Abstract
Deciphering AUA codons is a difficult task for organisms, because AUA and AUG specify isoleucine (Ile) and methionine (Met), separately. Each of the other purine-ending sense co-don sets (NNR) specifies a single amino acid in the universal genetic code. In bacteria and archaea, the cytidine derivatives, 2-lysylcytidine (L or lysidine) and 2-agmatinylcytidine (agm2C or agmatidine), respectively, are found at the first letter of the anticodon of tRNAIle responsible for AUA codons. These modifications prevent base pairing with G of the third letter of AUG codon, and enable tRNAIle to decipher AUA codon specifically. In addition, these modifications confer a charging ability of tRNAIle with Ile. Despite their similar chemical structures, L and agm2C are synthesized by distinctive mechanisms and catalyzed by different classes of enzymes, implying that the analogous decoding systems for AUA codons were established by convergent evolution after the phylogenic split between bacteria and archaea-eukaryotes lineages following divergence from the last universal common ancestor (LUCA).
General Principle of The Decoding System
In the universal genetic code, 20 species of amino acids are specified by 61 sense codons; 18 amino acids are encoded by multiple (2 to 6) codons, while Met and Trp are specified by AUG and UGG, respectively. The genetic code is essentially composed of family boxes and 2-codon sets. The first and second letters of a codon determine the species of amino acid. The third letter of a codon in a family box does not affect the amino acid specified. However, different species of amino acids are specified by pyrimidine-ending 2-codon sets (NNY) bearing U or C at the third letter, or by purine-ending 2-codon sets (NNR) bearing A or G at the third letter. Only Ile is encoded by 3 codons, AUU, AUC, and AUA.
Decoding takes place on the A-site of the ribosome. The A-site codon on mRNA is recognized by the anticodon of aminoacyl-tRNA. In this interaction, the second and first letters of the codon form base-pairs with the second and third letters (positions 35 and 36) of the anticodon, respectively, via Watson–Crick (WC) pairing rules. These two WC pairings in the codon-anticodon helix are specifically monitored by the conserved bases A1492, A1493, and G530 in the decoding center of 16S rRNA through a type of A-minor interaction.Citation1,2 These interactions trigger a conformational change of the 30S subunit from the open form to the closed form, directly stimulating GTP hydrolysis of EF-Tu, which eventually dissociates from the aminoacyl-tRNA on the ribosome.Citation3,4 On the other hand, non-WC pairing, such as G-U pairing, occurs between the third letter of the codon and the first letter of the anticodon (position 34). Such irregular pairing is called ‘wobble’ pairing.Citation5 Wobble pairing is a well-developed, sophisticated system by which 61 sense codons are deciphered by a limited number of tRNA species. In contrast to the first and second base pairs in the codon-anticodon helix, the third base pair is not strictly recognized by the residues of 16S rRNA and there is sufficient room in the decoding center to accept the wobble pairing.Citation1,2 Therefore, a number of modified bases, called wobble modifications, are found at position 34 of tRNAs.Citation6-8 The wobble modifications play critical roles in codon recognition, and allow organisms to develop their own decoding system.
AUA Decoding by Modified Bases in 3 Domains of Life
It is a difficult task for organisms to differentially decipher the AUA codon as Ile and the AUG codon as Met, because all other NNR sense codon sets specify just one amino acid in the universal genetic code. In general, tRNAs with a U or modified U (U*) at the wobble position can read both NNA and NNG codons.Citation6 For tRNAIle with the UAU or U*AU anticodon, both AUA and AUG codons are potentially deciphered as Ile, similar to other tRNAs responsible for NNR codons. However, there are exceptional organisms using the UAU anticodon (described later in this review). Thus, it is thought that organisms acquired unique mechanisms to separately decipher AUA codons and AUG codons. Although the mechanisms used to decipher AUA codons are different for each of the 3 domains of life, they all use modified bases at position 34 of tRNAIle.
In eukaryotes, inosine (I) () or pseudouridine (Ψ) () occurs at the wobble position of tRNAIle responsible for AUA codons.Citation6 I base-pairs with U, C, and A, facilitating the decoding of AUC, AUU and AUA codons by a single tRNAIle with an IAU anticodon (). Another tRNAIle with a ΨAΨ anticodon contributes to reading the AUA codon (), but it is not clear whether this anticodon can prevent AUG decoding.
Figure 1. Wobble modifications in tRNAs required to decipher AUA codons in 3 domains of life. (A) Chemical structures of modified nucleosides found at the first letter of anticodons in tRNAs responsible for AUA codons. (B) Anticodons with wobble modifications in tRNAs for AUA codons in 3 domains of life.
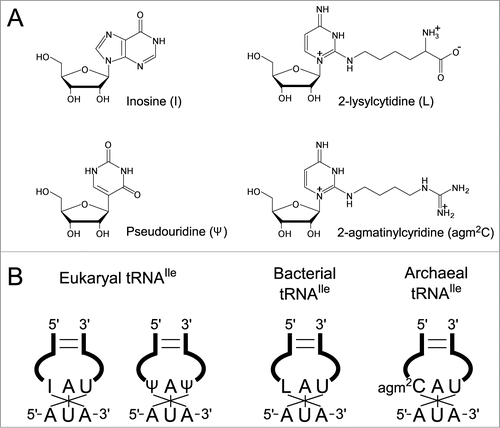
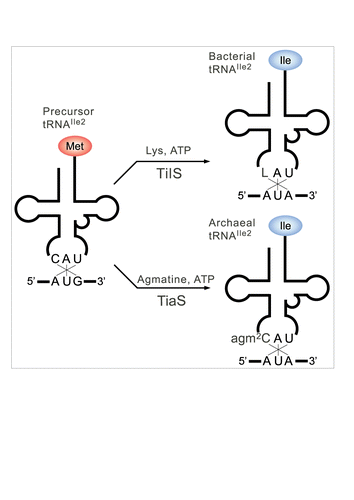
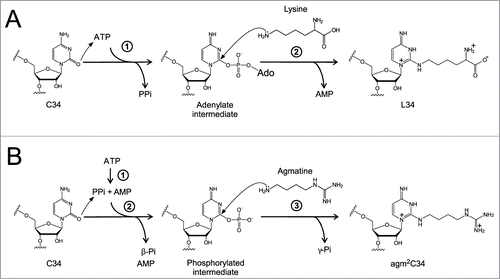
In almost all bacteria, lysidine (L) () occurs at the wobble position of tRNAIle responsible for the AUA codon.Citation9 L is a modified cytidine, in which the ϵ-amino group of lysine makes a covalent bond with the C2 carbon of cytosine. We previously discovered tRNAIle lysidine synthetase (TilS), which catalyzes L formation using ATP and L-lysine as substrates ().Citation10,11 The precursor tRNAIle bearing the CAU anticodon behaves like tRNAMet, because it can be recognized by methionyl-tRNA synthetase (MetRS) for charging Met and reading the AUG codon.Citation12 After L formation mediated by TilS, the LAU anticodon prevents recognition by MetRS, and in turn, works as a positive determinant for isoleucyl-tRNA synthetase (IleRS), enabling tRNAIle to charge Ile.Citation10 In addition, the LAU anticodon specifically recognizes the AUA codon by preventing recognition of the AUG codon.Citation12 Thus, a single L modification governs both amino acid and codon specificities. The requirement for the L modification for AUA decoding has been demonstrated biochemically as well as genetically, and tilS is an essential gene.Citation10,11
Figure 2. A single modification changes both codon and amino acid specificities of tRNAIle2. Precursor tRNAIle2 with CAU anticodon behaves like tRNAMet, since it accepts Met and decodes AUG codons. In bacteria, C34 is modified to L34 by TilS using Lys and ATP as substrates. tRNAIle2 with LAU anticodon accepts Ile and decodes AUA codons. In archaea, C34 is modified to agm2C34 by TiaS, using agmatine and ATP as substrates. tRNAIle2 with agm2CAU anticodon accepts Ile and decodes AUA codons.
In archaea, the mechanisms for decoding the AUA codon remained unknown for a long time. According to genome analyses, various archaea possessing tRNAIle genes responsible for the AUA codon have the CAT anticodon, implying the presence of a modified cytidine (C*) at the first letter of the anticodon. However, no L has been found in archaeal tRNAs,Citation13 and no homolog of tilS is found in any archaeal genome, which raises the enigmatic problem of how AUA codons are decoded in archaea. The presence of a modified C (C*) with an unknown structure was first reported in Haloferax volcanii tRNAIle,Citation14 and was also found in Haloarcula marismortui tRNAIle.Citation15 Several analytical methods were used to determine the atomic composition and chemical structure of C* isolated from H. marismortui (Euryarchaeota) and Sulfolobus tokodaii (Crenarchaeota), which results in its identification as 2-agmatinylcytidine (agm2C or agmatidine) ().Citation16 This was definitively confirmed by comparing it with the chemically-synthesized nucleoside. agm2C is a widely distributed modification in archaeal species, as evidenced by its occurrence in other Euryarchaeota including H. volcanii and Methanosarcina acetivorans.
Biogenesis of 2-Agmatinylcytidine
The chemical structure of agm2C led us to examine whether agmatine is a direct substrate for agm2C formation in the cell. We performed a metabolic labeling experiment to test for the incorporation of [13C, 15N]-labeled agmatine into agm2C of tRNAIle in S. tokodaii and M. acetivorans, and found that agmatine supplied in the medium was efficiently incorporated and used as a substrate for agm2C formation.Citation16 Agmatine is produced from arginine (Arg) via decarboxylation. We confirmed that agm2C could also be metabolically labeled with stable isotope-labeled Arg supplied in the medium. These results suggest that agm2C is generated from the extracellular agmatine or intracellular agmatine produced from Arg by decarboxylation.Citation16
To determine the enzyme responsible for agm2C formation, we used comparative genomics to narrow down the number of candidate genes, and identified COG1571, which encodes tRNAIle2 agm2C synthetase named TiaS.Citation16 After obtaining soluble recombinant protein of Archaeoglobus fulgidus TiaS, we successfully reconstituted agm2C on tRNAIle2 in the presence of ATP and agmatine as substrates ().Citation16 Agmatine is an intermediate metabolite for the major polyamines putrescine and spermidine.Citation17 These polyamines are important for a wide variety of cellular functions.Citation18 In particular, they contribute to adaptation to various stresses including high-temperature conditions.Citation19 Agmatine is mainly generated by the decarboxylation of arginine, which is catalyzed by arginine decarboxylase (pdaD). Disruption of pdaD in the hyperthermophilic euryarchaeon, Thermococcus kodakaraensis, resulted in growth only in media supplemented with agmatine,Citation20 demonstrating that agmatine is an essential metabolite for this archaeon. However, T. kodakaraensis with a deletion in pdaD was not rescued by supplying putrescine.Citation20 As spermidine can be produced from putrescine, the essentiality of agmatine cannot be explained by its supplier role for major polyamines. We clearly showed that extracellular or intracellular agmatine is directly used for agm2C modification of tRNAIle2. Based on these observations, the requirement of agmatine in euryarchaea can be attributed to its primary role in agm2C formation. tiaS was also found to be an essential gene in H. volcanii,Citation21 demonstrating that agm2C is an indispensable modification in archaeal species.
AUA Decoding by 2-Agmatinylcytidine
The ability to decipher the AUA codon by the agm2C modification was assessed in an in vitro assay of tRNA binding to the A-site of the ribosome.Citation16 In vitro transcribed A. fulgidus tRNAIle2 with a CAU anticodon specifically recognized the AUG codon, but did not bind to the AUA codon. The tRNAIle2 transcript was then modified in vitro by TiaS to form an agm2CAU anticodon. The modified tRNA recognized the AUA codon () but displayed reduced binding to the AUG codon, demonstrating that agm2C formation converted the codon specificity of tRNAIle2 from AUG to AUA. In addition, natural tRNAIle2 isolated from H. marismortui was charged with Ile (), and recognized the AUA codon on the ribosome.Citation22
L and agm2C have similar chemical structures (), in which the C2 carbon of the cytosine is conjugated with the primary amine of lysine or agmatine, respectively.Citation16 Conjugation of lysine or agmatine to the C2 carbon of cytosine by deoxidization induces a tautomeric change of cytosine from enamine to imine, with eventual protonation of N3. Thus, these modifications completely alter the proton donor-acceptor pattern of cytosine, preventing base-pairing with G, and enabling base-pairing with A. Two models of L-A base-pairing were proposed.Citation9 One model considers a canonical WC geometry such as U-A pairing (), whereas the other model suggests a wobble geometry such as U-G pairing (). However, structural study proposed the third model. The agm2C-A pair on the ribosome was visualized by solving the crystal structure of the Thermus thermophilus 70S ribosome in complex with archaeal tRNAIle2 and the AUA codon.Citation23 At the A-site, agm2C34 pairs with A3 of the AUA codon through a single hydrogen bond between N4 of agm2C34 and N1 of A3 (). This base-pair is stabilized by the interaction of the terminal amine of agm2C with the O4-oxygen of ribose at the second residue of the 3’ adjacent codon to the A-site codon. The configuration of the agm2C34-A3 pair is similar to that of the C34-A3 pair observed for the binding of the Hirsh suppressor tRNATrp to the UGA codon.Citation24 Considering that the C34-A3 pair is found in some mitochondrial decoding systems,Citation25 the C-A pair appears to be a natural decoding geometry.
Figure 3. Chemical structures of wobble cytidine modifications base-pairing with A. (A, B) Two models of L-A base pairings. As several resonance forms of L are possible, the positive charge of the base is delocalized. “R” represents a side chain of L. Imine tautomer of L pairs with A in a Watson-Crick geometry (A). Enamine tautomer of L pairs with A in a wobble geometry (B). (C) An agm2C34-A3 base pair at the ribosomal A-site observed in the crystal structure of a 70S ribosome in complex with tRNAIle2 and an AUA codon. An imine tautomer of agm2C is described as a representative of a number of possible tautomers. Coordinates are obtained from 3ZN7.
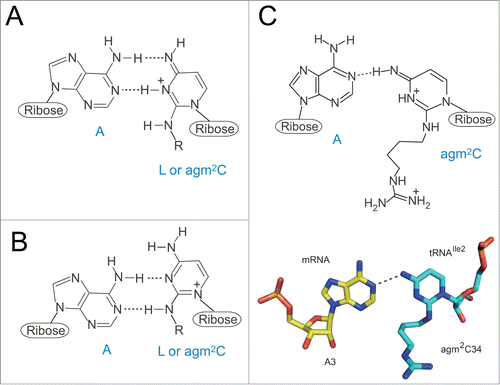
Although no structural information is available for the L34-A3 pairing so far, L34 has been suggested to recognize A3 with a similar geometry to that of agm2C34-A3 pair.Citation23 Crystal structures of 30 S subunit complexed with ASL bearing L and AUA codon, or 70 S ribosome complexed with intact tRNAIle2 with AUA codon will be necessary to solve this issue. Given that tautomerism of the modified base is susceptible to solvent condition with different pH and salt concentration,Citation26 further structural studies of the base pairing with different solvent conditions are needed for our deeper understanding of AUA decoding by the modified anticodons.
Role of Acceptor-stem recognition by TilS and TiaS
To date, the crystal structures are known for the ligand-free form of Escherichia coli TilS (EcTilS, Protein Data Bank [PDB] ID: 1NI5) and Aquifex aeolicus TilS (AaTilS, PDB ID: 1WY5),Citation27 AaTilS with AMPPNP (PDB ID: 2E21),Citation28 and with ATP, Mg2+ and L-lysine (PDB ID: 2E89),Citation28 and Geobacillus kaustophilus TilS (GkTilS) complexed with Bacillus subtilis tRNAIle2 (PDB ID: 3A2K) ().Citation29 GkTilS is composed of 3 structural domains. The N-type ATP pyrophosphatase in the N-terminal domain (NTD) is connected with 2 C-terminal domains, CTD1 and CTD2, through a helical linker (). EcTilS and GkTilS are Type-I TilS proteins that have both CTD1 and CTD2,Citation10 while AaTilS is a Type-II TilS that has only CTD1.Citation27 In the GkTilS-tRNAIle2 complex structure,Citation29 the NTD tightly recognizes the anticodon loop, while the CTD1 and CTD2 recognize the inner corner and the acceptor stem of tRNAIle2, respectively.
Figure 4 (See previous page). Comparison of tRNA recognition by TilS and TiaS. (A) Overall structure of the GkTilS-tRNAIle2 complex. The NTD, CTD1, and CTD2 are green, brown, and pink, respectively. The helical linker that connects NTD and CTD1 is gray. tRNAIle2 is yellow. (B) Overall structure of the AfTiaS in complex with tRNAIle2 and ATP. The TCKD, FLD, OBD, and ZRD are depicted in green, cyan, brown, and pink, respectively. ATP is represented as a stick model, and its carbon atoms are depicted in white. (C) Interactions between GkTilS CTD2 and the tRNAIle2 acceptor stem. (D) Interactions between AfTiaS ZRD and the tRNAIle2 acceptor stem. Hydrogen bonds are represented by dotted lines.
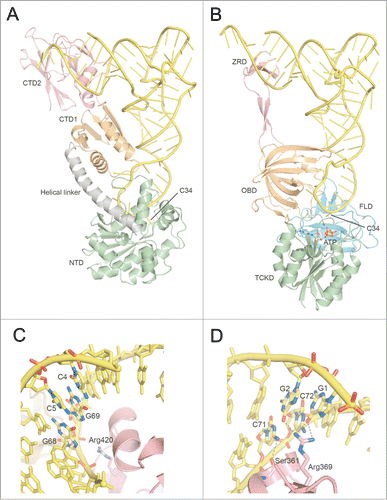
The ternary complex structure of A. fulgidus TiaS (AfTiaS) with tRNAIle2 and ATP (PDB ID: 3AMT), and the quaternary complex structure of AfTiaS with tRNAIle2, AMPcPP, and agmatine (PDB ID: 3AMU) were determined ().Citation30 TiaS folds into 4 structural domains, TCKD (Thr18-Cyt34 kinase domain), FLD (ferredoxin-like fold domain), OBD (OB fold domain), and ZRD (zinc ribbon-like domain). In the AfTiaS-tRNAIle2-ATP ternary complex,Citation30 the anticodon arm of tRNAIle2 is recognized by the interdomain groove formed by TCKD, FLD, and OBD, while the acceptor stem interacts with ZRD.
Since TilS and TiaS have no sequence similarity, and thus belong to non-related protein families, their 3-dimensional structures are quite different (). However, these 2 proteins associate with tRNAIle2 in a similar fashion; the C-terminal domains (CTD2 of GkTilS and ZRD of AfTiaS) protruding from these enzymes contact the acceptor stem of tRNAIle2 on the major groove side in a shape-complementary manner. This interaction allows the anticodon arm of tRNAIle2 to be directed toward the remaining parts of each protein, facilitating contact between the anticodon loop and the catalytic domain. The extensive interactions along the tRNA are unique to TilS and TiaS, because tRNA-modifying enzymes responsible for other wobble modifications, such as MnmACitation31 and TadA,Citation32 only recognize the anticodon arm, and not the other parts of tRNA.
Bacterial and archaeal tRNAIle2 bear the same anticodon-loop sequence as tRNAMet. Therefore, TilS and TiaS should be able to discriminate tRNAIle2 from the structurally-similar tRNAMet by recognizing region(s) other than the anticodon loop.Citation11,30 Mutation studies of tRNAs revealed that E. coli TilS strictly recognizes the 2 consecutive C-G pairs at the 4th (C4-G69) and 5th (C5-G68) base pairs from the top of the acceptor stem of tRNAIle2 (),Citation11 and these pairs are strictly conserved in bacteria bearing Type-I TilS proteins. Since the consecutive C-G pairs in the acceptor stem are not conserved at corresponding positions in tRNAMet, Type-I TilS cannot recognize tRNAMet bearing the same anticodon loop sequence with tRNAIle2.Citation11,33 In the crystal structure of the GkTilS-tRNAIle2 complex,Citation29 some basic residues in the helix-turn-helix motif of CTD2 come in close proximity to the consecutive C-G pairs (). A mutation analysis of GkTilS revealed an important role of Arg420 in L formation. However, the precise nature of the molecular interactions between CTD2 and the acceptor stem remain elusive, due to the medium resolution (3.65 Å) of the structural analysis.
The ternary complex of AfTiaS with tRNAIle2 and ATP clearly revealed that the side chain of Arg369 and the main chain carbonyl of Ser361 in ZRD specifically interact with the 2 consecutive G-C pairs (G1-C72 and G2-C71) at the top of the acceptor stem of tRNAIle2 ().Citation30 Arg369 is a critical residue for agm2C formation. In addition, the replacement of either the G1-C72 or G2-C71 base pair of tRNAIle2 with those of tRNAMet nearly eliminates the agmatine-accepting activity. Therefore, TiaS recognizes the 2 consecutive G-C base pairs at the top of the acceptor stem using Ser361 and Arg369 to discriminate tRNAIle2 from tRNAMet ().
Collectively, Type-I TilS and TiaS commonly discriminate tRNAIle2 from structurally-similar tRNAMet by recognizing the 2 consecutive base pairs and introduce a corresponding wobble modification. Despite the different determinants embedded in the tRNAIle2 acceptor stem, TilS and TiaS have acquired a similar strategy to select the substrate tRNA.
Anticodon Arm Recognition by TilS and TiaS
Upon interaction with the NTD of GkTilS, the anticodon loop of tRNAIle2 undergoes a drastic conformational change.Citation29 In the structure of the GkTilS-tRNAIle2 complex, the target residue C34 is flipped out and placed at the catalytic site where the PP-loop motif is located (). The mismatched base pair C32–A38, observed in the canonical tRNA structure, is disrupted by Arg142 in the NTD. This basic residue enters the anticodon-loop and expels A38 by mimicking base pairing with C32. The flipped-out A38 is accommodated in a hydrophobic pocket of the CTD1 comprised of aliphatic residues (Leu277 and Leu278). In fact, C32 and A38 are involved in lysine-accepting activity.Citation11 The functional importance of Arg142 and Leu277 is also supported by the results of mutational analysis.Citation11,29
Figure 5. Anticodon recognition and catalytic sites of TilS and TiaS. (A) Recognition of the anticodon loop of tRNAIle2 by NTD of GkTilS. (B) The structure of the active site of the GkTilS-tRNAIle2 complex, superimposed with the ATP bound to the catalytic site of AaTilS. The target nucleoside, C34, is represented as a yellow stick model. The ATP observed in the AaTilS-ATP complex is superimposed (white stick model). The ATP is situated near the N-type ATP pyrophosphatase motif (PP-loop) (blue). (C) Recognition of the anticodon loop of tRNAIle2 in the AfTiaS-tRNAIle2-ATP ternary complex structure. (D) The structure of the active site of the AfTiaS-tRNAIle2-ATP ternary complex structure. C34 and ATP are represented as yellow and white stick models, respectively. The catalytic residues (Asp8, Asp9, Asp11, and pThr18) are also represented as stick models. (E) The structure of the active site of the AfTiaS-tRNAIle2-AMPcPP-agmatine quaternary complex structure. C34, AMPcPP, and agmatine are represented as yellow, white, and light blue stick models, respectively. The catalytic residues (Asp8, Asp9, Asp11, and pThr18) are also represented as stick models. The coloring schemes of the proteins are the same as those in .
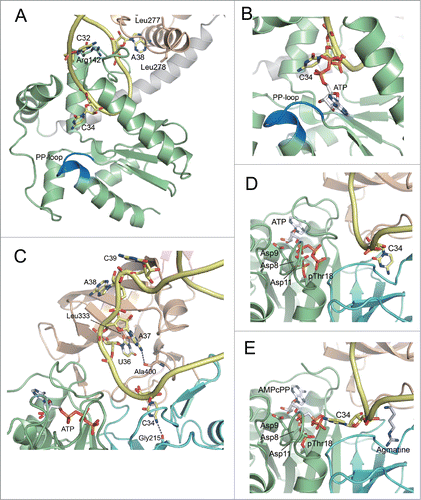
Similarly, AfTiaS binding to tRNAIle2 results in a dynamic conformational change in the anticodon arm ().Citation30 In the ternary complex structure, C34 is recognized by a hydrogen bond with the main chain carbonyl of Gly215. U36 and A37 of tRNAIle2 are also recognized by the main chain carbonyls of Leu333 and Ala400, respectively, in a hydrophobic pocket. A mutation study demonstrated the importance of U36 and A37.Citation30 Furthermore, A38 and C39 of tRNAIle2 are expelled from the anticodon arm and located at the OBD. This interaction allows A38 located onto a concave hydrophobic surface of the OBD. Biochemical data has indicated the critical importance of this region in AfTiaS for agm2C formation.Citation34
Molecular Mechanism of L Formation
In spite of the chemical similarity between L and agm2C, the catalytic domains of TilS and TiaS are quite different, suggesting that different catalytic mechanisms are employed by these 2 enzymes.
TilS has a highly conserved N-type ATP pyrophosphatase domain,Citation35 which catalyzes hydrolysis of ATP into AMP and pyrophosphate (PPi). TilS synthesizes L34 by a catalytic mechanism similar to that of other ATP pyrophosphatase family proteins including MnmACitation36 and GMP synthetase.Citation37 TilS synthesizes L34 on tRNAIle2 using Lys and ATP as substrates ().Citation11 First, TilS activates the C2 carbon of C34 by forming an adenylate intermediate, releasing PPi. Second, TilS catalyzes L34 formation by allowing the ϵ-amino group of lysine to attack the C2 carbon of the intermediate, releasing AMP. When the structures of the GkTilS-tRNAIle2 (PDB ID: 3A2K) and AaTilS-ATP complexes (PDB ID: 2E89) are superimposed (), the flipped-out C34 of tRNAIle2 is situated near the α-phosphate group of the ATP, supporting the biochemical evidence that TilS activates the C2 carbon of C34 by adenylation during L formation.Citation11 However, the binding site for Lys in the catalytic site remains unknown. Further study will be necessary to determine the detailed catalytic mechanism of L formation.
Figure 6. Reaction schemes for formation of L and agm2C (A) Two-step lysidine formation catalyzed by bacterial TilS. TilS activates the C2 carbonyl group of C34 by adenylation and releases PPi. Then, the ϵ-amino group of lysine attacks the adenylated C2 group to release AMP and complete lysidine formation. (B) The 3-step reaction of agm2C formation catalyzed by TiaS. First, TiaS hydrolyzes the α-β phosphodiester bond of ATP to produce AMP and PPi. Second, the C2 carbonyl oxygen of C34 attacks the γ-phosphorous atom to form the phosphorylated C34 (p-C34) intermediate, releasing β-Pi. Third, the primary amino group of agmatine attacks the C2 carbon of the p-C34 intermediate to release γ-Pi and form agm2C.
Molecular Mechanism of Agm2C Formation
TiaS synthesizes agm2C34 on tRNAIle2 via a unique reaction mechanism, which is distinct from the mechanism employed for TilS-catalyzed L34 formation. TiaS catalyzes agm2C synthesis in the presence of agmatine and ATP by 3 consecutive reactions ().Citation34 First, TiaS hydrolyzes ATP into AMP and PPi. Second, TiaS phosphorylates the C2 carbon of C34 using the γ-phosphate for activation. Finally, the amino group of agmatine attacks the C2 carbon to form agm2C, releasing the phosphate.
Although agm2C shows structural similarity to L, TiaS belongs to a family of enzymes unrelated to TilS. In terms of catalytic activation,Citation34 TilS activates the C2 carbon of C34 by adenylation (), whereas TiaS phosphorylates the same position (). Thus, TiaS has RNA kinase activity. In addition, a unique feature of agm2C formation is that TiaS hydrolyzes ATP into AMP and PPi before phosphorylating the C2 carbon. We call this reaction “idling," because TiaS hydrolyzes ATP even in the absence of tRNA. It is unclear why TiaS catalyzes such a non-productive reaction. However, agm2C formation is efficiently inhibited by AMPcPP, which is a non-hydrolyzable analog of ATP that cannot be cleaved at the α-β phosphodiester bond. TiaS mutants showing no idling reaction do not have the ability to form agm2C. In addition, any TiaS mutants do not produce ADP during the reaction. These facts strongly suggest that the “idling” reaction is essential for agm2C formation.
In addition to the RNA kinase activity of TiaS, which phosphorylates the C2 carbon of C34, we also found that TiaS autophosphorylates threonine (Thr) at position 18 ().Citation30,34 Therefore, TiaS has a novel kinase domain in the N-terminal region responsible for phosphorylating both C34 of tRNA and Thr18 of TiaS, which we named the Thr18-Cyt34 kinase domain (TCKD).Citation30,34 In the ternary complex (), ATP is specifically bound in a deep pocket in the TCKD, and its triphosphate group is surrounded by a number of residues highly conserved in TiaS family proteins. Mutations of these residues almost eliminate TiaS activity. In the ternary complex (), C34 of tRNAIle2 is located in the pocket of the FLD, which is far away (10 Å) from the γ-phosphate of ATP bound to the TCKD, suggesting that the phosphorylation of C34 does not take place when C34 is in this position. Intriguingly, in the quaternary complex (), agmatine binds exactly the same pocket used by C34 in the FLD in the ternary complex. Agmatine-binding to the FLD expels C34 from this pocket to place it near the γ-phosphate of AMPcPP in the TCKD, facilitating the phosphorylation of C34 with the ATP γ-phosphate.
Non-productive complex was observed in the absence of agmatine (). Considering the cellular concentration of agmatine and the kinetic parameters for agm2C formation,Citation34 TiaS is predicted to bind agmatine before interaction with tRNAIle2 under normal growth conditions. However, if the cellular concentration of agmatine drops as a result of nutrient starvation, C34 is trapped in the pocket of the FLD during agm2C formation, suspending the activation of C34. Given that agm2C is an essential for protein synthesis, this mechanism might function to attenuate cellular growth under some stress conditions.
We believe phosphorylated Thr18 has a catalytic role in agm2C formation. Phospho-Thr18 (pThr18) is located close to the ATP-binding region in the TCKD (). Phospho-Thr18 and 3 aspartates, Asp8, Asp9, and Asp11, are thought to coordinate Mg2+ ions and facilitate the phosphorylation of C34.Citation30 In addition, phospho-Thr18 might be involved in promoting the phosphorylation of C34 by inducing electrostatic repulsion between phospho-Thr18 and the γ-phosphate of ATP. Phosphorylated-C34 starts moving toward the FLD, where agmatine is bound to complete the reaction. Phospho-Thr18 also supports this relocation via electrostatic repulsion and may also be involved in clearance of the β-phosphate after the reaction, because β-phosphate is tightly recognized by the TCKD.
Natural Instance of AUA Decoding by The UAU Anticodon
As described above, most bacteria have a TilS homolog and a tRNAIle2 gene with a CAT anticodon. Thus, AUA codons are deciphered by tRNAIle2 bearing L at the wobble position. Similarly, most archaea have a TiaS homolog and a tRNAIle2 gene with a CAT anticodon. However, some bacteria and archaea lacking tilS or tiaS have been discovered.Citation33,38 According to the tRNA gene data base curated by experts (tRNADB-CE)Citation39 and information from the literatures,Citation33 no tilS homolog is found in 13 bacterial species () belonging to 3 bacterial clades, Tenericutes, Alphaproteobacteria and Actinobacteria, which possess tRNAIle genes with a TAT anticodon. In archaea, 2 archaeal species, Candidatus Korarchaeum cryptofilum OPF8 and Nanoarchaeum equitans, do not have a tiaS homolog (), but possess tRNAIle genes with TAT anticodons. These observation indicate that AUA codons are deciphered by UAU (or U*AU) anticodons in these species. Since organisms lacking tilS or tiaS sporadically appear in each clade, and have no common ancestor, AUA decoding by UAU anticodons might have arisen independently in each lineage they branched out from their ancestral clades, in which AUA codons are deciphered by tRNAIle2 with L or agm2C.
Table 1. Organisms lacking tilS or tiaS.
Among bacterial species lacking tilS, we studied, in detail, the molecular mechanism of AUA decoding by UAU anticodons in Mycoplasma mobile.Citation38 M. mobile has a tRNAIle2 with an unmodified UAU anticodon and IleRS that preferentially recognizes the UAU anticodon, whereas E. coli IleRS does not efficiently aminoacylate tRNAIle2UAU. A single Arg residue (position 865) in M. mobile IleRS is critical for recognition of the UAU anticodon of tRNAIle2. M. mobile tRNAIle2UAU efficiently recognizes both AUA and AUG codons on E. coli ribosomes. However, on M. mobile ribosomes, M. mobile tRNAIle2UAU specifically recognizes AUA codons, but not AUG codons, suggesting that M. mobile ribosomes have a property that prevents misreading of AUG codons by UAU anticodons.
The AUA decoding by the UAU anticodon was also demonstrated by a model experiment using Bacillus subtilis.Citation40,41 As tilS is an essential gene in B. subtilis Citation10, suppressor mutants with restored growth at a non-permissive temperature emerged spontaneously from the thermosensitive tilS (tilSts) strain. Citation41 One of the suppressors had a G-to-T point mutation at the wobble position of the tRNAIle1 gene, indicating that mutant tRNAIle1 bearing a UAU anticodon decodes the AUA codon instead of the hypomodified tRNAIle2 in the tilSts strain. An in vitro experiment revealed that the tRNAIle1 mutant with a UAU anticodon preferentially binds to the AUA codon but binds weakly to the AUG codon on the B. subtilis ribosome Citation40. Given that B. subtilis (Firmicutes) and M. mobile (Tenericutes) are phylogenetically closely related bacterial species, their ribosomes are predicted to have similar functional characteristics that allow the UAU anticodon to specifically recognize the AUA codon, and prevent misreading of the AUG codon.
Convergent Evolution of AUA Decoding Across Domains of Life
As described above, several organisms that do not possess tilS or tiaS are sporadically found in some clades of bacteria and archaea. AUA decoding by the UAU anticodon can be established in natural living organisms, though its occurrence is rare. We speculate that the AUA codon started to be decoded by a UAU anticodon without wobble modifications in the last universal common ancestor (LUCA) between bacteria and archaea, assuming that the primitive decoding system did not require any wobble modifications. However, the UAU anticodon can potentially recognize AUG codons by U-G wobble pairing, even if the ribosome gains the ability to protect against such misreading, as observed in M. mobile. In the presence of tRNAMet, which strongly recognizes the AUG codon, misreading is reduced to some extent. However, AUA decoding by the UAU anticodon is supposed to be a very unstable or risky mechanism to maintain accurate decoding in living organisms. During evolution of bacteria and archaea, acquisition of L or agm2C appears to have been beneficial for establishing an efficient and robust decoding system to maintain high fidelity in protein synthesis. TilS and TiaS are completely different classes of enzymes; nevertheless, L and agm2C share similar chemical structures. The decoding system for AUA codons might have occurred by convergent evolution after separation of bacteria and archaea from their common ancestor.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Suzuki lab members, especially T. Taniguchi, for critically reading the manuscript and for many fruitful discussions.
Funding
This work was supported by Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports, and Culture of Japan (to T.S.), and by a Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science (to T.N.).
References
- Ogle JM, Murphy FV, Tarry MJ, Ramakrishnan V. Selection of tRNA by the ribosome requires a transition from an open to a closed form. Cell 2002; 111:721-32; PMID:12464183; http://dx.doi.org/10.1016/S0092-8674(02)01086-3
- Ogle JM, Brodersen DE, Clemons WM Jr, Tarry MJ, Carter AP, Ramakrishnan V. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science 2001; 292:897-902; PMID:11340196; http://dx.doi.org/10.1126/science.1060612
- Schmeing TM, Voorhees RM, Kelley AC, Gao YG, Murphy FV 4th, Weir JR, Ramakrishnan V. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science 2009; 326:688-94; PMID:19833920; http://dx.doi.org/10.1126/science.1179700
- Voorhees RM, Schmeing TM, Kelley AC, Ramakrishnan V. The mechanism for activation of GTP hydrolysis on the ribosome. Science 2010; 330:835-8; PMID:21051640; http://dx.doi.org/10.1126/science.1194460
- Crick FH. Codon–anticodon pairing: the wobble hypothesis. J Mol Biol 1966; 19:548-55; PMID:5969078; http://dx.doi.org/10.1016/S0022-2836(66)80022-0
- Suzuki T. Biosynthesis and function of tRNA wobble modifications. in Topics in Current Genetics, Vol. 12 24-69 (Springer-Verlag, NY, 2005).
- Yokoyama S, Nishimura S. Modified nucleosides and codon recognition. in tRNA: Structure, Biosynthesis, and Function (ed. Soll, DR, U. L) 207-24 (American Society for Microbiology, Washington, D.C., 1995).
- Bjork G. Biosynthesis and function of modified nucleosides. in tRNA: Structure, Biosynthesis, and Function (ed. Soll, DR, U. L) 165-205 (American Society for Microbiology, Washington, D.C., 1995).
- Muramatsu T. Yokoyama S, Horie N, Matsuda A, Ueda T, Yamaizumi Z, Kuchino Y, Nishimura S, Miyazawa T. A novel lysine-substituted nucleoside in the first position of the anticodon of minor isoleucine tRNA from Escherichia coli. J Biol Chem 1988; 263:9261-7; PMID:3132458
- Soma A, Ikeuchi Y, Kanemasa S, Kobayashi K, Ogasawara N, Ote T, Kato J, Watanabe K, Sekine Y, Suzuki T. An RNA-modifying enzyme that governs both the codon and amino acid specificities of isoleucine tRNA. Mol Cell 2003; 12:689-98; PMID:14527414; http://dx.doi.org/10.1016/S1097-2765(03)00346-0
- Ikeuchi Y, Soma A, Ote T, Kato J, Sekine Y, Suzuki T. Molecular mechanism of lysidine synthesis that determines tRNA identity and codon recognition. Mol Cell 2005; 19:235-46; PMID:16039592; http://dx.doi.org/10.1016/j.molcel.2005.06.007
- Muramatsu T, Nishikawa K, Nemoto F, Kuchino Y, Nishimura S, Miyazawa T, Yokoyama S. Codon and Amino-Acid Specificities of a Transfer-Rna Are Both Converted by a Single Post-Transcriptional Modification. Nature 1988; 336:179-81; PMID:3054566; http://dx.doi.org/10.1038/336179a0
- Edmonds CG, Crain PF, Gupta R, Hashizume T, Hocart CH, Kowalak JA, Pomerantz SC, Stetter KO, McCloskey JA. Posttranscriptional modification of tRNA in thermophilic archaea (Archaebacteria). J Bacteriol 1991; 173:3138-48; PMID:1708763
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem 1984; 259:9461-71; PMID:6746655
- Kohrer C, Srinivasan G, Mandal D, Mallick B, Ghosh Z, Chakrabarti J, Rajbhandary UL. Identification and characterization of a tRNA decoding the rare AUA codon in Haloarcula marismortui. RNA 2008; 14:117-26; PMID:17998287; http://dx.doi.org/10.1261/rna.795508
- Ikeuchi Y, Kimura S, Numata T, Nakamura D, Yokogawa T, Ogata T, Wada T, Suzuki T, Suzuki T. Agmatine-conjugated cytidine in a tRNA anticodon is essential for AUA decoding in archaea. Nat Chem Biol 2010; 6:277-82; PMID:20139989; http://dx.doi.org/10.1038/nchembio.323
- Tabor CW, Tabor H. Polyamines in microorganisms. Microbiol Rev 1985; 49:81-99; PMID:3157043
- Groppa MD, Benavides MP. Polyamines and abiotic stress: recent advances. Amino Acids 2008; 34:35-45; PMID:17356805; http://dx.doi.org/10.1007/s00726-007-0501-8
- Terui Y, Ohnuma M, Hiraga K, Kawashima E, Oshima T. Stabilization of nucleic acids by unusual polyamines produced by an extreme thermophile, Thermus thermophilus. Biochem J 2005; 388:427-33; PMID:15673283; http://dx.doi.org/10.1042/BJ20041778
- Fukuda W, Morimoto N, Imanaka T, Fujiwara S. Agmatine is essential for the cell growth of Thermococcus kodakaraensis. FEMS Microbiol Lett 2008; 287:113-20; PMID:18702616; http://dx.doi.org/10.1111/j.1574-6968.2008.01303.x
- Blaby IK, Phillips G, Blaby-Haas CE, Gulig KS, El Yacoubi B, de Crécy-Lagard V. Towards a systems approach in the genetic analysis of archaea: Accelerating mutant construction and phenotypic analysis in Haloferax volcanii. Archaea 2010; 2010:426239; PMID:21234384; http://dx.doi.org/10.1155/2010/426239
- Mandal D, Köhrer C, Su D, Russell SP, Krivos K, Castleberry CM, Blum P, Limbach PA, Söll D, RajBhandary UL. Agmatidine, a modified cytidine in the anticodon of archaeal tRNA(Ile), base pairs with adenosine but not with guanosine. Proc Natl Acad Sci U S A 2010; 107:2872-7; PMID:20133752; http://dx.doi.org/10.1073/pnas.0914869107
- Voorhees RM, Mandal D, Neubauer C, Köhrer C, RajBhandary UL, Ramakrishnan V. The structural basis for specific decoding of AUA by isoleucine tRNA on the ribosome. Nat Struct Mol Biol 2013; 20:641-3; PMID:23542153; http://dx.doi.org/10.1038/nsmb.2545
- Schmeing TM, Voorhees RM, Kelley AC, Ramakrishnan V. How mutations in tRNA distant from the anticodon affect the fidelity of decoding. Nat Struct Mol Biol 2011; 18:432-6; PMID:21378964; http://dx.doi.org/10.1038/nsmb.2003
- Watanabe K. Unique features of animal mitochondrial translation systems. The non-universal genetic code, unusual features of the translational apparatus and their relevance to human mitochondrial diseases. Proc Jpn Acad Ser B Phys Biol Sci 2010; 86:11-39; PMID:20075606; http://dx.doi.org/10.2183/pjab.86.11
- Westhof E. Isostericity and tautomerism of base pairs in nucleic acids. FEBS Lett 2014; 588:2464-9; PMID:24950426; http://dx.doi.org/10.1016/j.febslet.2014.06.031
- Nakanishi K, Fukai S, Ikeuchi Y, Soma A, Sekine Y, Suzuki T, Nureki O. Structural basis for lysidine formation by ATP pyrophosphatase accompanied by a lysine-specific loop and a tRNA-recognition domain. Proc Natl Acad Sci U S A 2005; 102:7487-92. Epub 2005 May 13; PMID:15894617; http://dx.doi.org/10.1073/pnas.0501003102
- Kuratani M, Yoshikawa Y, Bessho Y, Higashijima K, Ishii T, Shibata R, Takahashi S, Yutani K, Yokoyama S. Structural basis of the initial binding of tRNA(Ile) lysidine synthetase TilS with ATP and L-lysine. Structure 2007; 15:1642-53; PMID:18073113; http://dx.doi.org/10.1016/j.str.2007.09.020
- Nakanishi K, Bonnefond L, Kimura S, Suzuki T, Ishitani R, Nureki O. Structural basis for translational fidelity ensured by transfer RNA lysidine synthetase. Nature 2009; 461:1144-8; PMID:19847269; http://dx.doi.org/10.1038/nature08474
- Osawa T, Kimura S, Terasaka N, Inanaga H, Suzuki T, Numata T. Structural basis of tRNA agmatinylation essential for AUA codon decoding. Nat Struct Mol Biol 2011; 18:1275-80; PMID:22002223; http://dx.doi.org/10.1038/nsmb.2144
- Numata T, Ikeuchi Y, Fukai S, Suzuki T, Nureki O. Snapshots of tRNA sulphuration via an adenylated intermediate. Nature 2006; 442:419-24; PMID:16871210; http://dx.doi.org/10.1038/nature04896
- Losey HC, Ruthenburg AJ, Verdine GL. Crystal structure of Staphylococcus aureus tRNA adenosine deaminase TadA in complex with RNA. Nat Struct Mol Biol 2006; 13:153-9; PMID:16415880; http://dx.doi.org/10.1038/nsmb1047
- Suzuki T, Miyauchi K. Discovery and characterization of tRNAIle lysidine synthetase (TilS). FEBS Lett 2010; 584:272-7; PMID:19944692; http://dx.doi.org/10.1016/j.febslet.2009.11.085
- Terasaka N, Kimura S, Osawa T, Numata T, Suzuki T. Biogenesis of 2-agmatinylcytidine catalyzed by the dual protein and RNA kinase TiaS. Nat Struct Mol Biol 2011; 18:1268-74; PMID:22002222; http://dx.doi.org/10.1038/nsmb.2121
- Bork P, Koonin EV. A P-loop-like motif in a widespread ATP pyrophosphatase domain: implications for the evolution of sequence motifs and enzyme activity. Proteins 1994; 20:347-55; PMID:7731953; http://dx.doi.org/10.1002/prot.340200407
- Kambampati R, Lauhon CT. MnmA and IscS are required for in vitro 2-thiouridine biosynthesis in Escherichia coli. Biochemistry 2003; 42:1109-17; PMID:12549933; http://dx.doi.org/10.1021/bi026536+
- Tesmer JJ, Klem TJ, Deras ML, Davisson VJ, Smith JL. The crystal structure of GMP synthetase reveals a novel catalytic triad and is a structural paradigm for two enzyme families. Nat Struct Biol 1996; 3:74-86; PMID:8548458; http://dx.doi.org/10.1038/nsb0196-74
- Taniguchi T, Miyauchi K, Nakane D, Miyata M, Muto A, Nishimura S, Suzuki T. Decoding system for the AUA codon by tRNAIle with the UAU anticodon in Mycoplasma mobile. Nucleic Acids Res 2013; 41:2621-31; PMID:23295668; http://dx.doi.org/10.1093/nar/gks1344
- Abe T, Inokuchi H, Yamada Y, Muto A, Iwasaki Y, Ikemura T. tRNADB-CE: tRNA gene database well-timed in the era of big sequence data. Front Genet 2014; 5:114; PMID:24822057; http://dx.doi.org/10.3389/fgene.2014.00114
- Kohrer C, Mandal D, Gaston KW, Grosjean H, Limbach PA, Rajbhandary UL. Life without tRNAIle-lysidine synthetase: translation of the isoleucine codon AUA in Bacillus subtilis lacking the canonical tRNA2Ile. Nucleic Acids Res 2014; 42:1904-15; PMID:24194599; http://dx.doi.org/10.1093/nar/gkt1009
- Fabret C, Dervyn E, Dalmais B, Guillot A, Marck C, Grosjean H, Noirot P. Life without the essential bacterial tRNA Ile2-lysidine synthetase TilS: a case of tRNA gene recruitment in Bacillus subtilis. Mol Microbiol 2011; 80:1062-74; PMID:21435031; http://dx.doi.org/10.1111/j.1365-2958.2011.07630.x
