nat Genet.201042544845320418888
- LevranO.O'HaraK.PelesE.et al ABCB1 (MDR1) genetic variants are associated with methadone doses required for effective treatment of heroin dependence.Hum Mol Genet.200817142219222718424454
- ThorgeirssonTE.GellerF.SulemP.et alA variant associated with nicotine dependence, lung cancer and peripheral arterial disease.Nature.2008452718763864218385739
- ChenAC.ManzN.TangY.et alSingle-nucleotide polymorphisms in corticotropin releasing hormone receptor 1 gene (CRHR1) are associated with quantitative trait of event-related potential and alcohol dependence.Alcohol Clin Exp Res.201034698899620374216
- SchrnidB.BlomeyerD.TreutleinJ.et alInteracting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds.Int J Neuropsychopharmacol.201013670371419607758
- TreutleinJ.KisslingC.FrankJ.et al Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples.Mol Psychiatry.200611659460216550213
- WetherillL.SchuckitMA.HesselbrockV.et alNeuropeptide Y receptor genes are associated with alcohol dependence, alcohol withdrawal phenotypes, and cocaine dependence.Alcohol Clin Exp Res.200832122031204018828811
- BhaskarLV.ThangarajK.KumarKP.PardhasaradhiG.SinghL.RaoVR.Association between neuropeptide Y gene polymorphisms and alcohol dependence: a case-control study in two independent populations.Eur Addict Res.201319630731323652361
- MutschlerJ.AbbruzzeseE.von der GoltzC.et alGenetic variation in the neuropeptide Y gene promoter is associated with increased risk of tobacco smoking.Eur Addict Res.201218524625222584873
