Abstract
Background
Endothelium-derived microparticles (EMPs) are submicron vesicles released from the plasma membrane of endothelial cells in response to injury, apoptosis or activation. We have previously demonstrated EMP-induced acute lung injury (ALI) in animal models and endothelial barrier dysfunction in vitro. Current treatment options for ALI are limited and consist of supportive therapies. We hypothesize that standard clinical continuous venovenous hemofiltration (CVVH) reduces serum EMP levels and may be adapted as a potential therapeutic intervention.
Materials and methods
EMPs were generated from plasminogen activation inhibitor-1 (PAI-1)-stimulated human umbilical vein endothelial cells (HUVECs). Flow cytometric analysis was used to characterize EMPs as CD31- and annexin V-positive events in a submicron size gate. Enumeration was completed against a known concentration of latex beads. Ultimately, a concentration of ~650,000 EMP/mL perfusate fluid (total 470 mL) was circulated through a standard CVVH filter (pore size 200 μm, flow rate 250 mL/hr) for a period of 70 minutes. 0.5 mL aliquots were removed at 5- to 10-minute intervals for flow cytometric analysis. EMP concentration in the dialysate was measured at the end of 4 hours to better understand the fate of EMPs.
Results
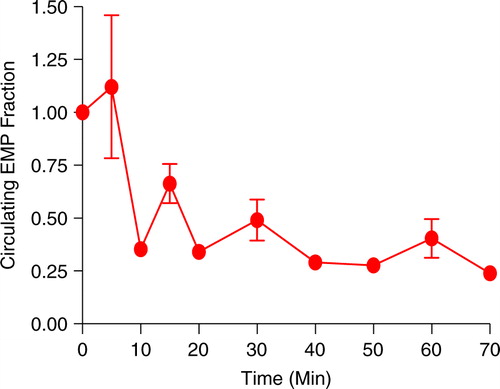
A progressive decrease in circulating EMP concentration was noted using standard CVVH at 250 mL/hr (a clinical standard rate) from a 470 mL volume modelling a patient's circulation. A 50% reduction was noted within the first 30 minutes. EMPs entering the dialysate after 4 hours were 5.7% of the EMP original concentration.
Conclusion
These data demonstrate that standard CVVH can remove EMPs from circulation in a circuit modelling a patient. An animal model of hemofiltration with induction of EMP release is required to test the therapeutic potential of this finding and potential of application in early treatment of ALI.
Acute lung injury (ALI) occurs in the setting of critical illness as a response to a myriad of physiologic insults, including sepsis, aspiration, transfusion reactions and others (Citation1). The mortality rate of the most serious form of ALI remains greater than 30% despite the best supportive care. Over 10% of the nearly one-million patients ventilated annually will develop ALI, which is a substantial and costly societal burden (Citation2–Citation4). Histologically, ALI is characterized by diffuse alveolar damage with neutrophil degranulation and dysregulation, cytokine release and endothelial dysfunction in the early stages of disease development (Citation2, Citation3). Neither biomarkers of those patients at highest risk nor targeted therapies have been identified, in part because the pathogenesis of ALI is incompletely elucidated and often occurs late in a disease process.
We have previously demonstrated the role of endothelium-derived microparticles (EMPs) as an inducer of ALI in rodent models (Citation5, Citation6). As submicron vesicles composed of a lipid bilayer and endothelial cell (EC) surface proteins (e.g. CD31, E-selectin, VE-cadherin), EMPs are released in high levels from the plasma membrane of ECs when stressed, apoptotic or injured. Elevated EMP levels have been identified in human disease states with pulmonary sequelae and ALI (Citation7–Citation58). The presence of endoplasmic reticulum proteins (e.g. BIP-2) and externalized annexin V suggest that EMP release is a deliberate compensation by stressed ECs (Citation59, Citation60). Endothelial dysfunction resulting from EMP exposure has been demonstrated both in vitro and in vivo (Citation6, Citation15) (Citation61). We have previously shown that EMPs increase pulmonary endothelial permeability and decrease eNOS phosphorylation, diminishing NO production (Citation5, Citation6).
Multiple studies of ALI in animal models and humans suggest a benefit of hemofiltration on lung function in the setting of ALI (Citation62–Citation68). This effect has been largely attributed to a decrease in venous pressure, an increase in circulatory hydrostatic pressure and clearance of inflammatory cytokines. We hypothesized that the CVVH benefit seen in ALI recovery may be attributable to the removal of EMPs from circulation. As a first step, this report tests our hypothesis by examining the efficacy of standard clinical CVVH dialysis in removing EMPs from a modelled patient circulation.
Methods
EMP generation, identification and enumeration
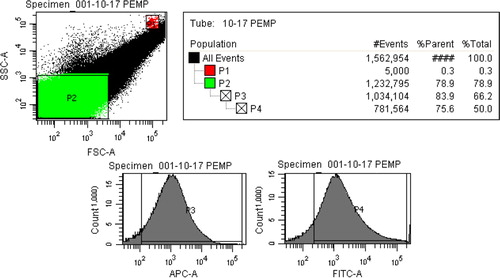
EMPs were generated using previously published protocols (Citation6). Human umbilical vein endothelial cells (HUVEC passage 4–6; Clonetics, Walkersville, MD) were grown to confluence, then stimulated with plasminogen activation inhibitor-1 (PAI-1, 10 ng/mL; American Diagnostica Inc., Stamford, CT). Supernatant was collected 3 hours after stimulation and centrifuged (145 g, 8 minutes) to remove cell debris. The supernatant was subsequently ultracentrifuged (100,000 g, 6 minutes, 4 degrees) and pelleted. EMPs were re-suspended in phosphate buffered saline (PBS) and stored at −80°C. Flow cytometric analysis was used to characterize and quantify EMPs based on CD31- and annexin V-positivity occurring in a size gate less than 1 µm. This size gate was set on forward versus side scatter using latex standard beads measuring 0.84 µm (Spherotech FP-0856-2). Enumeration was completed against a known concentration of 7.6 µm polystyrene beads placed within the sample (Spherotech PPS-4). The flow rate used for analysis was 12 µL/min ( and ).
Fig. 1. An EMP size gate was first defined using 0.84 µm latex beads. FSC vs. SSC was used to create this gate denoted as P2. 7.6 µm latex beads are shown in gate P1 and allowed for enumeration of EMPs.

Fig. 2. Within gate P2, events positive for annexin V (P3) and CD31 (P4) were enumerated against P1 to quantify EMP levels. The same settings were used to quantify EMPs in perfusate samples.
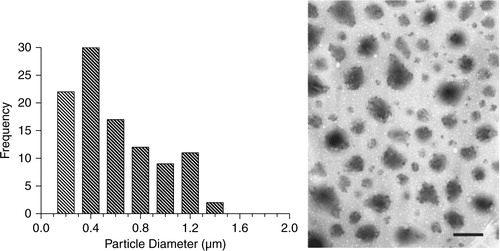
As a separate and confirmatory measure, transmission electron microscopy (TEM) was used to describe particle size distribution. A 25-µL drop of EMP in solution was pipetted onto a Formvar plastic coated grid (Ted Pella, Inc.) and allowed to air dry for 1 hour. The microparticle grid was examined and photographed by TEM with a JEOL 100 CX microscope ().
CVVH dialysis
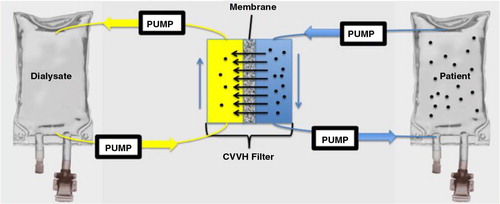
EMPs (300×106) were introduced to an IV bag (model patient) containing 300 mL of 0.9 NS (model patient) and manually agitated for 3 minutes. The IV bag was connected to a primed CVVH circuit containing 170 mL of 0.9 NS and mixed (~650×103 EMP/mL final mixed perfusate concentration) as shown in . After mixing, the perfusate was circulated through the CVVH filter (NxStage® PUREMA®, 200 µm pore size, 35 µm wall thickness, rate 250 mL/hour) for a period of 70 minutes. 0.5 mL aliquots were removed for flow cytometric analysis at 5–10 minute intervals for 70 minutes of dialysis. EMP levels were recorded and expressed as a fraction of starting concentration after mixing occurred. Three runs were completed and the fraction of EMPs versus time was plotted with standard error of the mean (SEM). At the end of 4 hours filtration, dialysate EMP concentration was measured using flow cytometry methods as previously described.
Fig. 4. Schema depicting CVVH circuit and model patient. A 300 mL IV bag of 0.9 NS served as the model patient and was spiked with EMPs. The circuit contained 170 mL 0.9 NS and ran counter-current against 1 L of 0.9 NS dialysate. After mixing, 0.5 mL aliquots were drawn from the “patient” for analysis as dialysis was completed via the filter.
Results
EMP clearance from our modelled patient can be seen in . The decline in EMP levels due to dialysis is rapid with 50% of clearance being seen in the first 30 minutes. Within 40 minutes, the circulating EMP levels were reduced to 29% of starting concentration and this level stayed relatively constant over the duration of the study. To determine the fate of the cleared EMPs, we measured EMP levels in the dialysate. The dialysate contained 5.7% of the original EMP concentration (~650×103 EMP/mL) at the end of 4 hours of uninterrupted dialysis. This suggests that the filter or tubing is entrapping EMPs.
Discussion
The data presented here demonstrate that counter-current dialysis using a standard clinical CVVH filter and under clinical conditions removes EMPs from a modelled patient's circulation. Given our previous evidence of EMP-induced ALI, these findings support the idea of CVVH as a meaningful therapeutic intervention in ALI due to clearance of EMPs from the patient circulation. The experimental conditions achieve the same plasma level of EMPs as seen in human clinical disease (1×106 per mL).
While this work demonstrates that a standard 200-µm CVVH circuit and filter entraps EMPs, there are significant limitations to the model presented. The observed EMP clearance is likely a saturable phenomenon, which would explain the relatively constant EMP concentration from 40 to 70 minutes and low dialysate EMP levels. The relative volume of human circulation (~5 L) to CVVH circuit (~200 mL) is much higher in the clinical condition as compared to our model (300 and 170 mL, respectively). While this would not affect a steady state of dialysis, a saturable filter EMP-binding would become relevant sooner in the clinical condition. Additionally, EMPs may continue to be generated in a patient and a non-saturable clearance method would be required to achieve a lower steady state EMP concentration. Further, this model examined only the diffusion force across a CVVH filter. Convection and pressure gradient forces were not modelled, as these interventions require replacement fluid and this would confound EMP measurement. However, if EMP charge and size are amenable, forced hindered diffusion via the application of convective and pressure forces coupled with replacement by EMP-free fluid would likely sustain clearance. In conjunction with particle trapping by the filter, the first order kinetics of EMP clearance could be prolonged at a minimum. Additionally, EMP interactions in vivo with plasma oncotic elements (circulating cells, albumin, etc.) are not modelled in this system. We do not feel that such elements have a major impact upon the steady state of EMPs in vivo, since characterized EMPs in human disease states using flow cytometry have identified the 0.5×106 per mL threshold injury value using flow cytometry of submicron events occurring in platelet-free plasma (Citation54). Thus, the absence of these elements in our model is representative of the steady state of unbound plasma EMPs present in human disease.
The low dialysate EMP level and plateauing circulation EMP level beyond 40 minutes suggest that EMP sequestration occurs within the CVVH filter or tubing and that this is a saturable phenomenon. Frequent changing of the CVVH filter is a logical step that may overcome this limitation. Additionally, more efficient and selective EMPs clearance can be realized by conjugating endothelium-specific antibodies to commercially available specialty CVVH filters (Aethlon ADAPT™). This approach would provide more selective binding sites and higher affinity for circulating EMPs. By combining a selective filter with convection and pressure gradients, EMP clearance could likely be substantially prolonged. Clinical data generally support the protective role of CVVH in ALI management. A clinical randomized trial and several animal trials demonstrate that the achievement of negative fluid balance is associated with improved outcomes from ALI (Citation62–Citation65, Citation67) (Citation68). In addition to its salutary effects on EMP levels, early CVVH may prevent or minimize volume overload that is commonly observed with the resuscitation phase of critical illness, particularly in persons suffering concomitant acute kidney injury (AKI). Thus, CVVH could be used as an effective tool targeting both the molecular machinery and iatrogenic components of ALI.
As a translatable next step, we plan to assay patients pre- and post-dialysis to observe EMP concentration effects. Ultimately, the application of dialysis to rodent models of EMP-induced ALI would examine efficacy in on-going diseases and EMP thresholds associated with worse injuries. While the utility of ultrafiltration can be tested in an animal model, diffusion appears to be enough to remove EMPs due to their small size: standard dialysis alone was effective in this in vitro model.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L et al. The American–European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994; 149: 818–24.
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005; 353: 1685–93.
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967; 2: 319–23.
- Goss CH, Brower RG, Hudson LD, Rubenfeld GD. Incidence of acute lung injury in the United States. Crit Care Med. 2003; 31: 1607–11.
- Buesing KL, Densmore JC, Kaul S, Pritchard KA Jr., Jarzembowski JA, Gourlay DM et al. Endothelial microparticles induce inflammation in acute lung injury. J Surg Res. 2011; 166: 32–9.
- Densmore JC, Signorino PR, Ou J, Hatoum OA, Rowe JJ, Shi Y et al. Endothelium-derived microparticles induce endothelial dysfunction and acute lung injury. Shock. 2006; 26: 464–71.
- Agouni A, Lagrue-Lak-Hal AH, Ducluzeau PH, Mostefai HA, Draunet-Busson C, Leftheriotis G et al. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am J Pathol. 2008; 173: 1210–9.
- Blann A, Shantsila E, Shantsila A. Microparticles and arterial disease. Semin Thromb Hemost. 2009; 35: 488–96.
- Forest A, Pautas E, Ray P, Bonnet D, Verny M, Amabile N et al. Circulating microparticles and procoagulant activity in elderly patients. J Gerontol A Biol Sci Med Sci. 2010; 65: 414–20.
- Guiducci S, Distler JH, Jungel A, Huscher D, Huber LC, Michel BA et al. The relationship between plasma microparticles and disease manifestations in patients with systemic sclerosis. Arthritis Rheum. 2008; 58: 2845–53.
- Kang P, Shen B, Yang J, Pei F. Circulating platelet-derived microparticles and endothelium-derived microparticles may be a potential cause of microthrombosis in patients with osteonecrosis of the femoral head. Thromb Res. 2008; 123: 367–73.
- Morel O, Ohlmann P, Epailly E, Bakouboula B, Zobairi F, Jesel L et al. Endothelial cell activation contributes to the release of procoagulant microparticles during acute cardiac allograft rejection. J Heart Lung Transplant. 2008; 27: 38–45.
- Ogura H, Tanaka H, Koh T, Fujita K, Fujimi S, Nakamori Y et al. Enhanced production of endothelial microparticles with increased binding to leukocytes in patients with severe systemic inflammatory response syndrome. J Trauma. 2004; 56: 823–30. discussion 30–1.
- van Beers EJ, Schaap MC, Berckmans RJ, Nieuwland R, Sturk A, van Doormaal FF et al. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009; 94: 1513–9.
- Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005; 16: 3381–8.
- Amabile N, Heiss C, Chang V, Angeli FS, Damon L, Rame EJ et al. Increased CD62e(+) endothelial microparticle levels predict poor outcome in pulmonary hypertension patients. J Heart Lung Transplant. 2009; 28: 1081–6.
- Amabile N, Heiss C, Real WM, Minasi P, McGlothlin D, Rame EJ et al. Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am J Respir Crit Care Med. 2008; 177: 1268–75.
- Arteaga RB, Chirinos JA, Soriano AO, Jy W, Horstman L, Jimenez JJ et al. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol. 2006; 98: 70–4.
- Bakouboula B, Morel O, Faure A, Zobairi F, Jesel L, Trinh A et al. Procoagulant membrane microparticles correlate with the severity of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008; 177: 536–43.
- Bandi VD, Munnur U, Matthay MA. Acute lung injury and acute respiratory distress syndrome in pregnancy. Crit Care Clin. 2004; 20: 577–607.
- Bastarache JA, Fremont RD, Kropski JA, Bossert FR, Ware LB. Procoagulant alveolar microparticles in the lungs of patients with acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2009; 297: L1035–41.
- Bernard S, Loffroy R, Serusclat A, Boussel L, Bonnefoy E, Thevenon C et al. Increased levels of endothelial microparticles CD144 (VE-Cadherin) positives in type 2 diabetic patients with coronary noncalcified plaques evaluated by multidetector computed tomography (MDCT). Atherosclerosis. 2009; 203: 429–35.
- Boulanger CM, Amabile N, Guerin AP, Pannier B, Leroyer AS, Mallat CN et al. In vivo shear stress determines circulating levels of endothelial microparticles in end-stage renal disease. Hypertension. 2007; 49: 902–8.
- Boulanger CM, Scoazec A, Ebrahimian T, Henry P, Mathieu E, Tedgui A et al. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation. 2001; 104: 2649–52.
- Bretelle F, Sabatier F, Desprez D, Camoin L, Grunebaum L, Combes V et al. Circulating microparticles: a marker of procoagulant state in normal pregnancy and pregnancy complicated by preeclampsia or intrauterine growth restriction. Thromb Haemost. 2003; 89: 486–92. [PubMed Abstract].
- Brodsky SV, Facciuto ME, Heydt D, Chen J, Islam HK, Kajstura M et al. Dynamics of circulating microparticles in liver transplant patients. J Gastrointestin Liver Dis. 2008; 17: 261–8. [PubMed Abstract].
- Brogan PA, Shah V, Brachet C, Harnden A, Mant D, Klein N et al. Endothelial and platelet microparticles in vasculitis of the young. Arthritis Rheum. 2004; 50: 927–36.
- Bulut D, Maier K, Bulut-Streich N, Borgel J, Hanefeld C, Mugge A. Circulating endothelial microparticles correlate inversely with endothelial function in patients with ischemic left ventricular dysfunction. J Card Fail. 2008; 14: 336–40.
- Carp H, Dardik R, Lubetsky A, Salomon O, Eskaraev R, Rosenthal E et al. Prevalence of circulating procoagulant microparticles in women with recurrent miscarriage: a case-controlled study. Hum Reprod. 2004; 19: 191–5.
- Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A. Endothelial microparticles in diseases. Cell Tissue Res. 2009; 335: 143–51.
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009; 19: 43–51.
- Combes V, Coltel N, Faille D, Wassmer SC, Grau GE. Cerebral malaria: role of microparticles and platelets in alterations of the blood–brain barrier. Int J Parasitol. 2006; 36: 541–6.
- Combes V, Simon AC, Grau GE, Arnoux D, Camoin L, Sabatier F et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999; 104: 93–102.
- Combes V, Taylor TE, Juhan-Vague I, Mege JL, Mwenechanya J, Tembo M et al. Circulating endothelial microparticles in malawian children with severe falciparum malaria complicated with coma. JAMA. 2004; 291: 2542–4. [PubMed Abstract].
- Dignat-George F, Camoin-Jau L, Sabatier F, Arnoux D, Anfosso F, Bardin N et al. Endothelial microparticles: a potential contribution to the thrombotic complications of the antiphospholipid syndrome. Thromb Haemost. 2004; 91: 667–73. [PubMed Abstract].
- Dursun I, Poyrazoglu HM, Gunduz Z, Ulger H, Yykylmaz A, Dusunsel R et al. The relationship between circulating endothelial microparticles and arterial stiffness and atherosclerosis in children with chronic kidney disease. Nephrol Dial Transplant. 2009; 24: 2511–8.
- Erdbruegger U, Grossheim M, Hertel B, Wyss K, Kirsch T, Woywodt A et al. Diagnostic role of endothelial microparticles in vasculitis. Rheumatology (Oxford). 2008; 47: 1820–5.
- Esposito K, Ciotola M, Giugliano F, Sardelli L, Maiorino MI, Beneduce F et al. Phenotypic assessment of endothelial microparticles in diabetic and nondiabetic men with erectile dysfunction. J Sex Med. 2008; 5: 1436–42.
- Esposito K, Ciotola M, Giugliano F, Schisano B, Improta L, Improta MR et al. Endothelial microparticles correlate with erectile dysfunction in diabetic men. Int J Impot Res. 2007; 19: 161–6.
- Esposito K, Ciotola M, Schisano B, Gualdiero R, Sardelli L, Misso L et al. Endothelial microparticles correlate with endothelial dysfunction in obese women. J Clin Endocrinol Metab. 2006; 91: 3676–9.
- Faure V, Dou L, Sabatier F, Cerini C, Sampol J, Berland Y et al. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost. 2006; 4: 566–73.
- Gelderman MP, Schiffmann R, Simak J. Elevated endothelial microparticles in Fabry children decreased after enzyme replacement therapy. Arterioscler Thromb Vasc Biol. 2007; 27: e138–9.
- Gonzalez-Quintero VH, Jimenez JJ, Jy W, Mauro LM, Hortman L, O'Sullivan MJ et al. Elevated plasma endothelial microparticles in preeclampsia. Am J Obstet Gynecol. 2003; 189: 589–93.
- Gonzalez-Quintero VH, Smarkusky LP, Jimenez JJ, Mauro LM, Jy W, Hortsman LL et al. Elevated plasma endothelial microparticles: preeclampsia versus gestational hypertension. Am J Obstet Gynecol. 2004; 191: 1418–24.
- Jimenez JJ, Jy W, Mauro LM, Horstman LL, Ahn YS. Elevated endothelial microparticles in thrombotic thrombocytopenic purpura: findings from brain and renal microvascular cell culture and patients with active disease. Br J Haematol. 2001; 112: 81–90.
- Jimenez JJ, Jy W, Mauro LM, Horstman LL, Bidot CJ, Ahn YS. Endothelial microparticles (EMP) as vascular disease markers. Adv Clin Chem. 2005; 39: 131–57. [PubMed Abstract].
- Kumpers P, Erdbrugger U, Grossheim M, Meyer GP, Hiss M, Gwinner W et al. Endothelial microparticles as a diagnostic aid in Churg-Strauss vasculitis-induced cardiomyopathy. Clin Exp Rheumatol. 2008; 26(3 Suppl 49): S86–9. [PubMed Abstract].
- Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000; 101: 841–3.
- Martinez MC, Tesse A, Zobairi F, Andriantsitohaina R. Shed membrane microparticles from circulating and vascular cells in regulating vascular function. Am J Physiol Heart Circ Physiol. 2005; 288: H1004–9.
- Nomura S, Inami N, Shouzu A, Urase F, Maeda Y. Correlation and association between plasma platelet-, monocyte- and endothelial cell-derived microparticles in hypertensive patients with type 2 diabetes mellitus. Platelets. 2009; 20: 406–14.
- Pihusch V, Rank A, Steber R, Pihusch M, Pihusch R, Toth B et al. Endothelial cell-derived microparticles in allogeneic hematopoietic stem cell recipients. Transplantation. 2006; 81: 1405–9.
- Pirro M, Schillaci G, Bagaglia F, Menecali C, Paltriccia R, Mannarino MR et al. Microparticles derived from endothelial progenitor cells in patients at different cardiovascular risk. Atherosclerosis. 2008; 197: 757–67.
- Shantsila E. Endothelial microparticles: a universal marker of vascular health?. J Hum Hypertens. 2009; 23: 359–61.
- Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003; 102: 2678–83.
- Simak J, Gelderman MP, Yu H, Wright V, Baird AE. Circulating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J Thromb Haemost. 2006; 4: 1296–302.
- Simak J, Holada K, Risitano AM, Zivny JH, Young NS, Vostal JG. Elevated circulating endothelial membrane microparticles in paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2004; 125: 804–13.
- Vanwijk MJ, Svedas E, Boer K, Nieuwland R, Vanbavel E, Kublickiene KR. Isolated microparticles, but not whole plasma, from women with preeclampsia impair endothelium-dependent relaxation in isolated myometrial arteries from healthy pregnant women. Am J Obstet Gynecol. 2002; 187: 1686–93.
- Werner N, Wassmann S, Ahlers P, Kosiol S, Nickenig G. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2006; 26: 112–6.
- Jimenez JJ, Jy W, Mauro LM, Soderland C, Horstman LL, Ahn YS. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res. 2003; 109: 175–80.
- Sander TL, Ou JS, Densmore JC, Kaul S, Matus I, Twigger S et al. Protein composition of plasminogen activator inhibitor type 1-derived endothelial microparticles. Shock. 2008; 29: 504–11.
- Klinkner DB, Densmore JC, Kaul S, Noll L, Lim HJ, Weihrauch D et al. Endothelium-derived microparticles inhibit human cardiac valve endothelial cell function. Shock. 2006; 25(6): 575–80.
- National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006; 354: 2564–75.
- Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010; 6: 107–15.
- Su X, Bai C, Hong Q, Zhu D, He L, Wu J et al. Effect of continuous hemofiltration on hemodynamics, lung inflammation and pulmonary edema in a canine model of acute lung injury. Intensive Care Med. 2003; 29: 2034–42.
- Ullrich R, Roeder G, Lorber C, Quezado ZM, Kneifel W, Gasser H et al. Continuous venovenous hemofiltration improves arterial oxygenation in endotoxin-induced lung injury in pigs. Anesthesiology. 2001; 95: 428–36.
- Elbahlawan L, West NK, Avent Y, Cheng C, Liu W, Barfield RC et al. Impact of continuous renal replacement therapy on oxygenation in children with acute lung injury after allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2010; 55: 540–5.
- Yan XW, Li WQ, Wang H, Zhang ZH, Li N, Li JS. Effects of high-volume continuous hemofiltration on experimental pancreatitis associated lung injury in pigs. Int J Artif Organs. 2006; 29: 293–302. [PubMed Abstract].
- Chon GR, Chang JW, Huh JW, Lim CM, Koh Y, Park SK et al. A comparison of the time from sepsis to inception of continuous renal replacement therapy versus RIFLE criteria in patients with septic acute kidney injury. Shock. 2012; 38: 30–6.