Abstract
Hepatocellular carcinoma (HCC) is a major pathological type of primary liver cancer. Sorafenib has demonstrated definite efficacy in targeted therapy for HCC. However, when treatment with sorafenib fails, suitable drugs must be found for further treatment. This article reports a case of an HCC patient who was treated with angiogenesis inhibitor axitinib and c-Met inhibitor cabozantinib following treatment with sorafenib. The report focuses on clinical treatment and toxicity. Rational application of targeted therapy is also explored.
Introduction
Primary liver cancer (PLC) is a malignant tumor with high incidence in China, and the number of new PLC cases and deaths in China each year accounts for approximately 54% of the total number of cases worldwide.Citation1 According to “2013 Annual Report of Cancer Cases Registration”, the incidence rate of liver cancer was 28.71/105 in China, taking the 4th place of all malignant tumors (11.60%), while the mortality of liver cancer was 23.76/10,Citation5 taking the 2nd place of all cancers (15.97%), only exceeded by lung cancer.Citation2 Hepatocellular carcinoma (HCC) is a major pathological type of PLC. Targeted therapy for HCC has attracted extensive attention in current clinical research. Although the use of sorafenib in clinical treatment has been well defined, most drugs are still at the clinical exploratory stage. This article reports a case of an HCC patient who was treated with angiogenesis inhibitor axitinib and c-Met inhibitor cabozantinib following treatment with sorafenib. The report focuses on clinical therapy and toxicity in the hope of providing a reference for future clinical activities. The patient's family was informed of this report and provided their consent. This report was also approved by the First Affiliated Hospital of Dalian Medical University.
Case Report
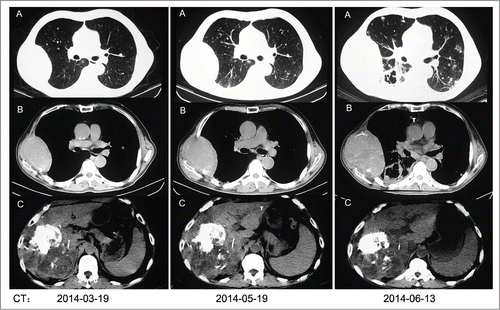
Male, aged at 64 y old, complained of discomfort in upper abdomen in early September 2013, without significant abdominal pain, fever, jaundice, nausea, diarrhea, or constipation. The patient did not pay much attention due to the mildness of the symptoms. The symptoms were not relieved after one week. Enhanced CT examination, performed on September 8, 2013, indicated a space-occupying mass in the right lobe of the liver, and liver cancer was considered. Multiple small nodules were found in the liver, suggesting intrahepatic metastases and multi-centered liver cancer. Alpha-fetoprotein (AFP): 2569 IU/ml, CA19-9: 54.79 U/ml. Alanine aminotransferase (ALT) 140 U/L, Aspartate aminotransferase (AST) 88 U/L. No surgical contradictions were found after group consultation. The patient underwent percutaneous transcatheter arterial chemoembolization on September 12, 2013. Celiac arteriography through the RH hepatic duct showed giant tumor staining in the right lobe of the liver, with the right hepatic artery as the feeding artery. Perfusion with a mixture of Oxaliplatin 100 mg+ Pirarubicin 10 mg+ camptothecin 5 mg was conducted using a Progreat microcatheter through the tumor feeding artery, followed by embolism with Lipiodol ultraliquid 25 ml+THP 10 mg+HCPT 5 mg and final embolism with 300–500 U PVA. Arteriography was repeated with satisfactory lipiodol accumulation. Chemotherapy or targeted therapy was recommended due to an AFP level of 1930 IU/ml 4 d after treatment, but the patient refused the treatment option. Therefore, interventional treatment was administered 3 times, with the last treatment on November 22. At the end of December, a gradually growing mass was found incidentally in the right side of the neck, indicating metastasis and disease progression. With his consent, the patient started to take sorafenib on January 4, 2014, and the dosing regimen was oral administration (po) of 40 mg twice a day (bid). Adverse reactions were observed, including ulcerative oral mucositis, rash, polyhidrosis, fatigue, loss of appetite, emesis, and elevated blood pressure, which were improved after symptomatic adjunct treatments. The patient did not show improvement in general condition after taking sorafenib for 1 month, with a hepatalgia of 5 on the numeric rating scale (NRS), hard masses in the right chest wall and neck with tenderness, and pain and numbness in the right upper arm of 7 on the NRS. Sustained-release morphine hydrochloride tablets were administered orally at a dose of 90 mg every 12 hours. On March 8, 2014, the AFP test was repeated, with a result of 6654 IU/ml. The masses in the right chest wall and neck grew significantly larger, indicating disease progression. The patient was offered 2 options, continued treatment with sorafenib 40 mg bid po for 1 month followed by efficacy evaluation or the use of targeted therapeutic drugs or chemotherapy. The patient refused to receive chemotherapy and started to take axitinib, 7 mg, bid, po, on March 17, 2014. The CT scan taken on March 19 indicated primary liver cancer, rib bone mass destruction, lung metastasis, and intrahepatic metastasis. Compared to previous conditions, the hepatic lesions did not show progression, even though distant and bone metastases were observed. The side effects of treatment with axitinib were somnolence and oral ulcers. During the treatment with axitinib, the patient experienced improved quality of life, with better physical conditions and an increase of 15 points in the Activities of Daily Living (ADL) score, improved appetite, and alleviated cancer pain. The dose of the analgesic sustained-release morphine hydrochloride tablets was reduced from 90 mg q12h po to 60 mg q12h po. On April 21, 2014, the AFP test was repeated and showed a level of 5173 IU/ml, indicating effective treatment. The patient continued the treatment with axitinib. On May 19, 2014, the masses in the neck and right chest wall became significantly larger, and the AFT level was found to be abnormally elevated at 6846 IU/ml. The CT scan was repeated on the same day, indicating aggravated rib bone mass destruction, enlarged soft tissue masses, increased number of lung metastasis loci, and disease progression. The patient was advised to switch to different targeted therapeutic drug. Cabozantinib 50 mg, qd, po was added on May 25, 2014, with concurrent dosing of axitinib 7 mg, bid, po. After treatment for 6 consecutive days, the patient exhibited symptoms of fatigue and hypodynamia. The patient subsequently stopped taking axitinib and continued with cabozantinib 50 mg bid for 2 consecutive days. The patient exhibited significantly aggravated hypodynamia and became bedridden, with nausea, emesis, somnolence, shallow breath, and a ZPS of 4. The patient was admitted to the ER on June 2, 2014. Blood gas analysis showed pH at 7.329, pO2 at 55 mmHg, pCO2 at 41.4 mmHg, SO2 at 82.9%, BE(B) at −4.0 mmol/l, and HCO3-act at 21.1 mmol/l. Biochemistry tests showed GLU at 5.50 mmol/l, urea at 25.4 mmol/l, CRE at 344 μmol/l, K+ at 6.50 mmol/l, ALB at 33.3 g/L, ALT at 309 U/L, AST at 771 U/L, ALP at 983 U/L, γ-GT at 812 IU/mL, and D-dimer at 3760 ug/L FUE. The patient exhibited impaired hepatic and renal functions, with a diagnosis of (1) lung and bone metastases of stage D primary liver cancer, (2) chronic type B hepatitis, (3) acute liver injury, (4) acute renal injury (AKI) stage 3, (5) type I respiratory failure, and (6) electrolyte disturbance with hyperkalemia. The patient was hospitalized to complete related tests and was administered symptomatic adjunct treatments including magnesium isoglycyrrhizinate and reduced glutathione for liver protection, Haikunshenxi capsules and dobesilate for renal protection, oxygen inhalation, analgesics, and nutrient supplement fluids to correct acid-base imbalance and electrolyte disturbance, with monitoring of hepatic and renal functions. During hospitalization, the patient exhibited difficulties in breathing. A CT scan indicated aggravated rib bone mass destruction, and the soft tissue masses grew larger, with a significantly increased number of lung metastasis loci showing formed cavities, indicating disease progression (). The tests on June 17, 2014 showed urea at 6.5 mmol/l, CRE at 55 μmol/l, K+ at 3.30 mmol/l, ALB at 21.1 g/L, ALT at 36 U/L, AST 58 U/L, ALP 241 U/L, and γ-GT at 348 IV/mL, indicating significantly improved hepatic and renal functions. The ZPS score was 3. The patient's family members requested active treatment, and the patient took axitinib 7 mg bid po without instruction of the physician. On July 4, 2014, the patient experienced breathing difficulty again and received symptomatic treatment at a local hospital. On July 10, the patient was declared clinically dead, with a total survival of 10 months.
Discussion
Clinical diagnosis and the formulation of a treatment plan for HCC are primarily based on imaging examination.Citation3 Early-stage HCC patients are mainly treated with surgery or local ablation, and advanced patients are usually treated through interventional therapy, radiotherapy, chemotherapy and targeted therapy to control disease progression and prolong survival.
The molecularly targeted therapy of HCC has drawn extensive attention in clinical studies, among which research on angiogenesis inhibitors is the most fruitful, highlighted by drugs such as sunitinib, brivanib, linifanib, regorafenib, ramucirumab, bevacizumab, and axitinib. Inhibitors of the epidermal growth factor receptor (EGFR), mTOR signaling pathway, c-Met, MEK, insulin-like growth factor (IGF) signaling pathway, and histone deacetylase (HDAC) are also under study as targeted therapies.Citation4
Sorafenib has a dual anti-tumor effect. First, it inhibits the RAF/MEK/ERK signal transduction pathway, directly suppressing tumor growth; second, it blocks tumor angiogenesis via inhibiting vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor (PDGF), indirectly suppressing tumor growth. Sorafenib is the only molecularly targeted drug with confirmed efficacy in the treatment of unresectable and distant metastatic advanced HCC.Citation5 It is worth investigating whether other anti-angiogenic drugs are available to control disease progression when treatment with sorafenib fails. In recent years, although the VEGFR and PDGFR targeted drugs sunitinibCitation6,7 and linifanibCitation8 and the VEGFR and fibroblast growth factor receptor (FGFR) targeted drug brivanibCitation9,10 were announced, they failed in the first-line treatment of liver cancer, and the combination of bevacizumab and erlotinib did not achieve significant efficacy in the Phase III clinical trial as a first-line treatment for advanced HCC,Citation11,12 encouraging achievements have been reported with other targeted therapeutic drugs.
Axitinib is a multi-target tyrosine kinase inhibitor, inhibiting vascular endothelial growth factor receptors VEGFR1, VEGFR2, VEGFR3, platelet-derived growth factor receptor PDGFR, and c-KIT. It has been approved by the US. Food and Drug Administration (FDA) for the treatment of advanced kidney cancer. Preclinical studiesCitation13 showed that the drug exhibited activity against liver cancer cells. A multi-center, global, randomized, double-blind study with advanced liver cancer patients who had undergone one failed anti-angiogenic therapy compared the efficacy of axitinib in combination with best supportive care (BSC) with the efficacy of placebo in combination with BSC. The results are still pending. The common adverse events in the axitinib group were hypertension, fatigue, dysphonia, and hypothyroidism, displaying good safety of axitinib. Another small molecule targeted drug, regorafenib, has been approved by the US. FDA for the treatment of metastatic colorectal cancer, in combination with chemotherapy. It exhibited good safety and anti-tumor efficacy as the second-line therapy for advanced HCC patients suffering progression after first-line treatment with sorafenib. Currently, regorafenib is in a global, multi-center, randomized, controlled Phase III clinical trial as a second-line HCC treatment.Citation14
Tyrosine kinase receptor c-Met is overexpressed and abnormally activated in most cancers and some sarcomas, playing a role in tumorigenesis, tumor progression, invasion, and metastasis as well as resistance to chemotherapy. As a critical node protein in tumor signaling pathways, c-Met has attracted extensive attention.Citation15,16 Santoro et al.Citation17 reported the efficacy and safety of a c-Met inhibitor, tivantinib, as a second-line treatment for advanced liver cancer in “Lancet Oncol” in January 2013. The results showed that in patients with a high level of c-Met expression, the median time to progression (TTP) of the patients receiving tivantinib and placebo were 2.7 months and 1.4 months (P = 0.03), respectively. The study subsequently reported the overall survival (OS) of patients. For HCC patients with high expression of c-Met, the OS was 7.2 months in the tivantinib group and 3.8 months in the placebo group (HR = 0.38, P = 0.01). Cabozantinib (XL184) is a multi-kinase inhibitor targeting MET, VEGFR2, AXL, Tie2, KIT, FLT3 and RET. Currently, Phase III clinical trials of the c-Met inhibitors tivantinib and cabozantinib as the second-line treatment for HCC with high c-Met expression are ongoing and are expected to bring new hope to cancer patients. The dose-limiting toxicities included hand-foot syndrome, mucositis, and abnormally elevated AST, ALT and lipase. Other common adverse reactions include diarrhea, hand-foot syndrome, loss of weight and appetite, nausea, and fatigue.
In this case study, the patient was treated with sorafenib for 2.5 months. The patient exhibited disease progression but no improvement in quality of life, which could be a result of possible primary drug resistance. Therefore, it was worthwhile to consider switching to an alternative anti-angiogenic drug to clinically control the disease progression. However, given the lack of special treatment measures and the patient's refusal of chemotherapy, axitinib was used for treatment. It was encouraging that the patient experienced improved quality of life, higher ADL score, lower AFP, alleviated pain, indicating that the treatment was effective. The side effects were clinically controllable. Unfortunately, the control of the disease lasted only a short time, as disease progression was found at the 2-month check-up. Considering disease progression, we did not immediately stop axitinib during the test of cabozantinib. We found that the disease progression was slow, with relatively small side effects, suggesting that axitinib could also play an active role in disease control. However, it was unexpected that the patient had acute hepatic and renal damage. After treatment, the patient was out of danger; the axitinib treatment resumed, and the patient's symptoms were slightly improved. Although elevated AST and ALT are the dose-limiting toxicity of cabozantinib, whether they are related to the combination with axitinib is still unclear. The combination of the 2 drugs may present some risks, which should be avoided clinically.
The treatment of primary liver cancer involves a variety of therapies and different specialties. However, the therapeutic efficacy has been unsatisfactory. The selection of appropriate treatments and timing requires strengthened communication and collaboration of relevant disciplines, which is an important way to further improve the therapeutic efficacy. The targeted therapy model has drawn significant attention, becoming a trend in treatment. What should receive greater focus is how to develop an optimal personalized comprehensive treatment plan and avoid improper treatment or over-treatment, which will continue to improve the final outcome of clinical efficacy and benefit more liver cancer patients.
Disclosures of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 6l:69-90; PMID:21296855; http://dx.doi.org/10.3322/caac.20107
- Chen WQ, Zhang SW, Zeng HM, Zheng RS, Zou XN, Zhao P, Wu LY, Li GL, Hao J. Report of Cancer Incidence and Mortality in China, 2010. China Cancer 2014; 23:1-10.
- Llovet JM, Ducreux M, Lencioni R, Di Bisceglie AM, Galle PR, Dufour JF, Greten TF, Raymond E, Roskams T, De Baere T, et al.. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56:908-943.
- Reataza M, Imagawa DK. Advances in managing hepatocellular carcinoma. Front Med 2014; 8:175-189; PMID:24810646; http://dx.doi.org/10.1007/s11684-014-0332-4
- Peck-Radosavljevic M. Drug therapy for advanced-stage liver cancer. Liver Cancer 2014; 3:125-131; PMID:24945003; http://dx.doi.org/10.1159/000343868
- Koeberle D, Montemurro M, Samaras P, Majno P, Simcock M, Limacher A, Lerch S, Kovàcs K, Inauen R, Hess V, et al. Continuous Sunitinib treatment in patients with advanced hepatocellular carcinoma: a Swiss Group for Clinical Cancer Research (SAKK) and Swiss Association for the Study of the Liver (SASL) multicenter phase II trial (SAKK 77/06). Oncologist 2010; 15:285-292; PMID:20203173; http://dx.doi.org/10.1634/theoncologist.2009-0316
- Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol 2013; 31:4067-4075; PMID:24081937; http://dx.doi.org/10.1200/JCO.2012.45.8372
- Llovet JM1, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res 2014; 20:2072-2079; PMID:24589894; http://dx.doi.org/10.1158/1078-0432.CCR-13-0547
- Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, Hsu CH, Hu TH, Heo J, Xu J, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol 2013; 31:3517-3524; PMID:23980084; http://dx.doi.org/10.1200/JCO.2012.48.4410
- Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, Kang YK, Assenat E, Lim HY, Boige V, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol 2013; 31:3509-3516; PMID:23980090; http://dx.doi.org/10.1200/JCO.2012.47.3009
- Philip PA, Mahoney MR, Holen KD, Northfelt DW, Pitot HC, Picus J, Flynn PJ, Erlichman C. Phase 2 study of bevacizumab plus erlotinib in patients with advanced hepatocellular cancer. Cancer 2012; 118:2424-2430; PMID:21953248; http://dx.doi.org/10.1002/cncr.26556
- Govindarajan R, Siegel E, Makhoul I, Williamson S. Bevacizumab and erlotinib in previously untreated inoperable and metastatic hepatocellular carcinoma. Am J Clin Oncol 2013; l 36:254-257; http://dx.doi.org/10.1097/COC.0b013e318248d83f
- Chen L1, Liu H, Liu J, Zhu Y, Xu L, He H, Zhang H, Wang S, Wu Q, Liu W, et al. Klotho endows hepatoma cells with resistance to anoikis via VEGFR2/PAK1 activation in hepatocellular carcinoma. PLoS One 2013; 8:e58413; PMID:23516476; http://dx.doi.org/10.1371/journal.pone.0058413
- Bruix J1, Tak WY, Gasbarrini A, Santoro A, Colombo M, Lim HY, Mazzaferro V, Wiest R, Reig M, Wagner A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer 2013; 49:3412-3419; PMID:23809766; http://dx.doi.org/10.1016/j.ejca.2013.05.028
- Scagliotti GV1, Novello S, von Pawel J. The emerging role of MET/HGF inhibitors in oncology. Cancer Treat Rev 2013; 39:793-801; PMID:23453860; http://dx.doi.org/10.1016/j.ctrv.2013.02.001
- Xiang Q, Chen W, Ren M, Wang J, Zhang H, Deng DY, Zhang L, Shang C, Chen Y. Cabozantinib Suppresses Tumor Growth and Metastasis in Hepatocellular Carcinoma by a Dual Blockade of VEGFR2 and MET. Clin Cancer Res 2014; 20:2959-2970; PMID:24700742; http://dx.doi.org/10.1158/1078-0432.CCR-13-2620
- Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethem JL, Van Vlierberghe H, Trojan J, Kolligs FT, Weiss A, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebocontrolled phase 2 study. Lancet Oncol 2013; 14:55-63; PMID:23182627; http://dx.doi.org/10.1016/S1470-2045(12)70490-4