Abstract
The quality traits of the fillets from tub gurnard (Chelidonichthys lucerna L.) fished in the mid-Adriatic Sea were investigated. Forty fishes per season were sampled to evaluate the proximate composition, cholesterol content and fatty acid profile of fillets. Seasons significantly affected the quality traits of flesh. The protein content ranged from 19.39% in winter to 19.67% in summer, without significant differences. Lipid content was notably higher in spring (2.28%) and summer (2.32%), compared to autumn (1.72%) and winter (1.31%). Energy content was significantly higher in spring (416.45 kJ/100 g) and summer (417.97 kJ/100 g) compared to autumn (391.35 kJ/100 g) and winter (372.79 kJ/100 g). Saturated fatty acid content was highest in spring (35.88%), whereas monounsaturated acid content was not influenced by season. The n-3 polyunsaturated fatty acid content exceeded 37% of total fatty acid content during summer. The n-6/n-3 ratio remained favourably low across all seasons (from 0.16 to 0.18), with a slight significant increase in autumn (0.31). In conclusion, this study indicates that the flesh of tub gurnard has high nutritional value year-round, with the best results (in terms of n-3 polyunsaturated fatty acids) being obtained in summer.
Introduction
According to the Food and Agriculture Organization (FAO, Citation2012), improvements to assure the quality and safety of seafood products from origin to the consumer represents the foremost challenge in forthcoming years. In Italy, this consideration is particularly relevant because of the high quantity of fish and fish products that is annually imported (>900,000 tons) to satisfy the domestic demand (Parisi et al., Citation2014). An official report (ISMEA, Citation2010) observed that consumers prefer national fresh products compared to imported seafood and, in particular, local fish compared to fish caught from other geographical areas.
The tub gurnard (Chelidonichthys lucerna L.) fishery is an important professional fishing activity of fleet of San Benedetto del Tronto (central Italy), which is one of the most important marine fishery districts in Italy, located in the coastal region of the mid-Adriatic Sea. Over the last decade, the tub gurnard fishery fleet from this harbour has maintained an annual catch rate of 38,000 kg of this species (data supplied by the wholesale fish market of San Benedetto del Tronto). Demand for this fish is continuously increasing because, in this territory (encompassing the entire Marche region, Italy), there is a strong tradition of consuming this species. To safeguard the natural stocks of gurnards, many fishermen cooperatives are participating in the design and management of marine protected areas, and promoting various forms of aquaculture for this species [Operational Programme of the European Fisheries Fund (PO FEP) Project]. To date, limited reports are available that contain data about the farming and flesh quality of tub gurnard. Previous studies (D’Andrea et al., Citation2012; Roncarati et al., Citation2013) evaluated the potential domestication of C. lucerna, focusing on the responses of this species to aquaculture practise. The possibility of rearing gurnards using commercial marine fish feeds was ascertained; however, feed conversion rates were not favourable. Hence, information about the feeding habits and spectrum of the species in the wild, along with the chemical composition of body tissues, is required to select suitable feed chemical compositions to establish optimal aquaculture practices for this fish species. It is important to obtain this information to formulate a balanced diet that includes feedstuffs able to cover metabolic requirements, and provide a quality end product that will be purchased by consumers. The feeding habits of tub gurnard has been investigated, and it has been ascertained that this species exhibits opportunistic foraging behaviour, mainly preying on epibenthic and nectobenthic organisms (Colloca et al., Citation1994; Morte et al., Citation1997; Serena et al., Citation1998; Stagioni et al., Citation2012) that are rich in essential fatty acids (Dalsgaard et al., Citation2003).
This study aimed to evaluate the fillet quality traits of tub gurnard, one of the most popular fish species eaten in Italy, caught in the mid-Adriatic Sea in different seasons.
Materials and methods
Fish samples
During the years 2012 (autumn: 1-5 October, winter: 10-15 February) and 2013 (spring: 2-4 May, summer: 3-6 July), tub gurnard were collected in the mid-Adriatic Sea. Commercial fishing vessels, equipped with trawling, were employed. After fish were captured, they were stored in tanks with sea water ice and, after arrival at the harbour of San Benedetto del Tronto, transferred to the laboratory by portable coolers containing ice. In the laboratory, each fish was weighed to the nearest 0.1 g fresh weight with an electronic balance (Mettler Toledo mod. 5000; Mettler-Toledo, Greifensee, Switzerland) and the total body length (from the most anterior extremity to tip of the caudal fin) was measured to the nearest millimetre using an ictiometre. The gurnards were then gutted and filleted.
Proximate composition, fatty acid profile and cholesterol content
A portion of about 30 g of skinless dorsal muscle was collected from a pool of 5 specimens (20 samples per group per seasonal sampling), homogenised, and subjected to proximate analysis (moisture, protein, lipid and ash content). The moisture percentage was determined in duplicate, according to the protocol of the Association of Official Analytical Chemists procedure (AOAC, Citation1990). Protein content was determined using the standard Kjeldahl copper catalyst method (AOAC, Citation1990). Ash content was determined using the procedure described by the AOAC (Citation1990).
Total lipid content was measured using the procedure described by Folch et al. (Citation1957). After determining total lipid content, fatty acids were converted to methyl esters following the method described by Christopherson and Glass (Citation1969). The separation of fatty acids was carried out by using a GC 3800 gas chromatograph (Varian Strumentazione, Cernusco sul Naviglio, Italy) with a WP-4 Shimadzu integration system (Shimadzu Corporation, Tokyo, Japan), which was equipped with a Supelco SPTM – 2340 capillary column (30 m × 0.25 mm i.d.; 0.25 μm film thickness; Supelco, Bellefonte, PA, USA) and a flame ionization detector. The operating conditions of the gas chromatograph were: the oven temperature was maintained at 170°C for 15 min, increased to 190°C at a rate of 1°C min–1, then increased to 220°C at a rate of 5°C min–1, and maintained at this temperature for 17 min. The temperature of the injector was 270°C, while that of the detector was 300°C. Helium was used as the carrier gas at a constant flow of 1.7 mL min–1. The identification of individual fatty acids was accomplished by comparing the retention times to fatty methyl esters of standard mixtures (37 FAME Mix and C22:5 n3, Supelco).
Cholesterol determination was carried out on the total lipid extract following the method described by Manzi et al. (Citation1996) by using a Shimadzu HPLC with a Shimadzu SPD-M10A Diode Array Detector equipped with a Alltech Nucleosil C18 column (150 mm length; 4.6 mm i.d.; 5 μm thickness; Alltech Italia Srl, Sedriano, Italy). The mobile phase was a ratio of methanol:water (97:3, vol/vol) at a flow rate of 1.5 mL min–1. The quantification of total cholesterol content was obtained by using an external calibration curve of cholesterol (Sigma C8667-1G, Grade ≥99%; Sigma-Aldrich, St. Louis, MO, USA).
Calculations and statistical analysis
The energy content of the different samples of tub gurnard was evaluated in kJ/100 g of flesh and it was determined using the equation reported by Food Standards Agency (Citation2002):
Energy content (kJ/100 g) = [(protein fraction × 4.0) + (fat fraction × 9.0) + (carbohydrate × 3.75) × 4.184]
The activity of Δ5- and Δ6-desaturase, the enzymes that catalysed the formation of long-chain n-6 and n-3 polyunsaturated fatty acids (PUFA) starting from the precursors C18:2n-6 and C18:3n-3, respectively, were calculated from the equation proposed by other authors (Sirri et al., Citation2010; Dal Bosco et al., Citation2013):
Δ5- desaturase plus Δ6-desaturase = [C20:2n - 6 + C20:4n - 6 + C20:5n - 3 + C22:5n - 3 + C22:6n - 3/C18:2n - 6 + C18:3n - 3 + C20:2n - 6 + C20:4n - 6 + C20:5n - 3 + C22:5n - 3 + C22:6n - 3] × 100
One-way analysis of variance using the SAS General Model procedure was conducted to detect differences in the quality traits of fillet characteristics of the tub gurnard captured across the four seasons. The means were separated by a Student Newmann Keuls test (SAS, Citation1988). Difference was considered significant at P<0.05.
Results
The body weight and size (mean±standard deviation) of the four seasonal groups of fish that were captured were: autumn: 182.3±9 g and 21.8±1 cm; winter: 195.6±5 g and 22±1 cm; spring: 190±12 g and 23.4±1 cm; summer: 202±11 g and 24±2 cm.
presents the proximate composition, energy and cholesterol content of flesh of tub gurnards sampled across the four seasons. Moisture content was significantly higher (P<0.05) in winter (77.95%) and autumn (77.46%) compared to spring (76.63%) and summer (76.58%). Protein content ranged from 19.39% in winter to 19.67% in summer. A noticeable change in lipid content (P<0.05) was observed across adjacent seasons: spring (2.28%) and summer (2.32%) followed by autumn (1.72%) differing from winter (1.31%). Significantly higher energy content was obtained in spring (416.45 kJ/100 g) and summer (417.97 kJ/100g) compared to autumn (391.35 kJ/100 g) and winter (372.79 kJ/100 g). Ash content did not exhibit any significant difference among seasons.
presents the data about the fatty acid composition of flesh tub gurnard. The highest percentages of total saturated fatty acids (SFA) were recorded in autumn (35.88%) compared to winter (34.07%) and the other two seasons (30 to 30.3%). Palmitic acid (C16:0) and stearic acid (C18:0) were the two most representative SFAs in all seasons, showing the same significant trend (P<0.05), being highest in autumn (7.15%), followed by winter (7.01%), spring (5.12%) and summer (5.18%).
Total monounsaturated fatty acids (MUFA) were not influenced by season. However, the oleic acid (C18:1) content (which is the most representative MUFA) was significantly lower during summer (11.49%) compared to the other seasonal groups (14.8 to 16.13%). The highest percentage of concerning polyenoic (n-6) fatty acids was recorded in autumn (8.45%), while the lowest was recorded in winter (5.02%). The highest total polyenoic (n-3) fatty acid content was recorded in summer (37.22%) and the lowest in autumn (27.06%). Docosahexaenoic acid (DHA) (C22:6 n-3) was the most important fatty acid in winter (24.81%) and summer (24.39%), when the values observed were both significantly different from those observed in spring (22.93%) and in autumn (19.84%). The highest percentage of eicosapentaenoic acid (EPA) (C20:5 n-3) was recorded in summer (8.91%), declined in autumn (5.14%) and winter (5.21%), and then rose noticeably in spring (7.40%). The Δ5-desaturase plus Δ6-desaturase index was the highest in summer (70.05), followed by spring (66.65), winter (59.72) and autumn (55.54) (). The n-6/n-3 ratio was favourably low in all seasons (0.16 to 0.18), with a slight significant increase in autumn (0.31).
Discussion
In the present work the possible changes in the proximate composition, cholesterol content and fatty acid profile of flesh of tub gurnard are investigated in relation to the season. This fish species has been studied since last years and reared in some Mediterranean plants where the optimisation of feed formulation and its effect on product quality need to pay attention. Among the macronutrients, the protein content showed high stability with levels similar to those reported in another paper about the proximate compositions of reared tub gurnard and wild fish caught from the Adriatic Sea (Roncarati et al., Citation2013) in which protein ranged around 19.5%. Concerning the lipid content, the highest percentage was recorded in spring and summer compared to autumn and winter, but always in accordance with those reported in a previous paper (Roncarati et al., Citation2013) in which the farmed gurnards were fatter compared to the wild-caught specimens, although the lipids of farmed fish were below 3.5%. Comparing tub gurnard with other fish species, the species demonstrates to have relatively lower lipid content (Murdock, Citation2002; Gogus and Smith, Citation2010). In several fish caught from fisheries the fat content changed with season (Osman et al., Citation2001; Zlatanos and Laskaridis, Citation2007; Celik, Citation2008), feeding habits (Campo et al., Citation2006), catch location (Roncarati et al., Citation2012; Brambilla et al., Citation2013), sexual maturity (Sargent et al., Citation2002; Zaboukas et al., Citation2006) and their interactions. Therefore, this species may be grouped among the leaner fish harvested in the Mediterranean. The low fat content of tub gurnard has the advantage of limiting energy intake by the consumer.
In the present study, the cholesterol content did not appear to be correlated with fat content. There is much controversy in the published literature over this compound, because some studies state that cholesterol is independent of lipid levels and seems to be genetically determined (Piironen et al., Citation2002), whereas other studies (Osman et al., Citation2001; Roncarati et al., Citation2010) have observed that an increase in the fat content of fish meat is followed by an increase in cholesterol. The fatty acid profile obtained in the current study showed that SFAs were more prevalent in autumn and winter compared to spring and summer, with n-3 PUFAs dominating in the latter seasons. In two mackerel species (Trachurus trachurus and Scomber japonicus) caught in the north-eastern Mediterranean Sea, the most significant changes in the fatty acid profile occurred in winter, with an increase in the degrees of unsaturation of the fatty acid profile due to the fish requiring PUFAs to provide adaptation to lower water temperatures (Celik, Citation2008). Other studies showed that lipids are important trophic markers for the study of predator-prey relationships through the season; the fatty acid composition of the summer diet of fish species, characterised by the ingestion of small benthic organisms (Crustacea, Amphipoda and Decapoda, such as Natantia), is rich in DHA (Dalsgaard et al., Citation2003). This feed spectrum affects the fatty acids content of the fish meat, with previous papers reporting that tub gurnard contains more than 1 g of total-n-3-fatty acid per 100 g fresh fish (Zlatanos and Sagredos, Citation2006).
Furthermore, the n-6/n-3 ratio and the Δ5-desaturase plus Δ6-desaturase index, used to estimate the long-chain n-6 and n-3 PUFA synthesis, confirmed the high content of polyunsaturated fatty acids; in particular, the Δ5- Δ6-desaturase index, related to flesh of tub gurnard analysed in all the seasons, showed lower activity compared to that determined in other fish species. In brown trout, Dal Bosco et al. (Citation2013), considering indexes of fatty acid metabolism, ascertained lower activity in wild subjects compared to farmed conspecific, explaining that it was mainly due to the feeding regimen.
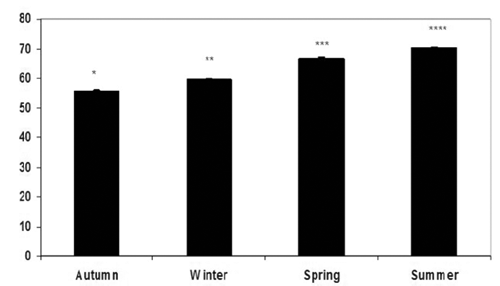
Table 1. Proximate composition, energy content and cholesterol content of flesh of tub gurnard (Chelidonichthys lucerna L.) captured in the mid-Adriatic Sea in relation to season (mean values and standard error).
Table 2. Fatty acid profile (% of total fatty acids) of flesh of tub gurnard (Chelidonichthys lucerna L.) captured in the mid-Adriatic Sea during different seasons (mean values and standard error).
Conclusions
The results obtained in this study about the nutritional traits of tub gurnard according to catch season in the mid-Adriatic may be used to compare the quality traits of wild and farmed tub gurnard in the field of aquaculture diversification. Because of the limited number of wild-caught tub gurnard, along with the general crisis in the fishery sector causing severe loss of peoples jobs, fishermen are exploring different strategies to address these issues, such as the management of natural fish stocks in marine protected areas and development of aquaculture systems.
Tub gurnard is of great socio-economic importance for Adriatic coastal communities, because this species could potentially facilitate the recovery and enhancement of traditional activities in the coastal regions of the mid-Adriatic. Important job opportunities may be generated by the characterisation of this fish product, which is in high demand by consumers. From the perspective of aquaculture diversification for this species, the high level of domestication of C. lucerna has been previously ascertained; hence, the following step involves optimising feed formulation to minimise the environmental impact and maximise the quality of captive fish production.
Acknowledgements
This research was partially supported by the Operational Programme of the European Fisheries Fund (PO FEP) Project n. 03/OPI/12. the authors would like to express their appreciation to Dr. Emanuele Troli and all the members of the Consortium of Address, Coordination and Management among Enterprises of the small-scale artisanal fishery (CO.GE.P.A) for the collection of fish samples.
References
- AOAC, 1990. Official methods of analysis. 15 th ed., Association of Official Analytical Chemists, Washington DC, USA.
- BrambillaG. Abete M.C. BinatoG. ChiaravalleE. CossuM. DellatteE. MinieroR. OrlettiR. PirasP. RoncaratiA. UbaldiA. ChessaG.,2013. Mercury occurrence in Italian seafood from the Mediterranean Sea and possible intake scenarios of the Italian coastal population. Regul. Toxicol. Pharm. 65:269-277.
- CampoD. MostardaE. CastriotaL. Scarabello M.P. AndaloroF.,2006. Feeding habits of the Atlantic bonito, Sarda sarda (Bloch, 1793) in the southern Tyrrhenian sea. Fish. Res. 81:169-175.
- CelikM.,2008. Seasonal changes in the proximate chemical compositions and fatty acids of chub mackerel (Scomber japonicus) and horse mackerel (Trachurus trachurus) from the north eastern Mediterranean Sea. Int. J. Food Sci. Tech. 43:933-938.
- Christopherson S.W. Glass R.L.,1969. Preparation of milk methyl esters by alcoholysis in an essentially non-alcoholic solution. J. Dairy Sci. 52:1289-1290.
- CollocaF. Ardizzone G.D. Gravina M.F.,1994. Trophic ecology of gurnards (Pisces: Triglidae) in the Central Mediterranean Sea. Mar. Life 4:45-57.
- D’AndreaM. RoncaratiA. MelottiP. Scarano M.T. PillaF.,2012. Development of 23 microsatellite markers for assessing genetic variability in the tub gurnard (Trigla lucerna L.). Anim. Genet. 43:650-651
- Dal BoscoA. MugnaiC. RosciniV CastelliniC.,2013. Fillet fatty acid composition, estimated indexes of lipid metabolism and oxidative status of wild and farmed brown trout (Salmo trutta L.). Ital. J. Food Sci. 25:83-89.
- DalsgaardJ. JohnM.St. KattnerG. Muller-NavarraD. HagenW.,2003. Fatty acid trophic markers in the pelagic marine environment. Adv. Mar. Biol. 46:225-340.
- FAO, 2012. The state of world fisheries and aquaculture. World review of fisheries and aquaculture. Available from: http://www.fao.org/docrep/016/i2727e/i2727e.pdf
- Food Standards Agency, 2002. McCance and Widdowson’s the composition of foods: summary edition. 6 th ed., Royal Society of Chemistry, Cambridge UK.
- FolchJ. LeesM. Sloane-Stanley G.H.,1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 60:497-509.
- GogusU. SmithC.,2010. n-3 omega fatty acids: a review of current knowledge. Int. J. Food Sci. Tech. 45:417-436.
- ISMEA, 2010. Il settore ittico in Italia; check-up 2010. Istituto di Servizi per il Mercato Agricolo Alimentare ed., Roma, Italy.
- ManziR. PanfiliG. PizzoferratoL.,1996. Normal and reversed-phase HPLC for more complete evaluation of tocopherois, retinols, carotenes and sterols in dairy products. Chromatographia 43:89-93.
- Morte M.S. Redon M.J. Sanz-BrauA.,1997. Trophic relationships between two gurnards Trigla lucerna and Aspitrigla obscura from the western Mediterranean. J. Mar. Biol. Assoc. U.K. 77:527-537.
- Murdock D.H.,2002. Encyclopedia of foods: a guide to healthy nutrition. Academic Press, San Diego, CA, USA.
- OsmanH. Suriah A.R. Law E.C.,2001. Fatty acid composition and cholesterol content of selected marine fish in Malaysian waters. Food Chem. 73:55-60.
- ParisiG. TerovaG. GascoL. PiccoloG. RoncaratiA. Moretti V.M. CentoducatiG. Gatta P.P. PaisA.,2014. Current status and future perspectives of Italian finfish aquaculture. Rev. Fish Biol. Fisher. 24:15-73.
- PiironenV. ToivoJ. Lampi A.M.,2002. New data for cholesterol contents in meat, fish, milk, eggs and their products consumed in Finland. J. Food Compos. Anal. 15:705-713.
- RoncaratiA. BrambillaG. MeluzziA. Iamiceli A.L. FanelliR. MoretI. UbaldiA. MinieroR. SirriF. MelottiP. di Domenico A.,2012. Fatty acid profile and proximate composition of fillets from Engraulis encrasicholus, Mullus barbatus, Merluccius merluccius and Sarda sarda caught in Tyrrhenian, Adriatic and Ionian seas. J. Appl. Ichthyol. 28:545-552.
- RoncaratiA. D’AndreaM. PillaF. FeliciA. MelottiP.,2014. Tub gurnard Chelidonichthys lucerna L.: a new fish species suitable for farming? First answers evaluating the growth of juveniles reared at different stocking densities, welfare and fillet quality. Aquac. Res. 44:1140-1151.
- RoncaratiA. SirriF. di Domenico A. BrambillaG. Iamiceli A.L. MelottiP. MeluzziA.,2010. Survey of qualitative traits of European sea bass cultivated in different rearing systems. Eur. J. Lipid Sci. Tech. 112:770-779.
- Sargent J.R. Tocher D.R. Bell J.G.,2002. The lipids. In:Halver J.E. Hardy R.W. ( eds.) Fish nutrition. 3 rd ed., Academic Press, New York NY, USA, pp. 181-257.
- SAS, 1988. Guide for Personal Computers, ver. 6.03. SAS Inst. Inc., Cary, NC, USA.
- SerenaF. VolaniA. AuteriR.,1998. Nursery areas and some biological information of tub gurnard (Trigla lucerna L., 1758) off Tuscany coast (Italy). Rapp. Comm. Int. Mer. Médit. 35:482-483.
- SirriF. CastelliniC. RoncaratiA. FranchiniA. MeluzziA.,2010. Effect of feeding and genotype on the lipid profile of organic chicken meat. Eur. J. Lipid Sci. Tech. 112:994-1002.
- StagioniM. MontaniniS. VallisneriM.,2012. Feeding of tub gurnard Chelidonichthys lucerna (Scorpae - niformes: Triglidae) in the north-east Mediterranean. J. Mar. Biol. Assoc. U.K. 92:605-612.
- ZaboukasN. MiliouH. MegalofonouP. Moraitou-ApostolopoulouM.,2006. Biochemical composition of the Atlantic bonito Sarda sarda from the Aegean Sea (eastern Mediterranean Sea) in different stages of sexual maturity. J. Fish Biol. 69:347-362.
- ZlatanosS. LaskaridisK.,2007. Seasonal variation in the fatty acid composition of three Mediterranean fish - sardine (Sardina pilchardus), anchovy (Engraulis encrasicholus) and picarel (Spicara smaris). Food Chem. 103:725-728.
- ZlatanosS. Sagredos A.N.,2006. The fatty acids composition of some important Mediterranean fish species. Eur. J. Lipid Sci. Tech. 95:66-69.
