Abstract
Low efficacy of targeted nanomedicines in biological experiments enforced us to challenge the traditional concept of drug targeting and suggest a paradigm of ‘addressed self-navigating drug-delivery vehicles,’ in which affinity selection of targeting peptides and vasculature-directed in vivo phage screening is replaced by the migration selection, which explores ability of ‘promiscuous’ phages and their proteins to migrate through the tumor-surrounding cellular barriers, using a ‘hub and spoke’ delivery strategy, and penetrate into the tumor affecting the diverse tumor cell population. The ‘self-navigating’ drug-delivery paradigm can be used as a theoretical and technical platform in design of a novel generation of molecular medications and imaging probes for precise and personal medicine.
The first medical nanovehicle was portrayed in the science fiction movie ‘Fantastic voyage’. It was a micron-size submarine navigating by a ‘miniaturized’ pilot along the patient's vasculature and boarded with a crew of sergeants aimed to destroy a clot in the patient's brain. Some characteristic features of the fantastic microsubmarine resemble desired vital technical characteristics of modern medical nanodevices. They include: autonomous self-navigation in the bloodstream and tissue, a means for destruction of biological barriers and tumor-surrounding threat cells, traceability for imaging of the nanovehicle location and capability to deliver a molecular cargo to the site of disease.
Appearance of drug-delivery nanovehicles (nanomedicines) provoked a new impulse toward development of targeted cancer chemotherapeutics. The first generation of nanoparticle therapeutics were autonomous non-navigating vehicles [Citation1]. They were proposed to take advantage of abnormalities in tumor vasculature for accumulation of drugs in tumor microenvironment exploring enhanced permeability and retention (EPR) effect [Citation2,Citation3]. In attempts to enhance their therapeutic efficacy, the second generation of nanomedicines explored the Ehrlich's ‘magic bullet’ concept for the nanomedicine targeting toward the vasculature and tumor cells [Citation4]. Discovery of unique, organ-specific vasculature receptors, called ‘zip codes’, suggested a new approach for organ- and tumor-targeted drug delivery, harnessing the power of the in vivo phage display technique–the concept developed by Pasqualini, Ruoslahti and colleagues [Citation4–10]. Since tumors were considered as a monocellular pathology, majority of anticancer therapeutics were designed to target exclusively cancer cells or cells of tumor vasculature [Citation1]. The concept of tumor-targeted nanomedicines has been around for years, but it has been underdeveloped because mostly relied on the assumed homogeneity of tumor cells and their proximity to the defective tumor epithelium, as illustrated in . The attempts of adapting Ehlich's ideas of direct tumor targeting have shown insignificant improvements [Citation11]. Analysis of nanoparticle delivery data accumulated during the past decade demonstrated that only a tiny portion of the administered nanoparticle dose is delivered to solid tumors that creates a critical hurdle for translating nanomedicines into the clinic [Citation12,Citation13]. Furthermore, based on numerous clinical studies, it was concluded that the foundation for the development of nanomedicines in cancer therapy–EPR effect, discovered and intensively studied in rodents [Citation14,Citation15], does not work in humans [Citation3]. The low performance of targeted nanomedicines in biological experiments compromised the fundamental concepts of EPR effect and tumor targeting, and forced researchers to pursue other ways of exploiting nanomedicine's pharmaceutical potential [Citation3,Citation16,Citation17]. Considering above-mentioned desired characteristics of the innovative medical nanodevices envisioned by the authors of Fantastic voyage, we focused on previously disregarded ability of medical nanovehicles to autonomous self-navigation in the patient body, and more specifically–in tumor microenvironment. As was noted by Maeda, one of the authors of EPR-based targeting concept [Citation14], “many experimental scientists and pharmacologists and nanotechnology engineers hold to the premise that solid tumors consist of uniform tissues, i.e., that tumors are homogeneous” [Citation15]. Currently, it is commonly recognized that solid tumors are nonhomogeneous and represent a well-organized ensemble of tumor and stromal cells, such as fibroblasts, smooth muscle, endothelial, vascular and immune cells accommodated in a network of extracellular matrix and secreted extracellular molecules [Citation18–20]. Proficient nanomedicines should run a long way though all these barriers to reach and attack the target tumor cells and further–to penetrate and bring the drug cargo to the target cellular compartment.
This paper suggests to reconsider the previously dominated approach to tumor ‘direct targeting’ based on the illusion that cancer is a monocellular disease and tumor cells are readily accessible to nanomedicines penetrating into the tumor compartment through the ‘king gate’ in the abnormal vasculature. We suggest a novel paradigm exploring ‘self-navigating drug-delivery’ model, based on the modern understanding of tumor microenvironment composition and function [Citation21]. The paper introduces also landscape phages and their fusion proteins as potentially invaluable versatile tools–‘explorers’ for the study of tumor microenvironment, and universal construction material for development of the third generation of ‘autonomous self-navigating’ cancer nanomedicines [Citation22]. Other required characteristics of modern nanomedical devices, mentioned above–“a means for destruction of biological barriers and tumor-surrounding threat cells”, and “traceability for imaging of the nanovehicle location”, are currently considered in the scope of one concept–‘theranostics’, and are developed and presented separately from this work [Citation23].
Development of autonomous self-navigating nanomedicines: change of the paradigm
Clarifying the composition of the tumor microenvironment has allowed to discover biological hurdles undermining the potential of targeted drug-delivery nanomedicines in human patients [Citation19,Citation20]. To overcome these difficulties in nanomedicine migration, it was suggested to supply the tumor-targeting vehicles with multiple ligands targeted to different components of tumor microenvironment [Citation24–28]. This modern trend of using ‘molecular cocktails’ for cancer drug targeting will likely prevail in the development of advanced targeted nanomedicines in a near future. Although the efficacy of dual-targeted nanomedicine was demonstrated in some in vitro and in vivo model experiments, they hardly can solve the problem of complete tumor eradication, because their ability to migrate toward the tumor cells depends on the fine balance of their affinities toward the intermedium cells. As was shown [Citation29], high-affinity probes occupy the target receptors of the cells that are reached in a single diffusion step. However, they would not be able to reach receptors of the next layer cells, and will be forced back out of the tumor. In opposite, low affinity probes can diffuse into the tumor using sequential binding to and dissociation from intermediate cell receptors.
This consideration led us to a novel selection strategy–the selection through migration. We suggested the distinctive ‘addressed self-navigating drug’ approach, in which peptides selected using traditional principles of affinity [Citation30] are substituted for ‘promiscuous phage’ particles discovered through ‘migration selection’ exploring their intrinsic capacity to extravasate from the blood stream to the tumor tissue, migrate through the tumor microenvironment, invade into the tumor and penetrate into the target tumor cells [Citation21]. We hypothesized that phages and their proteins discovered through migration selection in the specific tumor environment, under low pH and distinct medium composition, would serve as navigating ‘feelers’, or ‘sensors’ without any modification, or can be used as a reservoir of ‘elementary binding units’ that can help as ‘molecular LEGO’ in construction of proteins with expected tissue migrating propensity. For identification of motifs responsible for interaction with different components of tumor microenvironment, we use landscape phage libraries as collections of phage explorers, which themselves find a way to the tumor. In our new concept of self-navigating drug-delivery vehicles, we try mimicking some mechanisms of migration from the blood to the site of inflammation and cancer disorders, evolved in neutrophils, which have been used recently for anticancer drug delivery [Citation31].
Passive targeting of an actively targeted nanomedicine results in enhanced delivery and improved biological effect.
Adapted with permission from [Citation88] © TechConnect (2017).
![Figure 1. Schematic of nanomedicine deposition by passive and active targeting in the presence of an observable enhanced permeability and retention effect (leaky vasculature).Passive targeting of an actively targeted nanomedicine results in enhanced delivery and improved biological effect.Adapted with permission from [Citation88] © TechConnect (2017).](/cms/asset/e20084d7-42ee-4fd8-b043-c37b4a7d7065/itde_a_12366364_f0001.jpg)
Promiscous peptides & proteins: potential navigating sensors of ‘autonomous self-navigating’ nanovehicles
There are two types of promiscuous peptides: peptides recognizing distinct receptors in the same or foreign cells, and peptides recognizing the same receptor overexpressed in different cells [Citation32]. The promiscuous peptide of the first type–RGD (ArgGlyAsp)–has been discovered by Pasqualini and Ruoslahti in 1996 in their pioneering in vivo selection experiments [Citation4]. Since its discovery, promiscuous RGD motif-containing peptides, which bind a majority of known integrins [Citation33] of tumor endothelium and cancer cells have been widely used in laboratory experiments to study targeted delivery of nanomedicines both in vitro and in vivo [Citation34,Citation35]. A promiscuous peptide of the second type–surface nucleolin-binding octamer DMPGTVLP fused to the N-terminus of the p8 phage protein, was isolated from the landscape phage library f8/8 [Citation36] by its screening against breast cancer cells MCF-7 [Citation37]. Nucleolin is a multifaceted and ubiquitous nonhistone nucleolar phosphoprotein, which shuttles between the nucleus and the cytoplasm [Citation38]. Interestingly, the protein has been shown to be overexpressed on the surface of many different tumor cell types, among which are breast, prostate, leukemic, pulmonary, kidney, renal, cervical, intestinal, hepatic, colon cancer cells, gliomas and melanomas, and has been exploited as a biomarker for cancer diagnosis and development of targeted nanomedicines. Since surface nucleolin is overexpressed in a number of tumor and tumor-surrounding cells, the promiscuity of the DMPGTVLP peptide may expend to various cancer cells and cells of tumor microenvironment, such as activated lymphocytes, angiogenic endothelial cells within the tumor vasculature and others [Citation39]. It was shown to enhance antitumor activity of the phage-navigating nanomedicines in mice tumor models [Citation40,Citation41]. Promiscuous peptides of the first type have been selected in the intercellular selection scheme [Citation32] (). Promiscuity of these peptides is presumably lied in their mosaic structure. For example, heptapeptide EVNGRGD accommodates three overlapping motifs targeted to distinct receptors: NGR–aminopeptidase N (CD13) [Citation42]; RGD–integrins [Citation7] and EVN–unknown, (). Analyzing homology of multi-thousand population of tumor-binding peptides, selected as described below, we identified 3–4 amino acid residues motifs, which are assembled in the phage-displayed peptides during consecutive circles of affinity selection [Citation21,Citation32,Citation43].
Discovery of motifs serving as hypothetical ‘elementary binding units’ [Citation8,Citation44–47] inspired us to suggest the ‘addressed drug navigation’ concept based on the use of phages, phage proteins and distinct ‘molecular navigating motifs,’ identified from billion-clone landscape phage libraries used as a variety of ‘explorers’ in the tissue-migrating selection assay. In the suggested ‘self-navigating vehicles,’ promiscuous peptides and proteins of the first and second types are the ‘sensors’ that navigate the targeted vehicle from one cellular hub to another through the tumor environment to the final point of destination (). This novel tactic would allow replacing the existing mostly ineffective ‘point-to-point’ targeting concept for the innovative ‘autonomous self-navigating drug-delivery vehicle’-based’ paradigm that would allow design of a new generation of proficient self-navigating medicines capable to surmount physiological barriers that preclude their anticancer performance, and serve as a foundation in development of a novel generation of nanomedicines for personal precise medicine.
(A) Monocellular biopanning. (B) Intercellular migration selection in vitro. (C) Hypothetical ‘motif assembling’ mechanism operating during selection of migrating phage in vivo. (D) Examples of discovered motifs as molecular LEGO.
(A) Adapted with permission from [Citation87] © TechConnect (2017).
(B & C) Adapted with permission from [Citation88] © TechConnect (2017).
(D) Adapted with permission from [Citation88] © TechConnect (2017).
![Figure 2. Evolving of promiscuous peptides of the first type during intercellular selection. (A) Monocellular biopanning. (B) Intercellular migration selection in vitro. (C) Hypothetical ‘motif assembling’ mechanism operating during selection of migrating phage in vivo. (D) Examples of discovered motifs as molecular LEGO. (A) Adapted with permission from [Citation87] © TechConnect (2017). (B & C) Adapted with permission from [Citation88] © TechConnect (2017). (D) Adapted with permission from [Citation88] © TechConnect (2017).](/cms/asset/e764dd5a-1645-46af-8ddc-fd84463a5547/itde_a_12366364_f0002.jpg)
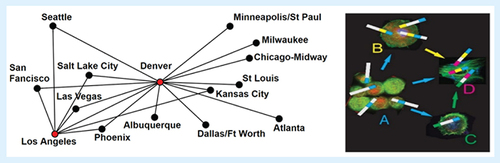
(Right) ‘Hub and spoke’ selection/delivery model of multimotif phages navigating through tumor microenvironment. ‘Promiscuous’ phages and their proteins, containing a set of motifs autonomously responsible for phage extravasation from tumor vasculature (1st phage-accumulating hub), and further migration through tumor stroma, serving as consecutive hubs during phage transportation toward cancer cells. (Left) Hub and spoke airline route structures. Los Angeles and Denver are used as hubs.
Adapted with permission from Elwood64151, at the English Wikipedia project, CC BY-SA 3.0, under permission of GNU Free Documentation License https://commons.wikimedia.org/w/index.php?curid=3456910
Landscape phages as selectable ‘explorers’ in development of ‘autonomous self-navigating drug-delivery vehicles’
Phage display technology is used for discovery of peptide ligands in majority of targeted drug/gene delivery projects [Citation30]. Filamentous phages, used in the traditional phage display systems, contain the major coat protein p8 (∼90% of the virion mass), and a few copies of the minor coat proteins at the ends of the virion [Citation48,Citation49] (). In phage display constructions, a foreign DNA segment is spliced in-frame into one of the phage coat protein genes, so that the ‘guest’ peptide encoded by that DNA segment is fused to the phage coat protein and thereby displayed on the surface of the phage virion [Citation30,Citation49,Citation50]. Phage display libraries are ensembles of up to about 10 billion such phage clones, each harboring a distinct foreign coding sequence, and therefore displaying a distinct fused peptide on the virion's surface [Citation51]. They serve as a versatile toolkit to identify fusion peptides binding the targeted organ tissues, tumors and their diverse cells [Citation30]. The minor (p3) and the major (p8) coat proteins, presented by 3–5 and approximately 2700 copies (∼4000 copies in fd-tet type ‘landscape phages’), respectively, are preferred in the mainstream of phage display projects [Citation50] ().
A potential role of filamentous phages M13 and fd as explorers in biological experiments was supported by their intrinsic physical stability in various aggressive media [Citation52] and at high temperature [Citation53,Citation54], and a relative inertness to their clearing by reticuloendothelial system, permitting plasma half-life of wild-type phages in mice up to 3.6–4.5 h [Citation55,Citation56]. Several minutes after intravenous injection of p3-type peptide fd-tet phage-displayed library [Citation57,Citation58], or M13 phage displaying p3-fused Fab domains 8 × 5 × 4 nm [Citation56], phage virions were associated with all surveyed organs, primarily lungs, kidneys, spleen and liver. Somewhat fewer phage particles were associated with colon and muscle [Citation56]. Immunohistochemical analysis of tissues evidenced that after 4 min of injection, phage particles accommodate on lumenal surface of the capillary endothelium in all organs, with the most intense staining found inside the glomerular capillary loops. No signs of tissue phage uptake were found at this point. Phage extravasation into the tissue was observed later, after 24 and 72 h, when phage virions were mostly eliminated from the blood [Citation56]. At 24 h, phage virions left the vascular compartment, and were found in the parenchyma of liver, the red pulp of spleen, the pulmonary interstitium, the proximal convoluted tubules of kidneys and in feces. The yield of recovered phage from each organ was decreased tenfold compared with the 4-min exposure, while remaining the major profile of phage distribution. No phage staining was observed in skeletal muscle and colon. The difference in phage distribution among different organs may be attributed to the higher perfusion and capillary permeability of the liver, lungs and kidneys when compared with colon and skeletal muscle [Citation59]. At 72 h, when phage particles were mostly eliminated from the circulation, only extravasated and trapped phage particles could be seen in the tissue. The results of the most comprehensive analysis of biodistribution and clearance of p3-fused fd-tet phage-displayed 15-mer peptide display library in normal (CF-1), athymic nude (nude-nu) T-cell deficient and ICR SCID mice lacking both T and B cells, were presented by Zou et al. [Citation57]. In consistence with other studies [Citation60], 1 h after injection of the phage library, infectious phage virions were revealed in all organs in the normal mice, however, with significant fluctuation of phage uptake per gram of tissue. As expected, liver and spleen harbored the most phage particles, while muscle, pancreas and brain–the least. In the mouse study [Citation61], proceeding the stage 1 clinical study [Citation62], Krag et al. were interested to imitate human studies so as to allow phage screening in cancer patients. In the study of the clearance of phage in organs, evaluated by phage tittering, they revealed that infective phage remains at 3 days in most tissues in most mice, blood become free of phage by 11 days and all tissues in most mice–by 3 weeks.
In phage biodistribution survey in a human comatose patient, intravenously injected p3-fused peptide fd-tet phage display library was revealed in several organs by tissue biopsies and phage tittering, and diversity of phage clones was analyzed by their DNA sequencing [Citation8]. It was found that phage virions were distributed approximately evenly in fat, skin, bone marrow, muscle and prostate tissues (∼106 TU/mg), with a significant dominance in liver. However, distribution of clones in different organs with fused random peptides was strongly biased, as was proved by assessments of the peptide 3-mer motif occurrences in a certain organ and in the native library. Optimization of phage survey by replacement of phage tittering and TU-counting with DNA extraction and quantitative (q)PCR allowed obtaining precise phage homing results in a few hours after removal of tissue, for dozens of samples in one setup [Citation63].
Development of phage screening technique in human patients is believed to lead to the molecular mapping of human blood vasculature toward a targeted medicines [Citation64]. In another set of phage screening experiments in humans, Krag et al. [Citation62] used minor coat protein p3-fused peptide/scFv libraries constructed in the fd-tet/fUSE5 vector system [Citation65]. In these experiments, results of 12 phage infusions in eight patients were analyzed. 30 min following phage infusion, a tumor nodule was surgically removed and processed to extract cell-associated phages. This first Phase I clinical study of phage library infusions in human cancer patients showed the safety of the procedure and a prospect for its further clinical translation. Several peptide motifs were identified among tumor-homing phages, proving their nonrandom tumor accumulation. In the most phage biodistribution experiments, an entire amount of phage particles associated with organ's vasculature is used for estimation of phage assimilation in a patient body and for selection of organ-specific phage binders. To move forward in obtaining a precise map of vascular receptors, Yao et al. combined in vivo phage display with laser microdissection, as exemplified by phage library screening in murine pancreas, led to discovery of peptide ligands for vascular receptors in the islets of Langerhans [Citation66].
Since the Folkman's hypothesis that “tumor growth is angiogenesis dependent and that inhibition of angiogenesis could be therapeutic” [Citation67], most in vivo phage screening efforts were focused on discovery of ligands specifically interacting with tumor vasculature (reviewed in [Citation60]). Starting from pioneering work of Pasqualini and Ruoslahti [Citation4], it is commonly accepted that phage display in vivo is a tool to recognize peptides targeting pathological endothelium. The identified fusion peptides are thought to provide molecular tools for targeting tumor vascular beds with diagnostic probes and therapeutic devices [Citation68]. In the new paradigm of ‘self-navigating tumor-targeting drugs’, the vasculature-targeting peptides can serve as ‘sensors’ initiating extravasation and migration of targeted medicines from blood stream toward tumor microenvironment. Thus, there is a need in new peptide ligands that would target receptors of cancer cells and cells that belong to the tumor microenvironment [Citation20]. Such peptides are also required in development of precise imaging and therapeutic nanodevices [Citation69]. In attempts to discover peptides that target the cancer cell, rather than the nearby vasculature cells, Landon and Deutscher suggested in vivo selection schemes that yield phage, which associates and penetrates into the tumor, and internalizes into cancer cells. To achieve this goal, phage libraries were depleted in normal mice to get rid of majority of vasculature-binding phage, and then were injected into prostate tumor-bearing mice. After 1 h, phage particles were eluted from tumors using mild detergent and the whole phage population captured in the tumor was amplified and analyzed. It was found that phage clones eluted from the prostate tissue demonstrated specific binding to the parental cancer cells initiated the tumor growth in mice, and some of them were able to internalized into the cancer cells [Citation69]. The success of this study first demonstrated that in vivo selection strategies can be tailored in different customer-determined ways to obtain peptides that bind the cancer cells and/or selected due to their capacity to internalize into the targeted cells. Phages that targeted pathological tumor endothelium were identified by screening 7-mer p3-fused peptide phage-displayed library in a model rat brain tumor C6 glioblastoma [Citation68]. After 6 h of phage circulation, some of them extravasated through the tumor endothelium into the parenchyma. Although distribution of extravasation ports in different tumor blood vessels were random, extravasated phages were conveyed or diffused themselves far into the surrounding tumor tissue, where they could persisted for several days. Visibly, only a small part of blood vessels were associated with phages and even distinct vessels with bound phages showed an irregular staining, demonstrating striking variability of tumor blood vessels detectable by tracing with phages.
To study effects of nanomedicine targeting in vitro and in vivo, we developed a toolkit based on the filamentous phage fd-tet [Citation70]. The whole architecture and function of the phage particles have been engineered through the reconstruction of their DNA [Citation30,Citation70,Citation71]. The majority of phage display projects use p3-displayed libraries to discover high-affinity peptides and antibodies using affinity selection technique (biopanning) [Citation30] (). As opposed to this traditional approach, in the phage nanobiotechnology [Citation72] relying mostly on the multivalent major coat protein p8 display, the entire phage proteins and whole virions are the goal of discovery [Citation73] (). Such phage constructs were named ‘landscape phage’ to highlight the vivid transformation of their surface design [Citation36].
The input in the affinity selection protocol is a phage library that encompasses billions of distinct phage clones, harboring trillions of mutations in gp3 or gp8 segments of the phage genome and displaying billions of unique peptides fused to p3 or p8 phage proteins. As a result of affinity selection, phage particles, whose displayed peptides bind the target cells are isolated. Phages with different profiles of interaction with cancer cells have been isolated using different methods of their extraction. For example, acid extraction is used for isolation of surface-binding phage; pH change–for isolation of phages from cytoplasm, and mild detergents–for extraction of membrane-bound phages [Citation37,Citation43]. The p8-expressing landscape phage libraries allow selecting peptides with lower affinities, as their dense arrangement on the virion's surface results in a stronger binding due to avidity effect [Citation74]. With a goal of using phages as tissue-navigating probes in vivo, p8 expressing phages become a pivotal research instrument because they can bind reversibly to polyvalent cellular receptors using short amino acid sequence motifs (hypothetical elementary binding units) accommodated on their fusion peptides. Because of their reversible binding propensity, p8-fusion proteins can rival high affinities and binding capacity of p3-fusion phage proteins, which would not allow to support and navigate the migration of the phage through diverse tumor environment–from vasculature to the target tumor cell. In this way, the migration of the p8-fusion phages mimics extravasation of leucocytes through the vasculature to the site of disease [Citation75]. In this concept, promiscuous phage proteins interact not only with receptors of the cancer cells but also with cells of tumor vasculature and microenvironment. P8-fusion phages demonstrate clear technological benefits: they can be directly used, without any reconstruction and processing, as a source of molecular probes, imaging devices and targeted medicines [Citation73].
(A) Phage vector fd-tet composed of 4000 copies of the p8 major coat protein (blue) and five copies of minor coat proteins p3 (pink), pVI (black), pIX (gray) and pVII (purple) each. (B & C) p3 and p8 phage display libraries. A random peptide (red) is fused to every copy of either p3 or p8 proteins. (D) Transmission electron microscopy of bacteriophage fd. (E) Electron density model of the major coat protein p8 in the fd phage. (F & G) Wire-stick and ball models of landscape phages (∼1% of the phage lengths is shown).
(A) Adapted with permission from [Citation88] © TechConnect (2017).
(B & C) Adapted with permission from [Citation88] © TechConnect (2017).
(D) Adapted with permission from [Citation89] © Elsevier (2003).
(E) Adapted with permission from [Citation89] © Elsevier (2003).
(F & G) Adapted with permission from [Citation88] © TechConnect (2017).
![Figure 4. Phage display toolkits. (A) Phage vector fd-tet composed of 4000 copies of the p8 major coat protein (blue) and five copies of minor coat proteins p3 (pink), pVI (black), pIX (gray) and pVII (purple) each. (B & C) p3 and p8 phage display libraries. A random peptide (red) is fused to every copy of either p3 or p8 proteins. (D) Transmission electron microscopy of bacteriophage fd. (E) Electron density model of the major coat protein p8 in the fd phage. (F & G) Wire-stick and ball models of landscape phages (∼1% of the phage lengths is shown). (A) Adapted with permission from [Citation88] © TechConnect (2017). (B & C) Adapted with permission from [Citation88] © TechConnect (2017). (D) Adapted with permission from [Citation89] © Elsevier (2003). (E) Adapted with permission from [Citation89] © Elsevier (2003). (F & G) Adapted with permission from [Citation88] © TechConnect (2017).](/cms/asset/12b6e802-f682-4e0d-852a-fefb8daf3a20/itde_a_12366364_f0004.jpg)
The major coat protein of landscape phages as antenna of tumor-targeted self-navigating vehicles
The major construction material that we use in the preparation of self-navigating vehicles is the major coat protein p8 [Citation22]. Proficiency of the major coat protein to associate with micelles and liposomes [Citation72,Citation76] emerges from its intrinsic function as a membrane protein during infection of the host E. coli and later–during the phage assembly [Citation77,Citation78] (). Spontaneous insertion of the major coat protein into lipid membranes is mediated by electrostatic, electrophoretic and hydrophobic interactions, as discussed in [Citation22]. Along with the use of the major coat protein for targeting of liposomes and micelles, fusion phage proteins can be used for encapsulation of DNA and RNA [Citation79]. This approach is based on the idea that phage coat protein acts as a natural vehicle for encapsulation of the phage DNA during phage proliferation [Citation48]. The fusion coat proteins with 8- or 9-mer inserts contain 55 amino acid residues and can accommodate several functional units (): membrane-binding segment, cancer cell-binding peptide, membrane-penetrating domain, DNA-binding peptide and fusogenic peptide [Citation80]. Their isolation in pure form is very fast, simple and inexpensive [Citation73]. It is important to note that capability of the selected landscape phage to specifically and selectively bind and penetrate into cancer cells is translated both to their individual proteins [Citation37] and to corresponding phage protein-navigated nanomedicines. Combinatorial approach allowed us to screen dozens of phage-driven nanomedicines in one experiment [Citation73]. Unique properties of landscape phages and their fusion proteins can justify their use as universal ‘explorers’ in migration selection and discovery of promiscuous proteins–‘sensing elements’ of self-navigating vehicles for drug/gene delivery, as discussed below.
In preliminary experiments, landscape phage libraries f8/8 and f8/9 were explored to discover phages specifically interacting with various type cancer cells [Citation22,Citation32,Citation43,Citation73]. It was demonstrated that phage fusion proteins selectively interacting with various cellular phenotypes are ideal construction material for preparation of targeted nanomedicine platforms with increased antitumor potential toward human prostate, breast, lung and pancreatic cancer cells [Citation40,Citation41,Citation81]. It was shown that the cell-binding specificity of selected phages and their proteins translates to the protein-decorated nanomedicines, enhancing their binding and cytotoxic potential toward the cancer cells. The major principle of the landscape phage-based approach is that targeted nanomedicines recognize the same receptors on the target cells, which were used in the phage selection.
(A) Multifunctional nucleolin-targeted micellar paclitaxel [Citation40,Citation90]. (B) Incorporation of major coat proteins p8 into the growing phage (The model is adapted with permission from [Citation91] © Elsevier [1998]).
![Figure 5. Association of the major coat protein p8 with micelles emerging from its intrinsic function as a membrane protein during phage assembly. (A) Multifunctional nucleolin-targeted micellar paclitaxel [Citation40,Citation90]. (B) Incorporation of major coat proteins p8 into the growing phage (The model is adapted with permission from [Citation91] © Elsevier [1998]).](/cms/asset/27b48594-358c-4600-a9b6-312202eb89dd/itde_a_12366364_f0005.jpg)
Migration selection versus affinity selection in preparation of self-navigating promiscuous landscape phages
In phage display, to discover the phage with desired characteristics, original population of phage virions with heavily mutated coat proteins genes is sorted to isolate a collection enriched for the variants with a better ‘fitness’, as defined by the formerly established principles. Since the appearance of phage display technology, the only commonly used sorting procedure has been affinity selection of the phage binders [Citation30]. However, with the development of a novel p8-type of phage display [Citation82] and design of p8-type libraries called ‘landscape libraries’ [Citation36,Citation83,Citation84] (), a new criteria of fitness can be imposed on the landscape phage population and new sorting principles can be applied. Thus, for example, landscape phages were sorted by their ‘migration selection’ in electric field, and by their exposure to high temperature, chloroform and gravitation [Citation30]. We envision that uniqueness of landscape phages and their libraries, comprising collections of billions of diverse nanomaterial species, rather than carriers of a few peptides or antibodies, would allow their sorting by selection during their migration through a tumor microenvironment, similar to their selection during migration in electric, magnetic and gravitational fields [Citation30,Citation85,Citation86]. We hypothesized that during a ‘fantastic voyage’ of billions of landscape phage particles harboring trillions of mutations in their gene 8, only species able to migrate through all biological barriers toward the site of disease would be discovered. To describe the iterative migration of phage from one cell to another in the tumor tissue, we used the spoke–hub distribution model, which is a delivery system of connections, as illustrated in . According to this model, ‘self-navigating molecular probes’ are recruited from a composition of motifs directed toward various cellular components of tumor microenvironment, responsible for phage transportation from the ‘departure’ vasculature cells toward ‘destination’ cancer cells. Phage extravasation and migration through tumor microenvironment are controlled by a set of motifs accommodated in promiscuous phage proteins, serving as ‘feelers’, which navigate phage vehicles through collecting discrete hubs during their traveling toward destination tumor cells. These navigating phages can be selected from landscape phage libraries using a procedure called ‘Micro-Biopanning’, which combines in vivo selection, laser capture microdissection and next-generation sequencing [Citation21,Citation63,Citation87]. Since ‘navigation selection’ in combination with ‘Micro-Biopanning’ analysis results in discovery of entirely unexpected motifs and their combinations that could not be revealed by other means, it may have a strong advantage in comparison with rational design of multifunctional compositions with desired characteristics, such as capacity to programmed navigation in the tumor microenvironment. We believe that the progression of the traditional drug-targeting concept, evolved into the self-navigating drug-delivery vehicle paradigm, can lead to ‘fantastic’ prospects for precise delivery of medicines to the patient's site of disease.
Conclusion & future perspective
The phage-programmed self-navigating nanomedicines are proposed to overcome biological and technical barriers that prevent tumor drug delivery. Using promiscuous fusion phage proteins and their combinations, selected during ‘migration selection’, as ‘navigating sensors’ would allow overwhelming the technically challenging complexity of rationally designed dual- and multiple-targeted nanomedicines, assumed to increase performance of nanomedicines. Taking into consideration the power of the ‘landscape phage’ selection and convenience of the fusion phage protein's self-assembly into the ‘autonomous self-navigating nanomedicines’, one can foresee a major breakthrough into a new age of perceptive, harmless, effective and inexpensive anticancer medications.
There is a need to reconsider the previously dominated approach to tumor ‘direct targeting’ based on the illusion that cancer is a monocellular disease and tumor cells are readily accessible to nanomedicines penetrating into the tumor compartment through the ‘king gate’ in the abnormal vasculature. We suggest a novel paradigm exploring ‘self-navigating drug-delivery’ model, based on modern understanding of tumor microenvironment composition and function.
The paper introduces landscape phages and their fusion proteins as potentially invaluable versatile tools–‘explorers’ for the study of tumor microenvironment, and universal construction material for development of the third generation of ‘autonomous self-navigating’ cancer nanomedicines.
In the novel ‘addressed self-navigating drug’ approach, peptides selected using traditional principles of affinity are substituted for ‘promiscuous phage’ particles discovered through ‘migration selection’ exploring their intrinsic capacity to extravasate from the blood stream to the tumor tissue, migrate through the tumor microenvironment, invade into the tumor and penetrate into the target tumor cells.
A toolkit for generation of autonomous self-navigating nanovehicles, including landscape phage libraries and discrete fusion phage major coat protein is described. Unique properties of landscape phages and their fusion proteins can justify their use as universal ‘explorers’ in migration selection and discovery of promiscuous proteins–‘sensing elements’ of self-navigating vehicles for drug/gene delivery.
Financial & competing interests disclosure
The author acknowledges the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under award numbers (1U54CA151881 & R01CA125063) and the Auburn University Research Initiative in Cancer (AURIC) for financial assistance. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health or Auburn University. The author states no conflicts of interest and has received no payment in the preparation of this manuscript.
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Shi J Kantoff PW Wooster R Farokhzad OC . Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer17 (1), 20–37 (2017).
- Danhier F Feron O Preat V . To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Rel.148 (2), 135–146 (2010).
- Danhier F . To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine?J. Control. Rel.244, 108–121 (2016).
- Pasqualini R Ruoslahti E . Organ targeting in vivo using phage display peptide libraries. Nature380 (6572), 364–366 (1996).
- Teesalu T Sugahara KN Ruoslahti E . Mapping of vascular zip codes by phage display. In : Methods in Enzymology: Protein Engineering for Therapeutics, Volume 203, Pt B. WittrupKDVerdineGL ( Eds). Elsevier Academic Press Inc., CA, USA, 35–56 (2012).
- Ruoslahti E Bhatia SN Sailor MJ . Targeting of drugs and nanoparticles to tumors. J. Cell Biol.188 (6), 759–768 (2010).
- Ruoslahti E . Drug targeting to specific vascular sites. Drug Discov. Today7 (22), 1138–1143 (2002).
- Arap W Kolonin MG Trepel M et al. Steps toward mapping the human vasculature by phage display. Nat. Med.8 (2), 121–127 (2002).
- Kolonin M Pasqualini R Arap W . Molecular addresses in blood vessels as targets for therapy. Curr. Opin. Chem. Biol.5 (3), 308–313 (2001).
- Kolonin MG Sun J Do KA et al. Synchronous selection of homing peptides for multiple tissues by in vivo phage display. FASEB J.20 (7), 979–981 (2006).
- Kwon IK Lee SC Han B Park K . Analysis on the current status of targeted drug delivery to tumors. J. Control Rel.164 (2), 108–114 (2012).
- Wilhelm S Tavares AJ Dai Q et al. Analysis of nanoparticle delivery to tumors. Nature Rev. Mat.1 (5), 12 (2016).
- Torrice M . Does nanomedicine have a delivery problem?Chem. Eng. News94 (25), 16–19 (2016).
- Matsumura Y Maeda H . A new concept for macromolecular therapeutics in cancer-chemotherapy–mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res.46 (12), 6387–6392 (1986).
- Maeda H . Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev.91, 3–6 (2015).
- Park K . The drug delivery field needs a well-diversified technology portfolio. J. Control Rel.245, 177 (2017).
- Lammers T Kiessling F Hennink WE Storm G . Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Control. Rel.161 (2), 175–187 (2012).
- Sagnella SM McCarroll JA Kavallaris M . Drug delivery: beyond active tumot targeting. Nanomedicine10 (6), 1131–1137 (2014).
- Roy A Li SD . Modifying the tumor microenvironment using nanoparticle therapeutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechno.8 (6), 891–908 (2016).
- Dai Y Xu C Sun X Chen X . Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumot microenvironment. Chem. Soc. Rev.46 (12), 3830–3852 (2017).
- Petrenko VA Gillespie JW . Paradigm shift in bacteriophage-mediated delivery of anticancer drugs: from targeted ‘magic bullets’ to self-navigated ‘magic missiles’. Exp. Opin. Drug Deliv.14 (3), 373–384 (2017).
- Petrenko VA Jayanna PK . Phage protein-targeted cancer nanomedicines. FEBS Lett.588 (2), 341–349 (2014).
- Yao VJ D'Angelo S Butler KS et al. Ligand-targeted theranostic nanomedicines against cancer. J. Control. Rel.240, 267–286 (2016).
- Murase Y Asai T Katanasaka Y et al. A novel DDS strategy, ‘dual-targeting’, and its application for antineovascular therapy. Cancer Lett.287 (2), 165–171 (2010).
- Takara K Hatakeyama H Kibria G Ohga N Hida K Harashima H . Size-controlled, dual-ligand modified liposomes that target the tumor vasculature show promise for use in drug-resistant cancer therapy. J. Control. Rel.162 (1), 225–232 (2012).
- Liu DX Auguste DT . Cancer targeted therapeutics: from molecules to drug delivery vehicles. J. Control. Rel.219, 632–643 (2015).
- Mei L Fu L Shi KR et al. Increased tumor targeted delivery using a multistage liposome system functionalized with RGD, TAT and cleavable PEG. Int. J. Pharmaceut.468 (1–2), 26–38 (2014).
- Daquinag AC Dadbin A Snyder B et al. Non-glycanated Decorin is a drug target on human adipose stromal cells. Mol. Ther. Oncolytics6, 1–9 (2017).
- Adams GP Schier R McCall AM et al. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res.61 (12), 4750–4755 (2001).
- Smith GP Petrenko VA . Phage display. Chemical Rev.97 (2), 391–410 (1997).
- Xue J Zhao Z Zhang L et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nano12 (7), 692–700 (2017).
- Gross AL Gillespie JW Petrenko VA . Promiscuous tumor targeting phage proteins. Protein Eng. Des. Sel.29 (3), 93–103 (2016).
- Ruoslahti E . RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol.12, 697–715 (1996).
- Wu CH Liu IJ Lu RM Wu HC . Advancement and applications of peptide phage display technology in biomedical science. J. Biomed. Sci.23, 14 (2016).
- Ruoslahti E . Tumor penetrating peptides for improved drug delivery. Adv. Drug Deliv. Rev. doi:10.1016/j.addr.2016.03.008 (2017) ( Epub ahead of print).
- Petrenko VA Smith GP Gong X Quinn T . A library of organic landscapes on filamentous phage. Protein Eng.9 (9), 797–801 (1996).
- Fagbohun OA Bedi D Grabchenko NI Deinnocentes PA Bird RC Petrenko VA . Landscape phages and their fusion proteins targeted to breast cancer cells. Protein Eng. Des. Sel.25 (6), 271–283 (2012).
- Mongelard F Bouvet P . Nucleolin: a multiFACeTed protein. Trends Cell Biol.17 (2), 80–86 (2007).
- Koutsioumpa M Papadimitriou E . Cell surface nucleolin as a target for anti-cancer therapies. Recent Pat. Anticancer Drug Discov.9 (2), 137–152 (2014).
- Wang T Yang S Mei LA et al. Paclitaxel-loaded PEG-PE-based micellar nanopreparations targeted with tumor-specific landscape phage fusion protein enhance apoptosis and efficiently reduce tumors. Mol. Cancer Ther.13 (12), 2864–2875 (2014).
- Wang T Hartner WC Gillespie JW et al. Enhanced tumor delivery and antitumor activity in vivo of liposomal doxorubicin modified with MCF-7-specific phage fusion protein. Nanomedicine10 (2), 421–430 (2014).
- Zou M Zhang L Xie Y Xu W . NGR-based strategies for targeting delivery of chemotherapeutics to tumor vasculature. Anticancer Agents Med. Chem.12 (3), 239–246 (2012).
- Gillespie JW Wei L Petrenko VA . Selection of lung cancer-specific landscape phage for targeted drug delivery. Comb. Chem. High Throughput Screen.19 (5), 412–422 (2016).
- Rajotte D Arap W Hagedorn M Koivunen E Pasqualini R Ruoslahti E . Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J. Clin. Invest.102 (2), 430–437 (1998).
- Meszaros B Simon I Dosztanyi Z . Prediction of protein binding regions in disordered proteins. PLoS Comput. Biol.5 (5), 18 (2009).
- Neduva V Russell RB . Linear motifs: evolutionary interaction switches. FEBS Lett.579 (15), 3342–3345 (2005).
- Vendruscolo M Paci E Dobson CM Karplus M . Three key residues form a critical contact network in a protein folding transition state. Nature409 (6820), 641–645 (2001).
- Marvin DA Symmons MF Straus SK . Structure and assembly of filamentous bacteriophages. Prog. Biophys. Mol. Biol.114 (2), 80–122 (2014).
- Smith GP . Chapter 1. The phage nanoparticle toolkit. In : Phage Nanobiotechnology. The Royal Society of Chemistry, 1–11 (2011).
- Opella SJ . Chapter 2. The roles of structure, dynamics and assembly in the display of peptides on filamentous bacteriophage. In : Phage Nanobiotechnology. The Royal Society of Chemistry, 12–32 (2011).
- Makowski L . Chapter 3. Quantitative analysis of peptide libraries. In : Phage Nanobiotechnology. The Royal Society of Chemistry, 33–54 (2011).
- Olofsson L Ankarloo J Andersson PO Nicholls IA . Filamentous bacteriophage stability in non-aqueous media. Chem. Biol.8 (7), 661–671 (2001).
- Holliger P Riechmann L Williams RL . Crystal structure of the two N-terminal domains of g3p from filamentous phage fd at 1.9 A: evidence for conformational lability. J. Mol. Biol.288 (4), 649–657 (1999).
- Brigati JR Petrenko VA . Thermostability of landscape phage probes. Anal. Bioanal. Chem.382 (6), 1346–1350 (2005).
- Molenaar TJ Michon I de Haas SA van Berkel TJ Kuiper J Biessen EA . Uptake and processing of modified bacteriophage M13 in mice: implications for phage display. Virology293 (1), 182–191 (2002).
- Yip YL Hawkins NJ Smith G Ward RL . Biodistribution of filamentous phage-Fab in nude mice. J. Immunol. Methods225 (1–2), 171–178 (1999).
- Zou J Dickerson MT Owen NK Landon LA Deutscher SL . Biodistribution of filamentous phage peptide libraries in mice. Mol. Biol. Rep.31 (2), 121–129 (2004).
- Pasqualini R Koivunen E Ruoslahti E . Alpha v integrins as receptors for tumor targeting by circulating ligands. Nat. Biotechnol.15 (6), 542–546 (1997).
- Clough G . Relationship between microvascular permeability and ultrastructure. Prog. Biophys. Mol. Biol.55 (1), 47–69 (1991).
- Babickova J Tothova L Boor P Celec P . In vivo phage display–a discovery tool in molecular biomedicine. Biotechnol Adv.31 (8), 1247–1259 (2013).
- Krag DN Fuller SP Oligino L et al. Phage-displayed random peptide libraries in mice: toxicity after serial panning. Cancer Chemother. Pharmacol.50 (4), 325–332 (2002).
- Krag DN Shukla GS Shen GP et al. Selection of tumor-binding ligands in cancer patients with phage display libraries. Cancer Res.66 (15), 7724–7733 (2006).
- Dias-Neto E Nunes DN Giordano RJ et al. Next-generation phage display: integrating and comparing available molecular tools to enable cost-effective high-throughput analysis. PLoS ONE4 (12), e8338 (2009).
- Staquicini FI Cardo-Vila M Kolonin MG et al. Vascular ligand-receptor mapping by direct combinatorial selection in cancer patients. Proc. Natl Acad. Sci. USA108 (46), 18637–18642 (2011).
- Scott JK Smith GP . Searching for peptide ligands with an epitope library. Science249 (4967), 386–390 (1990).
- Yao VJ Ozawa MG Trepel M Arap W McDonald DM Pasqualini R . Targeting pancreatic islets with phage display assisted by laser pressure catapult microdissection. Am. J. Pathol.166 (2), 625–636 (2005).
- Folkman J . Tumor angiogenesis: therapeutic implications. N. Engl. J. Med.285 (21), 1182–1186 (1971).
- Schluesener HJ Xianglin T . Selection of recombinant phages binding to pathological endothelial and tumor cells of rat glioblastoma by in-vivo display. J. Neurol. Sci.224 (1–2), 77–82 (2004).
- Landon LA Deutscher SL . Combinatorial discovery of tumor targeting peptides using phage display. J. Cell Biochem.90 (3), 509–517 (2003).
- Petrenko VA Smith GP . Vectors and modes of display. In : Phage Display in Biotechnology and Drug Discovery. SachdevSSidhuCRG ( Eds). CRC Press, Taylor & Francis Group, Boca Raton, London, New York (2015).
- Qi H Lu H Qiu HJ Petrenko V Liu A . Phagemid vectors for phage display: properties, characteristics and construction. J. Mol. Biol.417 (3), 129–143 (2012).
- Petrenko VA Jayanna PK . Phage-mediated drug delivery. In : Phage Nanobiotechnology. PetrenkoVASmithGP ( Eds). RSC Publishing, 55–82 (2011).
- Gillespie JW Gross AL Puzyrev AT Bedi D Petrenko VA . Combinatorial synthesis and screening of cancer cell-specific nanomedicines targeted via phage fusion proteins. Front. Microbiol.6, 628 (2015).
- Knez K Noppe W Geukens N et al. Affinity comparison of p3 and p8 peptide displaying bacteriophages using surface plasmon resonance. Anal. Chem.85 (21), 10075–10082 (2013).
- Xue J Zhao Z Zhang L et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol.12 (7), 692–700 (2017).
- Jayanna PK Torchilin VP Petrenko VA . Liposomes targeted by fusion phage proteins. Nanomedicine5 (1), 83–89 (2009).
- Opella SJ . The roles of structure, dynamics and assembly in the display of peptides on filamentous bacteriophage. In : Phage Nanobiotechnology. PetrenkoVASmithGP ( Eds). RSC Publishing, 12–32 (2011).
- Vos WL Nazarov PV Koehorst RB Spruijt RB Hemminga MA . From ‘I’ to ‘L’ and back again: the odyssey of membrane-bound M13 protein. Trends Biochem. Sci.34 (5), 249–255 (2009).
- Bedi D Gillespie JW Petrenko VA Jr et al. Targeted delivery of siRNA into breast cancer cells via phage fusion proteins. Mol. Pharm.10 (2), 551–559 (2013).
- Wang T Yang S Petrenko VA Torchilin VP . Cytoplasmic delivery of liposomes into MCF-7 breast cancer cells mediated by cell-specific phage fusion coat protein. Mol. Pharm.7 (4), 1149–1158 (2010).
- Sanchez-Purra M Ramos V Petrenko VA Torchilin VP Borros S . Double-targeted polymersomes and liposomes for multiple barrier crossing. Int. J. Pharm.511 (2), 946–956 (2016).
- Minenkova OO Ilyichev AA Kishchenko GP Petrenko VA . Design of specific immunogens using filamentous phage as the carrier. Gene128 (1), 85–88 (1993).
- Petrenko VA Smith GP Mazooji MM Quinn T . Alpha-helically constrained phage display library. Protein Eng.15 (11), 943–950 (2002).
- Kuzmicheva GA Jayanna PK Sorokulova IB Petrenko VA . Diversity and censoring of landscape phage libraries. Protein Eng. Design & Selection22 (1), 9–18 (2009).
- Tomkins MR Liao DS Docoslis A . Accelerated detection of viral particles by combining AC electric field effects and micro-Raman spectroscopy. Sensors (Basel)15 (1), 1047–1059 (2015).
- Yang SH Chung W-J McFarland S Lee S-W . Assembly of bacteriophage into functional materials. Chem. Record13, 43–59 (2013).
- Gillespie JW Petrenko VA . Molecular toolkits for engineering of self-navigating drug delivery vehicles. In : Conference: TechConnect World Innovation Conference & Expo TechConnect Briefs 2017, at Technical Proceedings of the TechConnect World Innovation Conference & Expo TechConnect Briefs 2017. TechConnect.org, ISBN 978–0–9988782–0–1, TechConnect, Gaylord National Hotel & Convention Center, Washington, DC (2017).
- Petrenko VA Gillespie JW . Self-navigating drug delivery nanovehicles driven by polyvalent multifunctional phages and their promiscuous proteins. In : Technical Proceedings of the 2017 TechConnect World, including the Nanotech 2017 Conference. TechConnect, Gaylord National Hotel & Convention Center, Washington, DC (2017).
- Petrenko VA Vodyanoy VJ . Phage display for detection of biological threat agents. J. Microbiol. Methods53 (2), 253–262 (2003).
- Wang T Petrenko VA Torchilin VP . Paclitaxel-loaded polymeric micelles modified with MCF-7 cell-specific phage protein: enhanced binding to target cancer cells and increased cytotoxicity. Mol. Pharm.7 (4), 1007–1014 (2010).
- Papavoine CH Christiaans BE Folmer RH Konings RN Hilbers CW . Solution structure of the M13 major coat protein in detergent micelles: a basis for a model of phage assembly involving specific residues. J. Mol. Biol.282 (2), 401–419 (1998).
