Abstract
Several authors demonstrated a strong linkage between proteose-peptones content and foaming properties of cow milk; this is of great interest for Italian dairy industries to create a new line of fresh milk characterized by a particular foaming property and, hence, particularly appreciate in catering industry. The aim of this trial was to quantify the relation between total concentration of proteose-peptones and the entity of foaming attitude in cow fresh milk. Ninety samples of raw bulk milk were analysed for proteose-peptones content, plasmin activity, fatty acid profile and foaming attitude. A negative relation was found among proteose-peptones percentage and foaming attitude which decreased with the increase of plasmin activity and somatic cell content in milk.
Introduction
Proteose-peptones (PP) fraction represents about 10% of whey protein and they are constituted by 38 components, several deriving from β-casein lysis as consequence of plasmin (PL) activity and others including different glycoprotein or hydrophobic constituents (Innocente et al., Citation1998; Andrew, Citation1978). PL is part of a complex system which primarily exists in a zymogen form (Plasminogen, PA) that is converted in PL by action of tissue-type and urokinase-type activators (Bastian and Brown, Citation1996). The conversion of PA in to PL occurs when milk is still in mammary lumen and continues after milking, during the storage of milk; the concentration of active PL depends on several factors as number and stage of lactation, somatic cells content and sanitary status of cows.
Andrew (Citation1983) demonstrated that components 5, 8 fast and 8 slow of PP (PP5, PP8f and PPs, respectively) increase during milk storage, principally as consequence of proteolytic activity of endogenous and bacterial enzymes, while the level of component 3 of PP (PP3) remains constant. Several authors demonstrated a strong linkage between PP content and foaming properties of cow milk (Haming and Srinivasan, Citation1994; Zhang and Goff, Citation2004; Caessens et al., Citation1999); this is of great interest for Italian dairy industries to create a new line of fresh milk characterized by a particular foaming property and, hence, particularly appreciated in the catering industry.
The aim of this trial was to study the relation between total concentration of PP and the entity of foaming attitude in cow fresh milk.
Materials and methods
Origin of milk samples
During a period of 12 weeks, at the moment of milk arriving at the industrial dairy processing plant located in Florence (Centrale del Latte di Firenze, Lucca e Pistoia), the milk samples were collected and immediately transported to laboratories for chemical analysis. More than one hundred bulk raw milk samples were evaluated for their foaming attitude but, to obtain a balanced experimental design, only ninety samples of them were considered and classified for foaming attitude. Milk was not pasteurized but used as raw for all determinations.
Measure of milk foaming attitude
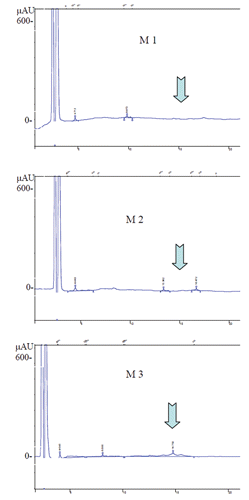
One hundred millilitres of raw milk in a graduated cylinder were insufflated with steam (100 mL min–1 of flow and 1.5 atm, for 40 sec) with the aim to produce the milk foam. After, on the base of foam volume, at each sample was attributed a score: score 1, if no foam is formed; score 2, if the final volume is doubled respect to the initial one, and score 3 if, at the end of test, the final volume is triplicate. Hence, the 90 milk samples were divided as follow: 30 milk samples lacking in foaming attitude were classified with score 1 (M1); 30 milk samples with a medium foaming attitude were classified with score 2 (M2); 30 milk samples having a high foaming attitude were classified with score 3, (M3). All 90 milk samples were analysed as described below.
Proximate analysis of milk samples
Milk samples were analyzed for fat and crude protein (CF, CP respectively) according to the official AOAC methods (Citation1990); milk caseins, urea and lactose contents were determined by infrared analysis (Milkoscan 133 B, Italian Foss Electric, Padova, Italy); somatic cell count (SCC) was performed by means of a Fossomatic 215 (Foss Electric, DK-3400, Hillerod, Denmark). Microbial cell count (MCC) was performed according to ISO 4833:003 procedure (ISO, Citation2003). The SCC and MCC data were expressed as Log10 with the aim to obtain a normal distribution of the data.
Preparation of proteose-peptones from milk samples
The extraction of PP was performed on fresh milk, immediately after sampling. Firstly, whey was obtained after centrifugation of 50 mL of milk (for 20 min at 3000 x g). Twenty mL of this solution was adjusted for pH=4.6 by 1M HCl. After centrifugation of the solution for 30 min at 5000 x g, 1 mL of the supernatant containing PP was collected in a vial, added with 3 mL of acetic buffer (0.1M, pH 4.6) and filtered (0.20 m) before the high-performance liquid chromatography (HPLC) injection.
HPLC of proteose-peptones
The HPLC analysis of milk was performed according to Innocente et al. (Citation2011) using a HPLC apparatus as follow: Varian Model 230 Pro Star (Varian Inc, Palo Alto, CA, USA) combined with a Rheodyne Model 7725i injector (Rheodyne, Cotati, CA, USA); the detector was a Varian Model 330 Å pro Star UV-Vis spectrophotometer set at 205 nm. The column was a PLRPS (4.6 nm id x 150 mm, 5 m, 300 from polymer Laboratories, Shropshire, UK), kept at 38°C during the elution. The eluting solvent A was HPLC-grade water (Fluka, Buchs, Switzerland) added with 0.1% (v/v) trifluoroacetic (Fluka) and solvent B was HPLC-grade acetonitrile (Sigma-Aldrich, St. Louis, MO, USA) added with 0.1% (v/v) trifluoroacetic. The elution gradient, as solvent B proportion was: 0-8 min, 25-35%; 8-10 min, 35-36%; 10-17 min, 36-38%; 17-23 min, 38-45%; 23-24 min, 45-100%; 24-26 min, min 0-100%; 26-27min, 100-25%. The follow rate was 1.0 ml min–1. Quantification of PP was carried out using solutions of α-lactoalbumin as external standards (α-LA, Sigma) obtained with 0.1 M phosphate buffer at pH 6.8 (1.108 mg mL–1, 0.554 mg mL–1, 0.277 mg mL–1, 0.1385 mg mL– 1,0.069 mg mL–1, 0.0345 mg mL–1) according to Innocente et al. (Citation2011). PP content has been expressed in mg mL–1.
Fatty acid analysis of milk fat
Fatty acids from milk samples were extracted according to Buccioni et al. (Citation2010). The fatty acid methyl esters (FAME) were prepared with a base catalyzed trans- esterification according to Christie (Citation1982). The FAME were separated on GC equipped with a capillary column (CP-Select CB for FAME Varian, Middelburg, the Netherlands: 100 m x 0.25 mm i.d.; film thickness 0.20 μm). The injector and flame ionization detector temperatures were respectively 270°C and 300°C. The programmed temperature was 40°C for 4 min, increased to 120°C at a rate of 10°C min–1, maintained at 120°C for 1 min, increased to 180°C at a rate of 5°C min–1, maintained at 180°C for 18 min, increased to 200°C at a rate of 2°C min–1, maintained at 200°C for 1 min, increased to 230°C at a rate of 2°C min–1 and maintained at this last temperature for 19 min. The split ratio was 1:100 and helium was the carrier gas with a flux of 1 mL min–1. Individual FAME were identified by comparison of the relative retention times of FAME peaks from samples, with those of the standard mixture 37 Component FAME Mix (Supelco, Bellefonte, PA, USA). Individual trans9 C18:1, trans11 C18:1, trans12 C18:1, trans13 C18:1 (Supelco), individual cis9, trans11 and trans10, cis12 C18:2 (Matreya Inc.), CLA mix standard (Sigma) were used to identify trans C18:1 and CLA isomers of interest. Nonadecanoic (C19:0) methyl ester was used as the internal standard (Sigma). All results concerning the fatty acid composition are expressed as g 100g–1 of milk fat.
Determination of plasminogen derived activity
Samples of fresh milk were added with a solution 0.4 M of Sodium citrate tribasic and centrifuged at 27,000 x g for 20 min at 4°C. The surface lipid layer was discarded, the supernatant (milk serum fraction) was considered for the following test. PL plus PA activities were determined in milk according to Politis et al. (Citation1992): PA-derived activity was defined as PL activity generated after addition of 150 plough units of Urokinase (Cod Z00054; GenScript Corporation, Piscataway, NJ USA) to 50 L of whey buffered with TRIS (pH 7.4); to favourite the conversion of PA in to PL, the reaction mixture was incubated for 1 h at 37°C in a water bath. After, the absorbance at 405 nm was measured at 30 min intervals. PL activity (units mL–1) was measured in the same reaction mixture without added urokinase. Derived PA activity (units mL–1) was calculated as difference.
Statistical analysis
Data were processed by GLM of SAS (Citation1999) using the following linear model
where yij is the observation; μ is the overall mean; Ri the foaming attitude (j=1 to 3), eij the residual error. The differences were considered statistically significant for P≤0.05.
Results and discussion
Among samples, no differences were found neither in urea nor in crude protein content, but casein fraction is significantly higher in M3, as reported in .
M3 samples showed, also, a significant lower value for somatic cells content () and free fatty acids concentration (). However, all samples showed values of SCC under limit established by law for milk commercialization (EC regulation 853/2004). Crude fat, fatty acid profile and microbial cells counts were similar among samples, as reported in , and . Main differences among samples involved PP concentration because it was significantly low in M3 than M1 (, ), following an inverse trend respect to foaming attitude of milk.
Probably, it can be related mainly to the higher activity of PA-PL system that induced in M1 a major lyses of casein according to several authors (Adrews et al., Citation1978; Bastian et al.,Citation1996). Literature documented that proteolytic activity in milk is strongly related to SCC level that cause a decrement of caseins as consequence of the presence of proteolytic enzymes (Ali et al., Citation1980; Verdi and Barbano, Citation1991; Albenzio et al., Citation2004; Kelly et al., Citation2000). In particular, Politis et al. (Citation1989) reported that when SCC content in milk increases, also the activity of PA-PP complex becomes higher. In our samples, SCC values were higher in milks characterized by a lower foaming attitude and our data ( and ) agree with those reported by Summer et al. (Citation2003) who suggested that milk PP concentration varies in relation to SCC content; in particular, these authors reported that when SCC value was low (about 4.998±0.199 log mL–1) the total content of PP reached 0.11 mg mL–1. According to Kennedy and Kelly (Citation1997), the PL activity in milk samples was accompanied by the highest value of SCC, approximately twice than that found in samples with the lower SCC content. However, in literature is reported that other factors can influence the entity of PA-PL activity, as the stage and lactation number of cows. At the end of lactation, in fact, PL activity is higher as consequence of a greater contribution of this enzyme in the mammary gland rather than an increase of PA activation (Richardson et al., Citation1983). Also seasonal and physiological factors are important (Sevi et al., Citation2001, Citation2002). Moreover, Bastian et al. (Citation1996) reported that PL activity is higher in older cows, even if an interaction between age and stage of lactation has been demonstrated. It could be the reason because at industrial level, the foaming attitude of bulk milk is greatly variable, often also during the same month of milk collection. In fact, dairy plants received milk from a wide area of production characterized by farms with different characteristics of production (i.e., stage of lactation, sanitary status of animals, age, breed, animal diet, etc.). Moreover, when SCC content is high, PL activity is extremely heat stable and PA activators derived from SCC appear to be likewise very heat stable. Hence, pasteurization, used largely to sanitize milk in dairy industry, is not effective to limit PL activity (Kennedy and Kelly, Citation1997).
Previous studies demonstrated that PP are responsible for emulsifying power, foaming properties and the spontaneous lipolysis control in milk (Girardet and Linden, Citation1996; Innocente et al., Citation1999), still to be used as emulsifiers in ice cream preparation (Innocente et al., Citation2002). PP are a mixture of heterogeneous proteins and peptides, each characterized by different functional properties some of which already not well known. Innocente et al. (Citation1999) published that PP are responsible of foaming attitude of milk. Our data showed a negative relation between these two aspects. PP, in fact, can be divided in two groups of proteins: the first is constituted by peptides originated by lysis of and β caseins and at this group belongs the fragments PP5, PP8f and PP8s (so called according to their electrophoretic mobility); the other group is formed by PP3 (Lactophorin) and several glycoproteins. PP3 seems to be the main responsible for the emulsifying and foaming properties of milk. Moreover, Innocente et al. (Citation1999) showed that emulsion obtained in presence of PP3 were more stable over time respect to those containing the un-purified fraction of PP. In this trial the PP profile was not determined, but only the total content has been considered (). Hence, the slow foaming attitude of M1 samples could be related to the higher presence of PP in which, probably, the PP3 component was not in prevalence. PP3 is expressed exclusively in mammary tissue of ruminants and it is not formed after the milk ejection (Pedersen et al., Citation2011). Several studies showed that bovine PP3 is a good substrate for PL that reduces the lactophorin to a shorter fragment (residue 54-135) that has lost the emulsifying properties (Sørensen and Petersen, Citation1993). The mechanism involved in the processes that lead to single PP are not well known, but certainly not only animal factor is involved but also condition of milk conservation or industrial treatment have to be considered. In , large differences in PP content between samples with poor attitude to foam and samples that easily form foam were reported. Hence, this parameter could be used to select bulk milk with the aim to use it for a line destined to a specific production.
Table 1. Crude protein, total casein and urea content in milk samples.
Table 2. Somatic cell and microbial counts in milk samples.
Table 3. Crude fat and free fatty acid content in milk samples.
Table 4. Fatty acids profile of milk samples (g 100 g–1 of milk fat).
Table 5. Proteose-peptone content in milk samples.
Table 6. Plasminogen derived activity in milk samples.
Conclusions
Raw bulk milk was characterized by an high variability in foaming attitude strongly liked to PP content; factors affecting PP concentration in milk are numerous and several of them not directly linked to animal status. Moreover, other aspects of chemical milk composition could play an important role in this aspect. Our data encourage to focussed the attention to a rapid quantification of PP, during the phases of milk selection destined to fresh comsumption.
Acknowledgments
The authors thank Mr. Andrea Bartoletti of Centrale del latte of Firenze, Prato and Pistoia laboratories, for technical assistance.
References
- AlbenzioM.CaropreseM.SantilloA.MarinoR.TaibiL.SeviA. 2004. Effects of somatic cell counts and stage of lactation on the plasmin activity and cheese making properties of ewe milk. J. Dairy Sci. 87:533-542.
- AliA.E.AndrewT.A.CheesemanG.C. 1980. Influence of elevated somatic cell count on casein distribution and cheesemaking. J. Dairy Res. 50:45-55.
- AndrewT.A. 1978. The composition, structure and origin of proteose-peptone component 5 of bovine milk. Eur. J. Biochem. 90:59-65.
- AndrewT.A. 1983. Proteinase in normal bovine milk and their action on caseins. J. Dairy Res. 50:45-55.
- AOAC, 1990. Official Methods of Analysis, vol 1, 15th ed. Association of Official Analytical Chemists, Arlington, VA, USA.
- BastianE.D.BrownR.J. 1996. Plasmin in milk and dairy products: an update. Int. Dairy J. 6:435-457.
- BuccioniA.RapacciniS.AntongiovanniM.MinieriS.ConteG.MeleM. 2010. Conjugated Linoleic Acid (CLA) and C18:1 isomers content in milk fat of sheep and their transfer to Pecorino Toscano D.O.P. cheese. Int. Dairy J. 20:190-194.
- CaessensP.W.J.R.VisserS.GruppenH.van AkenG.A.VoragenA.G.J. 1999. Emulsion and foam properties of plasmin derived β-casein peptides. Int. Dairy J. 9:347-351.
- ChristieW.W. 1982. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 23:1072-1075.
- European Commission, 2004. Regulation n. 853/2004 of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs. In: Official Journal, L 139, 30/04/2004, pp 22-82.
- GirardetJ.M.LindenG. 1996. PP3 component of bovine milk: A phosphorylated whey glycoprotein. J. Dairy Res. 63:333-350.
- HamingZ.SrinivasanD. 1994. Protease peptones and physical factors affect foaming properties of whey protein isolate. J. Food Sci. 59:554-556.
- InnocenteN.BiasutiM.BleckerC. 2011. HPLC profile and dynamic surface properties of the proteose-peptone fraction from bovine milk and from whey protein concentate. Int. Dairy J. 21:222-228.
- InnocenteN.ComparinD.CorradiniC. 2002. Proteose-peptone whey fraction as emulsifier in ice cream preparation. Int. Dairy J. 12:69-74.
- InnocenteN.CorradiniC.BleckerC.PaquotM. 1998. Dynamic Surface properties of proteose peptone fraction of bovine milk. J. Dairy Sci. 81:1833-1839.
- InnocenteN.CorradiniC.BleckerC.PaquotM. 1999. Emulsifyng properties of the total fraction and the hydrophobic fraction of bovine milk proteose-peptones. Int. Dairy J. 8:981-985.
- ISO, 2003. Microbiology of food and animal feeding-stuffs. Horizontal method for the enumeration of microorganism: colonycount technique at 30°C. Norm ISO 4833:2003. International Organization for Standardization Publ., Geneva, Switzerland.
- KellyA.L.TiernanD.O’SullivanC.JoyceP. 2000. Correlation between bovine milk somatic cell count and polymorphonuclear leukocyte level for samples of bulk milk and milk from individual cows. J. Dairy Sci. 83:300-304.
- KennedyA.KellyA.L. 1997. The influence of somatic cell count on the heat stability of bovine milk plasmin activity. Int. Dairy J. 7:717-721.
- PedersenL.R.L.NielsenS.B.HanstedJ.G.TorbenE.PetersenT.E.OtzenD.E.SørensenE.S. 2011. PP3 forms stable tetrameric structures through hydrophobic interactions via the C-terminal amphipathic helix and undergoes reversible thermal dissociation and denaturation. FEBS J. 279:336-347.
- PolitisI.HangN.G.K. 1989. Environmental factors affecting plasmin activity in milk. J. Dairy Sci. 72:1713-1718.
- PolitisI.BarbanoD.M.GorewitR.C. 1992. Distribution of plasminogen and plasmin in fractions of bovine milk. J. Dairy Sci. 75:1402-1410.
- RichardsonB.C. 1983. The proteinases of bovine milk and the effect of pasteurization on their activity. New Zeal. J. Dairy Sci. 18:233-245.
- SAS, 1999. SAS/STAT User’s Guide, ver. 8.1. SAS Inst. Inc., Cary, NC, USA.
- SeviA.AlbenzioM.AnnicchiaricoG.CaropreseM.MarinoR.TaibiL. 2002. Effects of ventilation regimen on the welfare and performance of lactating ewes in summer. J. Anim. Sci. 80:2362-2372.
- SeviA.TaibiL.AlbenzioM.AnnicchiaricoG.MuscioA. 2001. Airspace effects on the yield and quality of ewe milk. J. Dairy Sci. 84:2632-2640.
- SørensenE.S.PetersenT.E. 1993. Purification and characterization of three proteins isolated from the proteose peptone fraction of bovine milk. J. Dairy Res. 60:189-197.
- SummerA.FormaggioniP.FranceschiP.MalacarneM.MarianiP. 2003. Proteosepeptone content in the milk of Italian Friesian cows with moderate and hight somatic cell values. Ital. J. Anim. Sci. 2(Suppl.1):266-268.
- VerdiR.J.BarbanoD.M. 1991. Properties of proteases from milk somatic cells and blood leukocytes. J. Dairy Sci. 74:2077-2081.
- ZhangZ.GoffH.D. 2004. Protein distribution at air interface in dairy foams and ice cream as affected by casein dissociation and emulsifiers. Int. Dairy J. 14:647-657.