Abstract
Neurons are highly polarized specialized cells. Neuronal integrity and functional roles are critically dependent on dendritic architecture and synaptic structure, function and plasticity. The cadherins are glycosylated transmembrane proteins that form cell adhesion complexes in various tissues. They are associated with a group of cytosolic proteins, the catenins. While the functional roles of the complex have been extensively investigates in non-neuronal cells, it is becoming increasingly clear that components of the complex have critical roles in regulating dendritic and synaptic architecture, function and plasticity in neurons. Consistent with these functional roles, aberrations in components of the complex have been implicated in a variety of neurodevelopmental disorders. In this review, we discuss the roles of the classical cadherins and catenins in various aspects of dendrite and synapse architecture and function and their relevance to human neurological disorders. Cadherins are glycosylated transmembrane proteins that were initially identified as Ca2+-dependent cell adhesion molecules. They are present on plasma membrane of a variety of cell types from primitive metazoans to humans. In the past several years, it has become clear that in addition to providing mechanical adhesion between cells, cadherins play integral roles in tissue morphogenesis and homeostasis. The cadherin family is composed of more than 100 members and classified into several subfamilies, including classical cadherins and protocadherins. Several of these cadherin family members have been implicated in various aspects of neuronal development and function.Citation1-3 The classical cadherins are associated with a group of cytosolic proteins, collectively called the catenins. While the functional roles of the cadherin-catenin cell adhesion complex have been extensively investigated in epithelial cells, it is now clear that components of the complex are well expressed in central neurons at different stages during development.Citation4,5 Recent exciting studies have shed some light on the functional roles of cadherins and catenins in central neurons. In this review, we will provide a brief overview of the cadherin superfamily, describe cadherin family members expressed in central neurons, cadherin-catenin complexes in central neurons and then focus on role of the cadherin-catenin complex in dendrite morphogenesis and synapse morphogenesis, function and plasticity. The final section is dedicated to discussion of the emerging list of neural disorders linked to cadherins and catenins. While the roles of cadherins and catenins have been examined in several different types of neurons, the focus of this review is their role in mammalian central neurons, particularly those of the cortex and hippocampus. Accompanying this review is a series of excellent reviews targeting the roles of cadherins and protocadherins in other aspects of neural development.
The Cadherin Superfamily
The human genome encodes 115 members of cadherin superfamily.Citation5-7 They vary greatly in size and structure and based on the sequence similarities can be further grouped into several subfamilies: classical cadherins (type I and II), desmosomal cadherins, and protocadherins (clustered and non-clustered), and atypical members including FATs, Flamingo/Celsrs and calsyntenins ( and ). A common feature of members of the superfamily is that they share characteristic extracellular cadherin (EC) domain repeats that range in number between 1 and 34. The structural characteristics of the EC domains and their tandem repeats increase the number of possible adhesive interactions and also allow them to protrude across cell-cell interface and provide for different levels of mechanical stability. While at least classical and desmosomal cadherins function as cell adhesion molecules, many others appear to play more diverse roles initiated by homo- or occasional heterophilic interactions at cell-cell interfaces.
Table 1. Cadherin Superfamily Members. The classification is based on 5, but for simplicity several subfamilies are merged under the Atypical group. Non-clustered protocadherins are classified according to18 EC# specifies the number of Extracellular Cadherin repeats on each subfamily or member
Figure 1. Cadherin superfamily members. Members of the cadherin superfamily vary greatly in size and structure and based on the sequence similarities can be further grouped into several subfamilies. Members have varying numbers of Extracellular Cadherin (EC) repeat domains (green oval). Typically EC domains are preceded by unique prodomains (yellow diamond). Both classical cadherins (type I and II) and desmosoal cadherins have 5 EC domains. Clustered protocadherins (α, β, and γ) have 6 EC domains. Nonclustered protocadherins are grouped into δ1 (7 EC), δ2 (6 EC), and others (varius EC). A couple of atypical subfamilies are are also illustrated: FAT (34 EC), Flamingo/CELSRS (9 EC), Calsyntenin (2 EC).
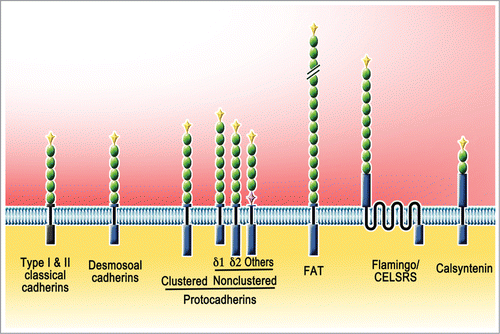
Many of these molecules are expressed in the nervous system, implying their contribution to neural development and function. The most extensively investigated cadherin superfamily members in central neurons are the classical cadherins and the protocadherins. Different classical cadherins are named according to the tissues from which they were first isolated. For example, E-, N-, and R-cadherins were derived from epithelial, neural, and retinal tissues, respectively. However, it is now clear that these cadherins have wider tissue distribution and functional roles than originally anticipated.
Cadherin Mediated Adhesion Mechanisms
Cadherins mediate calcium dependent cell adhesion in the cis- and trans- configurations. The ability of the members of the cadherin superfamily to mediate adhesion is mainly dependent on the EC domains. Each EC domain is composed of about 110 amino acid residues, which fold into 7 anti-parallels β-strands that assemble 2 β-sheets. Three Ca2+ bind to the interdomain of each consecutive EC domain pair and rigidify the connection between neighboring EC domains, which imparts a strong curvature to the entire extracellular domain (, reviewed inCitation8) Thus Ca2+ binding stabilizes the whole ectodomain structure and facilitates homophilic interaction with other cadherin monomers from the apposing membrane. Removal of Ca2+ leads to disordering of the ectodomain orientations and results in the loss of adhesive trans-dimers.Citation9
Figure 2. cis- and trans- interaction of type I classical cadherins. The extracellular domain of cadherin is flexible in absence of Ca2+. After binding to Ca2+, it adopts a curved rod-like structure which is relatively rigid. Ca2+-bound cadherin monomers from 2 neighboring cells first form trans-dimers through the interaction of EC1 domains. These cadherin trans-dimers interact laterally (cis-interaction) to form a lattice by nonsymmetrical binding of EC1 of one cadherin and the EC2-EC3 region of its cis-binding partner.
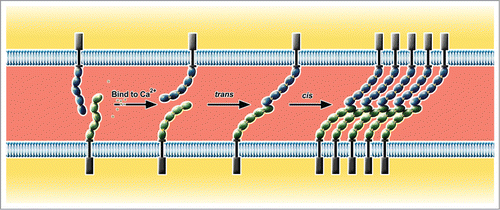
The type I classical cadherins, best characterized among the cadherin superfamily, have 5 EC domains, membrane-distal EC1 to membrane-proximal EC5 (). Though the details of adhesive binding mechanisms are not yet conclusive, currently favored view is “strand swapping” between 2 dimers to achieve trans-homodimerization. In this model, the 2 cadherins from the apposing membranes align their EC1 and EC2 in parallel, in spite of their overall antiparallel orientations. The parallel alignment, enabled by the characteristic curvature of the ectodomains, allows a transient X-shaped intermediate near EC1 and EC2. The X-dimer exchanges the tryptophan 2 (Trp2) residues on EC1, which derives “strand swapping” between the trans-dimer. The resulting strand-swapped trans-dimers are less flexible, i.e. less diffusible than monomers, thus allow lateral oligomerization to further strengthen the adhesion of 2 apposing membranes.Citation10
The strand-swap binding mechanism is apparently also conserved among classical type II and desmosomal cadherins. However, protocadherins and other cadherins with large ectodomains do not have the conserved sequences required for similar binding mechanisms and probably engage different and quite diverse binding mechanisms. For further detailed information on structures of cadherin superfamily, we refer the reader to 2 recent excellent reviews dedicated to this topic.Citation8,11
Cadherins in Central Neurons
Several members of the cadherin superfamily are expressed in central neurons. The most well studied ones include the classical cadherins and protocadherins.
Classical cadherins
As the predominantly expressed cadherin family in vertebrates, the classical cadherins have undertaken critical roles in cell-cell adhesion, which mediates the majority of vertebrate tissue-specific interactions and thus has direct effects on development and cancer (reviewed inCitation12) The human genome encodes 18 classical cadherin members that have highly conserved cytoplasmic domains (∼150 amino acids), which contain binding sites for catenins and form cadherin-catenin complexes. Classical cadherins can be further grouped into type I and type II, with higher sequence homology of cytoplasmic domains within each subfamily. Type I subfamily comprises of E-, N-, P-, R- and M-cadherin, and most type II cadherins are numerically denoted, such as cadherin-6, -7, and -8. In general, each type I cadherin protein binds homophillically and form a homodimer, and the affinity for each dimerization seems variable. For example, N-cadherin dimerization has higher affinity than E-cadherin dimerizationCitation13. When both N-cadherin and E-cadherin were present together in proximity, N- and E-cadherins formed heterodimers with an intermediate affinity of their respective homodimersCitation13, whereas another study found the heterodimer formation to be still less frequent than homodimerizationCitation14. Interactions among type II cadherins are more complicated. All type II cadherins have similar level of homophilic affinity comparable to E-cadherin, but certain pairs of heterodimers like cadherin-7 and -14, and cadherin-8 and -11, have as high affinities as respective homodimers.Citation15
Protocadherins
Protocadherins constitute the largest cadherin family, with 68 members in humans (). They are single-pass transmembrane proteins with 6 or 7 EC domains, and further grouped into 2 subfamilies, clustered and nonclustered. In humans, 53 clustered protocadherins (Pcdhs) are encoded in a single genomic locus of ∼1 Mb on chromosome 5q31, assembled in tandem into α-, β-, and γ-Pcdh clusters. In contrast, the nonclustered Pcdh subfamily has about 15 genes scattered over different chromosomes. A remarkable difference in the genomic structures of both Pcdh subfamilies from classical cadherins is the presence of unusually large exons in Pcdh genes, which typically encode the entire ectodomain comprising 6–7 EC domain repeats as well as the transmembrane domain and short cytoplasmic part.Citation16
Both Pcdh subfamilies are expressed predominantly in the nervous system, and many of them have the capacity for homophilic interaction albeit with reduced affinity compared to classical cadherins. A recent cell-aggregation study of γ-Pcdhs showed that unlike classical cadherins, EC2 and EC3, rather than EC1, are critical in homophilic bindings.Citation17
Non-clustered protocadherins
Nonclustered protocadherins show diverse spatiotemporal expression patterns. Notable expressions have been detected in cortical areas during early postnatal period and in caudate putamen/hippocampal formation of mature brain tissues. Among non-clustered protocadherins, not only the number of extracellular EC domains but the cytoplasmic domains are relatively variable, suggesting that nonclustered Pcdhs act as regulators via interactions with diverse intracellular molecules.Citation18
Other cadherins in central neurons
In addition to classical cadherins and protocadherins, there are 21 unique cadherins with varying number of EC repeats. Some of them constitute small subfamilies of 2–4 members (). Flamingo/Celsr subfamily has 3 members with unique 7-pass transmembrane domain and 9 EC repeats. FAT subfamily has 4 members with 34 EC repeats that interact with Dachsous members with 27 EC repeats. The calsyntenin subfamily has 3 member with 2 EC repeats. Several of these subfamilies have been implicated in neuronal morphogenesis during development.Citation5
Thus, a variety of cadherins are expressed in central neurons. However, our knowledge of the functional roles of the cadherins, particularly the non-clustered cadherins and other cadherins in dendrite, spine and synapse morphogenesis and plasticity remains incomplete.
Cadherin-Catenin Complex in Central Neurons
In epithelial cells, the adherens junction (AJ) is a type of cell-cell adhesion structure, characterized by parallel plasma membranes apposed with a distance of 10–30 nm and linked by rod-shaped cadherin molecules. AJ is connected to actin cytoskeleton at the cytoplasmic side and assumes different morphologies depending on the cell types. In neurons AJ is restricted in the synaptic junctions and named ‘puncta adherentia’.Citation19 At these junctions, cadherins form trans-dimers and bridge the intermembrane space via their ectodomains, while their cytoplasmic domains, through direct binding to catenins, become linked to intracellular signaling pathways and the cytoskeleton. In neurons, the complex is highly receptive to synaptic activity mediated neuronal signaling mechanisms that allow it to function in regulating various aspects of neuronal morphogenesis and function.Citation20
In central neurons, both type I and type II cadherins are localized in both pre- and post- synaptic compartmentsCitation21 and also border the neurotransmitter release zone.Citation19,22 They form trans- and cis-interactions and are associated with catenins and synaptic complex components,Citation23,24,25particularly at excitatory synapses.Citation26
The catenins are a group of cytosolic proteins that are associated with the cadherins and link cadherins to actin and microtubules to provide mechanical support. The α-, β- and γ-catenins were initially identified by immunoprecipitation against E-cadherin.Citation27 The catenins are subdivided into 3 groups in mammals: 2 β-catenin-like proteins (β-catenin and plakoglobin/γ-catenin), 3 α-catenins (αN, αE, and αT) and 4 p120ctn-related proteins (p120ctn/p120-catenin, NPRAP/δ-catenin, p0071/Pkp4, and ARVCF). β-catenin binds to the membrane-distal region of the cadherin cytoplasmic domain (CBD: catenin binding domain), whereas the p120ctn family members bind to the cadherin's membrane-proximal region (JMD: juxtamembrane domain). The classical model of the complex suggested that the complex links to the actin cytoskeleton via a mechanism that involves direct interaction of β-catenin to cadherin and interaction of α-catenin to β-catenin the actin cytoskeleton. However, recent studies have challenged this model and indicate that the binding of α-catenin to β-catenin and the actin cytoskeleton is mutually exclusive,Citation28,29 thus pointing to alternate mechanisms for linking the complex to the actin cytoskeleton. Consistent with this model, αE-catenin can regulate the dynamics of the actin cytoskeleton independent of cell adhesion mediated by cadherin.Citation30 It has been proposed that α-catenin links to the actin cytoskeleton via actin binding molecules including vinculin and EPLIN, however the significance of these interactions in central neurons remains unclear.Citation5
The structure of β-catenin includes 12 armadillo (ARM) repeats composed of central ∼520 amino acid residues that are responsible for binding to the intracellular region of cadherin. The C-terminal region binds to various PDZ domain-containing proteins. In addition to its role in the cadherin-catenin complex, β-catenin participates in a variety of signaling pathways regulating cell proliferation, survival, migration, differentiation during the embryonic development and tissue regeneration in adult organisms.Citation31 Some of these functional roles are mediated by its ability to regulate transcriptional signaling through the Wnt signaling pathway. Unlike the multitude of cadherins, there is only a single β-catenin protein that is ubiquitously expressed and obligatory for all classical cadherin-catenin complex formation.
The p120ctn family of proteins includes p120ctn, δ-catenin, ARVCF and p0071 (plakophilin-4).Citation32 The structure of the proteins include 9-10 arm repeat domains flanking N- and C-termini. All members of the family, excluding p120ctn, also include a PDZ motif at the C-terminus. All of these family members have a wide tissue distribution, except for δ-catenin.
α-catenin, in mammals, is encoded by 3 genes that encode αE-catenin in epithelial tissues, αN-catenin in the nervous system, and αT-catenin in the heart and the testis. They are not structurally related to other catenins. Unlike other catenins, α-catenin has 4 domains of helix bundles and lack arm domains. Their N-terminus binds to β-catenin and C-terminus has F-actin-binding domain. In addition, α-catenins bind to other actin-binding proteins including vinculin, EPLIN, α-actinin and formins and can form dimers that have distinct affinity for cadherin-catenin complex.Citation33 The most recent study of α-catenin suggests that the monomeric form of mouse αE-catenin binds to β-catenin and this complex has little affinity for the actin cytoskeleton, while the dimeric form links to the actin cytoskeleton. Thus, it is not entirely clear how the complex links to the actin cytoskeleton.Citation34 The precise control of α-catenin and its diverse interacting partners supports its critical role in dynamic regulation of actin cytoskeleton in response to extracellular cadherin interaction with adjacent cells.
Thus, the cadherin catenin complex is in a key position to transduce extracellular signals into intracellular signaling events and orchestrate alterations in the cytoskeleton in response to various external stimuli, thus placing it in a position to regulate dendrite and spine morphogenesis in central neurons.
The Cadherin-Catenin Complex in Dendrite Morphogenesis
Dendrite outgrowth is a process that requires adhesion mediated contacts that guide dendrite arborization. Given the adhesive functional roles and ability to modulate the cytoskeleton of the cadherin-catenin complex, it is not surprising that they regulate dendrite morphogenesis. Growth cones represent the major sites of attachment of developing dendrites. The force necessary for propulsion of the growth cone is governed by adhesion based mechanisms that involve the actin cytoskeleton. While several studies indicate roles for N-cadherin, R-cadherin, E-cadherin, cadherin-7, and cadherin-11 in neurite outgrowth,Citation5 these cadherins appear to have a limited role in dendrite outgrowth and arborization in hippocampal and cortical neurons. Treatment of hippocampal slices with a peptide blocking N-cadherin prevents further dendritic elaboration and leads to dendritic retraction in CA3 neurons.Citation35 Expression of a construct expressing the extracellular and transmembrane domains of N-cadherin in hippocampal neurons leads to a modest increase in dendritic branching.Citation36 N-cadherin mediated extracellular interaction has also been implicated in activity-mediated dendritic outgrowth.Citation37 These functional effects of N-cadherin are mediated by an activity dependent increase in the surface levels of N-cadherin and through a link between αN-catenin and the actin cytoskeleton. Given the link between the cadherin-catenin cell adhesion complex and the actin cytoskeleton, it is perhaps surprising that genetic loss of N-cadherin does not lead to abnormal dendritic branching or extension.Citation38 Given the repertoire of cadherin family members in neurons, one likely explanation for this is that compensatory mechanisms exist that allow neurons to override the loss of N-cadherin.
The catenins appear to have critical roles in dendrite development and arborization. Overexpression of β-catenin in hippocampal neurons promotes dendritic outgrowthCitation36 by promoting the formation or stabilization of short dendritic branches. In addition, β-catenin is also a component of the signaling pathway that promotes activity mediated dendrite arborization.Citation36
Loss p120ctnCitation39 or δ-cateninCitation40,41 severely impair dendrite outgrowth in developing neurons. The role of ARVCF and p0071 in dendrite outgrowth remain undefined. The loss of p120ctn or δ-catenin in vivo leads to reduced levels of N-cadherin, consistent with a predicted functional role for p120ctn and δ-catenin in the trafficking and stabilization of N-cadherin.Citation42 The small GTP binding proteins, Rac and Rho have well defined critical roles in dendrite outgrowth and branching.Citation43 Interestingly, while both p120ctnCitation44 and δ-cateninCitation45 influence the levels of active Rac and increases the levels of active Rho,Citation39 a δ-catenin mouse model does not have any alterations in the global levels of Rac and Rho. Moreover, the LAP family protein, erbinCitation40 and cortactinCitation41 are critical regulators of the ability of δ-catenin to influence dendrite arborization. This suggests that while the members of the p120ctn family of proteins may regulate dendrite arborization and N-cadherin stability, they may recruit different signaling pathways to influence dendrite outgrowth. It would be interesting to determine the evolutionary significance of differential signaling by distinct members of the same family of proteins.
The Cadherin-Catenin Complex in Spine and Synapse Morphogenesis
The functional roles of the cadherin-catenin complex have been more widely investigated at excitatory synapses, particularly of those in the hippocampus and cortex. Components of the complex have critical roles in synapse formation/maintainance, synaptic transmission and synaptic plasticity.
Cadherin-catenin complex in synapse formation/maintenance
The localization of N-cadherin at excitatory synapses is maintained thorough development.Citation26 The localization of N-cadherin to pre- and post-synaptic structures suggests that their functional role may not be in target recognition at synapses, however their presence has been suggested to provide a permissive environment for maintaining synaptic contact. By taking advantage of technical advances in structured illumination microscopy, recent studies indicate that N-cadherin demonstrates a spectrum of localization patterns at synapses including clusters adjacent to the active zone or even distribution along the active zone with variations between these extremes.Citation46
Inhibition of cadherin function using a dominant negative approach, results in abnormal morphogenesis of spines, including filopodium-like elongation and spine head bifurcation.Citation47 However, the genetic deletion of N-cadherin using a Cre-loxP mediated approach in cultured neurons indicated no abnormalities in synaptic structure or function in the absence of N-cadherin.Citation38 Similar results were observed in mice in which N-cadherin was genetically ablated in late postnatal forebrain synapses.Citation48 However, in this case, the levels of the GluA1 receptor and PSD95, an excitatory postsynaptic scaffolding protein, were decreased. Interestingly, these changes were accompanied by an increase in the density of inhibitory synapse markers, suggesting a role for N-cadherin in maintaining an appropriate balance between excitatory and inhibitory synapses. The mechanisms underlying this functional role of N-cadherin need further dissection, since it has been previously described that N-cadherin is lost from inhibitory synapses as development proceeds.Citation26
N-cadherin is also a key player in the control of presynaptic vesicle clustering.Citation49 N-cadherin cooperates with another cell adhesion molecule, Neuroligin -1, to perform this functional role. Interestingly, this cooperation involves a trans-synaptic mechanism in which N-cadherin promotes postsynaptic accumulation of Neuroligin-1 and subsequent signaling by S-SCAM that in turn promotes presynaptic vesicle clustering.
Recent studies indicate that long-term asymmetric overexpression of N-cadherin in embryonic stem cell derived neurons leads to synapse elimination.Citation50 The mechanism underlying this functional ability of N-cadherin remains unclear, but it has been suggested that cis-interactions of N-cadherin with non-cadherin receptors or lack of trans-synaptic N-cadherin signaling promotes retrograde signaling via unknown signaling pathways. Further experimental evidence is necessary to determine if either of these models is pertinent. Synaptic development proceeds through a number of stages including both synapse formation and elimination. Given these studies, it is tempting to suggest that asymmetric N-cadherin expression may be a trigger for synaptic elimination during development.
N-cadherin has also been implicated in regulating spine dynamics and spine stability.Citation51 Acute interference of the functional role of N-cadherinCitation52 using a peptide mediated approach promotes spine motility followed by decrease in spine length and spine loss, suggesting that N-cadherin may have a key role in stabilizing spines. These functional roles may also require intracellular signaling by N-cadherin since the expression of a protein expressing a significant deletion of the extracellular region of N-cadherin also interferes with spine stabilization.Citation53
N-cadherin is synthesized in the endoplasmic reticulum. Cleavage of its signal sequence leads to the generation of a prodomain form of N-cadherin, proN-cadherin,that is then further processed to generate the mature N-cadherin. Contrary to the expectation that proN-cadherin is not localized to cell surface, recent data indicate that not only is proN-cadherin localized to cell surfaces in hippocampal neurons, but also is a critical regulator of synapse formation.Citation54 Overexpression of a proN-cadherin form inhibits synapse formation concomitant with a reduction in synaptic function.
The classical cadherins also participate in regulating inhibitory synapses. There is evidence to indicate that postsynaptic loss of E-cadherin leads to a reduction in the density of inhibitory synapses, without affecting the density of excitatory synapses,Citation55 suggesting that E-cadherin participates in retrograde signaling to regulate inhibitory synapses. The molecular partners that cooperate with E-cadherin to regulate its functional role at inhibitory synapses remain unclear, although the catenins are attractive candidates.
In addition to N-cadherin, recent studies indicate that other type 2 cadherins also actively participate in synapse development. Cadherin-8 is required for formation of synapses in the hippocampal mossy fibers.Citation35 Cadherin-11 and -13 have been shown are required for both glutamatergic and GABAergic synaptogenesis.Citation56 Cadherin-9 regulates the formation of the DG-CA3 hippocampal synapses in vitro and in vivo.Citation25 Cadherin-7 regulates the formation of synapses between the pontine nucleus and the granule cells in the cerebellum.Citation57
N-cadherin also cooperates with other cell adhesion molecules, for example, neuroliginCitation58 and the protocadherin arcadlin,Citation59 to regulate excitatory synapses. Thus, the classical cadherins participate in synapse formation and maintainance by a variety of different mechanisms that may or may not rely solely on their ability to interact with the catenins.
β-catenin regulates synaptic structure and function at the pre and postsynaptic sites by several different mechanisms. The mRNA encoding β-catenin localizes to recently formed presynaptic boutons, where it is locally translated and regulates the dynamics of synaptic vesicles.Citation60,61 Targeted loss of β-catenin in glutamatergic neurons in a mouse model leads to an increase in the number of synapses concomitant with a reduction in the non docked pool of synaptic vesicles with no alterations in the docked pool of vesicles.Citation61 This was accompanied by a functional reduction in the replenishment of the readily releasable pool using a prolonged stimulation protocol, suggesting that loss of β-catenin perturbs both synaptic structure and function presynaptically. The ability of β-catenin to regulate synaptic vesicle localization is mediated in part by its ability to recruit with PDZ domain containing proteins, including scribble.Citation62 β-catenin is also localized to inhibitory synapses, however its functional roles at these synapses remain undefined. Postsynaptic genetic loss of β-catenin reduces the amplitude of mEPSCs in a manner dependent on the arm and C-terminal PDZ-binding motifs of β-catenin.Citation63
The loss of αN-catenin in a mouse model leads to an increase in spine motility and turnover. Conversely, overexpression of αN-catenin promotes spine density and stabilizes spines. This ability of αN-catenin is dependent on its ability to interact with various cytoskeletal proteins.Citation64
Genetic loss of p120ctn in a mouse models leads to a reduction in the density of excitatory spines and synapses. These alterations are also associated with alterations in the spine length and headwidth, both at developing and mature synapses. The ability of p120ctn to regulate spine density is independent of its ability to interact with cadherin. The small G-proteins Rac and Rho have well-established functional roles in spine and synapse morphogenesis. Loss of p120ctn results in increased levels of active RhoA and reduction in active Rac1 and perturbations in the levels of these small G-proteins contributes to the ability of p120ctn to regulate spine density and architecture. Thus, p120ctn can regulate spine density and architecture via cadherin dependent and independent mechanisms.
There is some discrepancy in literature about the functional roles of δ-catenin in spine morphogenesis. One study indicated that overexpression of δ-catenin leads to filopodia like protrusions in a manner consistent with functional cooperation of δ-catenin with the small GTP binding proteins of the Rho family,Citation45 while knockdown of δ-catenin leads to a decrease in mature spines and increase in filopodia. In contrast, another study indicated that loss of δ-catenin the δ-catenin N-term mouse model or knockdown in cultured primary neurons leads to an increase in spine and synapse density and function during development. This functional role is independent of the ability of δ-catenin to bind cadherin. Interestingly, no differences in the levels of total active Rac and Rho were observed in cultured neurons from the control and δ-catenin N-term mouse model. It is unclear what the molecular bases of these conflicting results are if they are related to experimental conditions. However, they do suggest a critical role for δ-catenin in regulating spine density and architecture. In the adult mouse model of δ-catenin, dendritic complexity and spine density are unchanged in comparison to control mice. However, later δ-catenin N-term mice experience progressive dendritic retraction, a reduction in spine density and stability. These studies argue for distinct functional roles for δ-catenin spine formation during development and maintainance in the adult. It remains to be determined if the mechanisms underlying the ability of δ-catenin to regulate spines at different stages is mediated by different effectors.
δ-catenin is also a critical regulator of glutamate receptor trafficking, particularly the GluR2 subunit via interactions with (AMPAR)-binding protein ABP and glutamate receptor (GluR)-interacting protein GRIP.Citation65,66 These functional roles are mediated by PDZ-mediated interactions. The roles of ARVCF and p0071 in spine and synapse organization remain undefined. However, both of these proteins are expressed in the hippocampus and have PDZ motifs at their C-termini similar to δ-catenin. Given the critical roles of p120ctn and δ-catenin in regulating excitatory synapses, it would be interesting to determine the contribution of the other family members to excitatory synapse development and maintainance in hippocampal neurons and examine their functional redundancy.
The cadherin-catenin complex in synaptic transmission and synaptic plasticity
The cadherin-catenin complex is in a key position to coordinate the conversion of extracellular stimuli to alterations in cell signaling pathways. Given this ability, it is perhaps not surprising that the components of the complex are key regulators of synaptic plasticity in central neurons.
Mice that are genetically null for N-cadherin are lethal. By taking advantage of in vitro differentiation of embryonic stem cells to neurons, it has been demonstrated that N-cadherin presynaptically regulates synaptic vesicle availability at glutamatergic synapses, particularly during enhanced activity.Citation67 The absence of N-cadherin in these neurons also leads to an enhancement of synaptic depression and an alteration in short-term plasticity. In addition, facilitation was converted to depression under specific stimulation conditions. In this system, asymmetric loss of N-cadherin restricted to postsynaptic regions also affects functional synapse formation, consistent with a trans-synaptic functional role in regulating synaptic plasticity. However, the molecular mechanisms that underlie this functional ability of N-cadherin remain unexplored.
Synaptic activity is a key factor in long-term synapse stabilization. The induction of long-term potentiation (LTP) promotes the formation of N-cadherin clusters in spines that are stimulated. Consistently, expression of N-cadherin promotes synapse stability while expression of the a mutant form of N-cadherin expressing the intracellular domain or knockdown of N-cadherin prevents LTP-induced long-term synapse stabilization.Citation53
One of the key features underlying the ability of synaptic plasticity is structural plasticity of spines. N-cadherin is a key regulator of depolarization mediated increase in spine headwidth via coupling to the actin cytoskeletonCitation68 in cultured primary neurons. Loss of N-cadherin in a mouse model at mature CA1 synapses, suggests that N-cadherin is required for persistence, but not induction or initial expression, of LTP and spine enlargement.Citation69 Synaptic activity, particularly the activation of the NMDA receptor, inhibits the endocytosis of N-cadherin resulting in its surface accumulation. This is dependent on the ability of N-cadherin to interact with β-catenin. The stabilization of surface N-cadherin inhibits NMDA-dependent synaptic plasticity. Thus N-cadherin provides a link between structural alterations and synaptic plasticity.Citation70
N-cadherin is modulated by synaptic activity. Synaptic activity promotes dimerization of the protein resulting in a trypsin-resistant form.Citation71 Recent studies also indicate that synaptic stimulation causes a relocalization of N-cadherin to the peripheral regions of the synapse while recovery causes translocation to a more central location within the synapse.Citation46 The molecular mechanisms that underlie the ability of N-cadherin to relocate likely involve association with catenins.
The localization of αN-catenin at synapses is regulated by synaptic activity. In cultured primary hippocampal neurons, tetrodotoxin inhibits the synaptic accumulation of αN-catenin, while bicuculline, a GABAA receptor antagonist, enhances total and synaptic levels of αN-catenin. Overexpression of αN-catenin renders spines resistant to tetrodotoxin induced enhancement of spine length. Thus, αN-catenin is a key component of the machinery that underlies activity induced structural plasticity of spines.
β-catenin is also a critical regulator of activity dependent synaptic size and strength.Citation63 Synaptic activity drives the relocalization of β-catenin from dendritic shafts to spine heads with enhanced interaction with cadherin. This functional role is critically dependent on the ability of β-catenin to be dephosphorylated at Y654 that affects its ability to interact with cadherin.Citation72
Enhanced synaptic activity promotes the transient palmitoylation of δ-catenin that in turn leads to it enhanced interaction with cadherin.Citation73 The palmitoylated form of δ-catenin stabilizes N-cadherin at synapses and promotes spine enlargement and insertion of the GluA1 and GluA2 subunits into the synaptic membrane, accompanied by an increase in the amplitude of mEPSCs. Thus, δ-catenin is a key regulator of activity dependent synaptic alterations at synapses and mediates this functional role via interaction with cadherin. It would be interesting to determine if the other members of the δ-catenin family of proteins regulate synaptic structure and function in a similar manner.
Thus, the cadherins and catenins are key regulators of dendrite and synapse structure, function and plasticity. Consistent with the key roles of these proteins in morphological and functional alterations associated with higher level behavior, mouse models of these proteins have aberrations in learning and other forms of higher level behavior including cognitive dysfunction,Citation74 memory consolidation and cognitive flexibility.Citation75
Cadherins in Neural Disorders
The human brain has about one hundred billion (1011) neurons, and each neuron makes on average 7,000 synaptic connections to other neurons. Estimates for the total number of synapses in the brain ranges from 1014 to 1015 depending on the age.Citation76 Therefore, subtle changes in the connectivity patterns of a subset of synapses or in their basal synaptic transmission and plasticity, particularly during development, might dramatically alter the neuronal circuitry, contributing to neural disorders. The current prevailing etiology model of neuropsychiatric disorders such as schizophrenia and autism suggests subnormal or abnormal functional connectivity and coherent information processing in affected patients. Considering the direct involvement of cadherin-based adhesive system and signaling pathways to neurodevelopment and synaptic transmission and plasticity, the cadherin superfamily genes are excellent candidates for causal mutations of neurological, neurodevelopmental, and neuropsychiatric disorders. Indeed in many genome-wide association studies cadherins have been associated with autism, bipolar disease or schizophrenia as well as with Alzheimer's disease. It is noteworthy that not only cadherin superfamily members but also the catenins, αN-catenin, β-catenin and δ-catenin, have been repeatedly associated with these neural disordersCitation77-79 ().
Table 2. Cadherin superfamily members implicated in neuronal disorders
Neuropsychiatric disorders
Several classical cadherins have been associated with autism spectrum disorders. A common genetic variant in an intergenic region between CDH9 and CDH10 has shown robust association in 2 independent cohorts.Citation80 Although the transcript level of both genes appeared similar regardless of the variant genotype in adult brain tissues, their developmental expression pattern still awaits further investigation. It is noteworthy that CDH10 but not CDH9 expression is high in orbitofrontal and frontal cortex, brain regions implicated in autism pathophysiology. Microdeletions involving CDH8 have been detected in 2 independent families with autism and learning disability, though they are not completely penetrant.Citation81 In some schizophrenic patients, an intergenic deletion between classical CDH12 and CDH18 was identified.Citation82 With bipolar disorders, CDH7 shows robust repeated associationsCitation83 and CDH11 is associated with comorbidity of alcoholism and bipolar disorders.Citation84
Similarly, both clustered and nonclustered protocadherins have been identified for genetic association with autism. A deletion of PCDH10Citation85 has been reported in a rare case of autism. From the clustered protocadherins, a de novo deletion in PCDHA13 has been observed in autism,Citation86 and small nucleotide polymorphisms in the middle of PCDHACitation87 cluster (α-protocadherin) appear to have strong association with autism.
FAT, a member of the atypical cadherins, has been associated with bipolar disorder and its expression was down-regulated in response to therapeutic lithium treatment in mice.Citation88 CDH13 (T-cadherin), the truncated cadherin member lacking transmembrane or cytoplasmic domain with negative regulatory function in axonal growth, has been identified in multiple genome-wide association studies of different psychiatric disorders including schizophrenia, bipolar disorder, and ADHD (attention deficit hyperactivity disorder).Citation89
Notably in a similar pattern to CDH13, CTNNA2, the αN-catenin gene, has been repeatedly identified from multiple genome-wide association studies of similar psychiatric disorders. It also has been verified to have differential gene expression in schizophrenia patients.Citation90
Intellectual disability
Heterozygous missense mutations in M-cadherin gene (CHD15, classical type I) have been identified in autosomal dominant intellectual disability. Three of the mutations were mapped in the N-terminal ectodomain, EC1, which is critical for homophilic dimerization and shown to reduce cell adhesion in in vitro functional expression studies. Despite M-cadherin's high expression in skeletal muscle and cerebellum, the 4 M-cadherin mutations in humans were identified in individuals with mild to severe mental retardation without any obvious skeletal muscle defects.Citation91
The cri-du-chat syndrome, also known as chromosome 5p deletion syndrome, is associated with an unusual high-pitched cry at birth, facial dysmorphology, poor growth, and severe mental retardation. The gene encoding δ-catenin (CTNND2) maps to a specific region in 5p15.2 implicated in the mental retardation phenotype of cri-du-chat syndrome. There is a strong correlation between hemizygous loss of CTNND2 and severity of mental retardation phenotype.Citation77
Alzheimer's disease
The association of δ-catenin with Alzheimer's disease has long been suspected since its gene was discovered. δ-catenin was initially identified in a yeast 2-hybrid screen for its interaction with presenilin-1 (PS1), the molecule most frequently mutated in familial Alzheimer's disease. More recently, a genome wide association study implicated strong co-heritability of age-related cortical cataract (CC) and Alzheimer's disease-related brain MRI traits, especially temporal horn volume (THV) measurements. CTNND2 SNPs were significantly associated with the bivariate outcome of CC and THV.Citation92 Additionally, a rare CTNND2 missense mutation was shown to cause altered δ-catenin localization and increase fibrillogenic amyloid-β1–42 (Aβ42) secretion in neuronal cell culture. These findings suggest that genetic variation in δ-catenin may underlie both cortical lens opacities in mid-life and subsequent MRI and cognitive changes that presage the development of Alzheimer's disease.
Likewise, N-cadherin has drawn attention for its direct interactions with presenilin-1/γ-secretase complex and its modulation of Aβ peptide formation, which plays a critical role in Alzheimer's disease etiology.Citation93 Interestingly, the blockade of N-cadherin function accelerated Aβ synaptotoxicity, and furthermore patients with Alzheimer's disease have increased level of proteolytically-cleaved N-cadherin C-terminal fragment 194. However, no evidence for its genetic association with the disease has yet been reported.
Epilepsy
PCDH19 on chromosome Xq22.3 encodes protocadherin-19, a member of the δ2 subclass of nonclustered protocadherins. Several heterozygous missense and nonsense mutations of PCDH19 have been found in unrelated patients with the syndrome of female-restricted epilepsy and mental retardation (EFMR), also known as early infantile epileptic encephalopathy-9, affecting only heterozygous females and sparing hemizygous males.Citation95 Most mutations appear to affect the function of PCDH19 by early truncation or influencing its adhesiveness on the ectodomain.Citation96 Since the PCDH19 gene is subject to X-inactivation, affected females are likely to be mosaics comprising PCDH19-mutant and PCDH19-wildtype cells, whereas hemizygous asymptomatic males have a homogeneous population of PCDH19-mutant cells. This tissue mosaicism in females may scramble cell-cell communication (termed cellular interference), which then manifests clinically as early infantile epileptic encephalopathy. Yet, mosaic males, though rarely found, can also be affected, supporting cellular interference as the pathogenic mechanism. Recently, mutations in PCDH19, mostly occurring de novo, were shown to be a frequent cause of sporadic infantile-onset epileptic encephalopathy in females and its clinical features overlapping with Dravet syndrome, the most common type of epilepsy. PCDH19 mutations were also identified in epileptic females without cognitive impairment and it has become the second most causative gene in epilepsy after SCN1A.
Perspectives for the Future
In the past several years, we have made significant progress in dissecting the functional roles of the cadherins and catenins in various aspects of neuronal morphogenesis and synaptic plasticity. However, several exciting questions remain.
It is now clear that the classical cadherins are key regulators of synaptic structure and function. The existence of a vast repertoire of members of the cadherin superfamily and their expression patterns in central neurons suggests that several of them may have functional roles in regulating dendrite and spine architecture and function that remain to be fully dissected. Moreover, it is not entirely clear how the catenins contribute to the functional roles of the non-classical cadherins in central neurons. The vast majority of studies have also focused mainly on excitatory synapses and our knowledge of the contribution of the cadherin catenin complex to inhibitory synaptogenesis remains incomplete.
Cadherin based interactions are dependent on calcium. Synaptic activity rapidly alters the calcium content of the synaptic cleft. How these alterations in calcium are translated into adhesive changes mediated by cadherin remains unexplored. It would be interesting to determine if cadherins function as transducers of information encoded by alterations in extracellular calcium.
While the classical composition of the cadherin catenin complex has been suggested to include cadherin, α-catenin, β-catenin and a member of the p120ctn family of proteins, it is now becoming clear that other proteins may compete with the catenins for binding to cadherins since they share the site of interaction. For example, one of these proteins includes Vangl2, a component of the Wnt/planar cell polarity pathway. Interestingly, Vagnl2 also regulates spine and synapse morphogenesis and can compete with β-catenin for binding to N-cadherin.Citation97 Thus, in addition to regulating various aspects of neuronal morphogenesis through components of the classical cadherin-catenin cell adhesion complex, components of the complex may recruit other proteins to regulate neuronal structure and function. Future studies might thus be directed toward closely elucidating neuron sub-type specific complex composition and function, determining the functional significance of these interactions, dissecting out these functional roles in vivo in a neuron-type specific manner and determining the functional significance of cross talk between multiple signaling pathways in a neuron-type specific manner.
The roles of the p120ctn family of proteins appear to be somewhat functionally redundant, since their loss results in similar phenotypes. However, knockout of one member of the family does not appear to be compensated by upregulation of other members of the family. This suggests that in addition to functional roles that might overlap with other family members, each protein may either have unique binding partners that might regulate its ability to function in other neuronal signaling pathways. Moreover, there is some evidence to indicate that while the loss of function phenotypes may be similar for p120ctn and δ-catenin and presumably the other members of the family, the underlying signaling mechanisms that relay information to the cytoskeleton may vary. This may reflect yet unappreciated functional roles of these proteins in various aspects of neuronal morphogenesis. Accepting the challenge of dissecting out these signaling pathways and functional roles may lead to critical insights into the functional roles of these proteins in central neurons.
In addition to their association and functional roles as components of the cadherin-catenin complex, the catenins participate in other signaling mechanisms in a cadherin-dependent or independent manner.Citation98 Several of these functional roles have also been examined in non-neuronal cells. For example, β-catenin regulates transcriptional effects via the TCF/Lef signaling pathway. Several components of the pathway participate in the Wnt signaling pathway that in turn regulates a variety of functional roles. p120ctn interacts with kaiso, a transcriptional repressor in the nucleus, thus regulating transcription. Thus, by virtue of their ability to participate in multiple signaling pathways in a cadherin dependent or independent manner, the catenins are uniquely suited to mediate crosstalk between multiple signaling pathways. Association with cadherins uniquely positions them to function in relaying information from the environment to intracellular signaling pathways to elicit functional responses. Hence, they are also uniquely suited to participate in transducing external stimuli through alternate signaling pathways and mediate cross talk between signaling pathways, thus allowing finer control of responses to synaptic stimuli. The challenge is to determine the identity of stimuli specific signaling pathways, their outcomes and contribution to neuronal development and neurological disorders.
Alterations in dendritic and spine architecture and function have been implicated in a variety of neurodevelopmental, neuropsychiatric and neurological disorders. Cadherins and catenins have been implicated in neurodevelopmental disorders, particularly those associated with learning disabilities. Given the determined and predicted functional roles of cadherins and catenins in various aspects of neuronal development, cadherin and catenin family members are good candidates for neuronal disorders associated with aberrations in dendrite and synapse architecture and function. Dissecting out the signaling pathways that are aberrant with mutations or alterations in cadherins and catenins may offer vital insights into therapeutic approaches for these devastating disorders.
We are now uniquely positioned to address these challenges given the advances in technological innovations that allow the generation of mouse models, advances in DNA sequencing, including Next gen and Deep sequencing, single cell gene sequencing, advances in proteomics and bioinformatics and the tools of microscopy. By taking advantage of these technical advances, we anticipate that the next several years will bring forth a cascade of data that will significantly enhance our understanding of the role of the cadherin-catenin complex in central neurons and aid in the generation of therapeutic approaches for neurodevelopmental and neurological disorders associated with aberrations in components of this complex.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Deans, MR, Krol A, Abraira VE, Copley CO, Tucker AF, Goodrich LV. Control of neuronal morphology by the atypical cadherin Fat3. Neuron 2011; 71:820-32; PMID:21903076; http://dx.doi.org/10.1016/j.neuron.2011.06.026 S0896-6273(11)00556-3 [pii]
- Matsunaga E, Nambu S, Oka M, Iriki A. Differential cadherin expression in the developing postnatal telencephalon of a New World monkey. J Comp Neurol 2013; 521:4027-60; PMID:23784870; http://dx.doi.org/10.1002/cne.23389
- Li Y, Xiao H, Chiou TT, Jin H, Bonhomme B, Miralles CP, Pinal N, Ali R, Chen WV, Maniatis T, De Blas AL, et al. Molecular and functional interaction between protocadherin-gammaC5 and GABAA receptors. J Neurosci 2012; 32:11780-97; PMID:22915120; http://dx.doi.org/10.1523/JNEUROSCI.0969- 12.2012 32/34/11780 [pii]
- McLachlan IG, Heiman MG. Shaping dendrites with machinery borrowed from epithelia. Curr Opin Neurobiol 2013; 23:1005-10; PMID:23871793; http://dx.doi.org/10.1016/j.conb.2013.06.011 S0959-4388(13)00130-X [pii]
- Hirano S, Takeichi M. Cadherins in brain morphogenesis and wiring. Physiol Rev 2012; 92:597-634; PMID:22535893; http://dx.doi.org/10.1152/physrev.00014.2011 92/2/597 [pii] (2012)
- Takeichi M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol 2014; 15:397-410; PMID:24824068; http://dx.doi.org/10.1038/nrm3802 nrm3802 [pii]
- Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci 2007; 8:11-20; PMID:17133224; nrn2043 [pii] http://dx.doi.org/10.1038/nrn2043
- Shapiro L, Weis WI. Structure and biochemistry of cadherins and catenins. Cold Spring Harb Perspect Biol 2009; 1:a003053; http://dx.doi.org/10.1101/cshperspect.a003053
- Troyanovsky RB, Sokolov E, Troyanovsky SM. Adhesive and lateral E-cadherin dimers are mediated by the same interface. Mol Cell Biol 2003; 23:7965-72
- Harrison OJ, Bahna F, Katsamba PS, Jin X, Brasch J, Vendome J, Ahlsen G, Carroll KJ, Price SR, Honig B, Shapiro L et al. Two-step adhesive binding by classical cadherins. Nat Struct Mol Biol 2010; 17:348-57; PMID:20190754; http://dx.doi.org/10.1038/nsmb.1784 nsmb.1784 [pii]
- Brasch J, Harrison OJ, Honig B, Shapiro L. Thinking outside the cell: how cadherins drive adhesion. Trends Cell Biol 2012; 22:299-310; doi:10.1016/j.tcb.2012.03.004 S0962-8924(12)00052-9 [pii]
- Batlle E, Wilkinson DG. Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Cold Spring Harb Perspect Biol 2012; 4:a008227; http://dx.doi.org/10.1101/cshperspect.a008227 a008227 [pii] 4/1/a008227 [pii]
- Katsamba P, Carroll K, Ahlsen G, Bahna F, Vendome J, Posy S, Rajebhosale M, Price S, Jessell TM, Ben-Shaul A, et al. Linking molecular affinity and cellular specificity in cadherin-mediated adhesion. Proc Natl Acad Sci U S A 2009; 106:11594-9; PMID:19553217; http://dx.doi.org/10.1073/pnas.0905349106 0905349106 [pii]
- Ounkomol C, Yamada S, Heinrich V. Single-cell adhesion tests against functionalized microspheres arrayed on AFM cantilevers confirm heterophilic E- and N-cadherin binding. Biophys J 2010; 99:L100-102; PMID:21156120; http://dx.doi.org/10.1016/j.bpj.2010.11.013 S0006-3495(10)01382-2 [pii]
- Shimoyama Y, Tsujimoto G, Kitajima M, Natori M. Identification of three human type-II classic cadherins and frequent heterophilic interactions between different subclasses of type-II classic cadherins. Biochem J 2000; 349:159-67; PMID:10861224
- Wu Q, Maniatis T. Large exons encoding multiple ectodomains are a characteristic feature of protocadherin genes. Proc Natl Acad Sci U S A 2000; 97:3124-9; PMID:10716726; http://dx.doi.org/10.1073/pnas.060027397 060027397 [pii]
- Schreiner D, Weiner JA. Combinatorial homophilic interaction between gamma-protocadherin multimers greatly expands the molecular diversity of cell adhesion. Proc Natl Acad Sci U S A 2010; 107:14893-8; http://dx.doi.org/10.1073/pnas.1004526107 1004526107 [pii]
- Kim SY, Yasuda S, Tanaka H, Yamagata K, Kim H. Non-clustered protocadherin. Cell Adh Migr 2011; 5:97-105; PMID:21173574; http://dx.doi.org/ 14374 [pii]
- Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol 1996; 135:767-79.
- Arikkath J, Reichardt LF. Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci 2008; 31:487-94; doi:10.1016/j.tins.2008.07.001 S0166-2236(08)00166-5 [pii]
- Redies C, Inuzuka H, Takeichi M. Restricted expression of N- and R-cadherin on neurites of the developing chicken CNS. J Neurosci 1992; 12:3525-34
- Bozdagi O, Shan W, Tanaka H, Benson DL, Huntley GW. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron 2000; 28:245-59; http://dx.doi.org/ S0896-6273(00)00100-8 [pii]
- Fannon AM. Colman DR. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron 1996; 17:423-34; http://dx.doi.org/ S0896-6273(00)80175-0 [pii]
- Manabe, T, Togashi H, Uchida N, Suzuki SC, Hayakawa Y, Yamamoto M, Yoda H, Miyakawa T, Takeichi M, Chisaka O. Loss of cadherin-11 adhesion receptor enhances plastic changes in hippocampal synapses and modifies behavioral responses. Mol Cell Neurosci 2000; 15:534-46; http://dx.doi.org/10.1006/mcne.2000.0849 S1044-7431(00)90849-2 [pii]
- Williams, ME, Wilke SA, Daggett A, Davis E, Otto S, Ravi D, Ripley B, Bushong EA, Ellisman MH, Klein G, et al. Cadherin-9 regulates synapse-specific differentiation in the developing hippocampus. Neuron 2011; 71:640-55; http://dx.doi.org/10.1016/j.neuron.2011.06.019 S0896-6273(11)00547-2 [pii]
- Benson DL, Tanaka H. N-cadherin redistribution during synaptogenesis in hippocampal neurons. J Neurosci 1998; 18:6892-904; PMID:9712659
- Ozawa M. Baribault H. Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J 1989; 8:1711-17
- Drees F. Pokutta S. Yamada S. Nelson WJ. Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 2005; 123:903-15; http://dx.doi.org/ S0092-8674(05)00975-X [pii] 10.1016/j.cell.2005.09.021
- Yamada S. Pokutta S. Drees F. Weis WI. Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell 2005; 123:889-901; http://dx.doi.org/ S0092-8674(05)00974-8 [pii] 10.1016/j.cell.2005.09.020
- Benjamin JM, Kwiatkowski AV, Yang C, Korobova F, Pokutta S, Svitkina T, Weis WI, Nelson WJ. AlphaE-catenin regulates actin dynamics independently of cadherin-mediated cell-cell adhesion. J Cell Biol 2010; 189:339-52; http://dx.doi.org/10.1083/jcb.200910041 jcb.200910041 [pii]
- Heuberger J. Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol 2010; 2:a002915; http://dx.doi.org/10.1101/cshperspect.a002915
- Carnahan RH. Rokas A. Gaucher EA. Reynolds AB. The molecular evolution of the p120-catenin subfamily and its functional associations. PLoS One 2010; 5:e15747; http://dx.doi.org/10.1371/journal.pone.0015747
- Maiden SL. Hardin J. The secret life of alpha-catenin: moonlighting in morphogenesis. J Cell Biol 2011; 195, 543-52; http://dx.doi.org/10.1083/jcb.201103106 jcb.201103106 [pii]
- Desai, R, Sarpal R, Ishiyama N, Pellikka M, Ikura M, Tepass U. Monomeric alpha-catenin links cadherin to the actin cytoskeleton. Nat Cell Biol 2013; 15, 261-273; http://dx.doi.org/10.1038/ncb2685 ncb2685 [pii]
- Bekirov IH, Nagy V, Svoronos A, Huntley GW, Benson DL. Cadherin-8 and N-cadherin differentially regulate pre- and postsynaptic development of the hippocampal mossy fiber pathway. Hippocampus 2008; 18:349-363; http://dx.doi.org/10.1002/hipo.20395
- Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci 2003; 6, 1169-1177; http://dx.doi.org/10.1038/nn1132 nn1132 [pii]
- Tan ZJ, Peng Y, Song HL, Zheng JJ, Yu, X. N-cadherin-dependent neuron-neuron interaction is required for the maintenance of activity-induced dendrite growth. Proc Natl Acad Sci U S A 2010; 107, 9873-78; http://dx.doi.org/10.1073/pnas.1003480107 1003480107 [pii]
- Kadowaki M, Nakamura S, Machon O, Krauss S, Radice GL, Takeichi M. N-cadherin mediates cortical organization in the mouse brain. Dev Biol 2007; 304, 22-33; http://dx.doi.org/ S0012-1606(06)01432-1 [pii] 10.1016/j.ydbio.2006.12.014
- Elia LP, Yamamoto M, Zang K, Reichardt LF. p120 catenin regulates dendritic spine and synapse development through Rho-family GTPases and cadherins. Neuron 2006; 51, 43-56; http://dx.doi.org/ S0896-6273(06)00410-7 [pii] 10.1016/j.neuron.2006.05.018
- Arikkath, J, Israely I, Tao Y, Mei L, Liu X, Reichardt LF. Erbin controls dendritic morphogenesis by regulating localization of delta-catenin. J Neurosci 2008; 28:7047-56; PMID:18614673; http://dx.doi.org/10.1523/JNEUROSCI.0451-08.2008 28/28/7047 [pii]
- Martinez MC, Ochiishi T, Majewski M, Kosik KS. Dual regulation of neuronal morphogenesis by a delta-catenin-cortactin complex and Rho. J Cell Biol 2003; 162:99-111; PMID:12835311; http://dx.doi.org/10.1083/jcb.200211025 jcb.200211025 [pii]
- Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol 2003; 163:525-534; PMID:14610055; http://dx.doi.org/10.1083/jcb.200307111 jcb.200307111 [pii]
- de Curtis I. Functions of Rac GTPases during neuronal development. Dev Neurosci 2008; 30:47-58; PMID:18075254; http://dx.doi.org/ 000109851 [pii] 10.1159/000109851
- Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB. Inhibition of RhoA by p120 catenin. Nat Cell Biol 2000; 2, 637-644; http://dx.doi.org/10.1038/35023588
- Abu-Elneel K, Ochiishi T, Medina M, Remedi M, Gastaldi L, Caceres A, Kosik KS. A delta-catenin signaling pathway leading to dendritic protrusions. J Biol Chem 2008; 283:32781-91; http://dx.doi.org/10.1074/jbc.M804688200 M804688200 [pii]
- Yam PT, Pincus Z, Gupta GD, Bashkurov M, Charron F, Pelletier L, Colman DR, N-cadherin relocalizes from the periphery to the center of the synapse after transient synaptic stimulation in hippocampal neurons. PLoS One 2013; 8, e79679; http://dx.doi.org/10.1371/journal.pone.0079679 PONE-D-13-23642 [pii]
- Togashi H. Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron 2002; 35:77-89; http://dx.doi.org/ S0896627302007481 [pii]
- Nikitczuk JS. Patil SB, Matikainen-Ankney BA, Scarpa J, Shapiro ML, Benson DL, Huntley GW. N-cadherin regulates molecular organization of excitatory and inhibitory synaptic circuits in adult hippocampus in vivo. Hippocampus 2014; 24:943-62; http://dx.doi.org/10.1002/hipo.22282
- Stan A, Pielarski KN, Brigadski T, Wittenmayer N, Fedorchenko O, Gohla A, Lessmann V, Dresbach T, Gottmann K. Essential cooperation of N-cadherin and neuroligin-1 in the transsynaptic control of vesicle accumulation. Proc Natl Acad Sci U S A 2010; 107:11116-21; http://dx.doi.org/10.1073/pnas.0914233107 0914233107 [pii]
- Pielarski KN. van Stegen B, Andreyeva A, Nieweg K, Jüngling K, Redies C, Gottmann K. Asymmetric N-cadherin expression results in synapse dysfunction, synapse elimination, and axon retraction in cultured mouse neurons. PLoS One 2013; 8, e54105; http://dx.doi.org/10.1371/journal.pone.0054105 PONE-D-12-24919 [pii]
- Wang X, Cahill ME, Werner CT, Christoffel DJ, Golden SA, Xie Z, Loweth JA, Marinelli M, Russo SJ, Penzes P, et al. Kalirin-7 mediates cocaine-induced AMPA receptor and spine plasticity, enabling incentive sensitization. J Neurosci 2013; 33:11012-22; http://dx.doi.org/10.1523/JNEUROSCI.1097-13.2013 33/27/11012 [pii]
- Mysore SP, Tai CY, Schuman EM. Effects of N-cadherin disruption on spine morphological dynamics. Front Cell Neurosci 2007; 1, 1; http://dx.doi.org/10.3389/neuro.03.001.2007
- Mendez P, De Roo M, Poglia L, Klauser P, Muller D. N-cadherin mediates plasticity-induced long-term spine stabilization. J Cell Biol 2010; 189:589-600; http://dx.doi.org/10.1083/jcb.201003007 jcb.201003007 [pii]
- Reines A, Bernier LP, McAdam R, Belkaid W, Shan W, Koch AW, Séguéla P, Colman DR, Dhaunchak AS. N-cadherin prodomain processing regulates synaptogenesis. J Neurosci 2012; 32:6323-34; http://dx.doi.org/10.1523/JNEUROSCI.0916-12.2012 32/18/6323 [pii]
- Fiederling A, Ewert R, Andreyeva A, Jungling K, Gottmann K. E-cadherin is required at GABAergic synapses in cultured cortical neurons. Neurosci Lett 2011; 501:167-72; http://dx.doi.org/10.1016/j.neulet.2011.07.009 S0304-3940(11)01042-1 [pii]
- Paradis S, Harrar DB, Lin Y, Koon AC, Hauser JL, Griffith EC, Zhu L, Brass LF, Chen C, Greenberg ME. An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron 2007; 53:217-32; http://dx.doi.org/ S0896-6273(06)00997-4 [pii] 10.1016/j.neuron.2006.12.012
- Kuwako K, Nishimoto Y, Kawase S, Okano HJ, Okano H. Cadherin-7 regulates mossy fiber connectivity in the cerebellum. Cell Rep 2014; 9, 311-23; PMID:25284782; http://dx.doi.org/; http://dx.doi.org/10.1016/j.celrep.2014.08.063 S2211-1247(14)00739-6 [pii]
- Aiga M, Levinson JN, Bamji SX. N-cadherin and neuroligins cooperate to regulate synapse formation in hippocampal cultures. J Biol Chem 2011; 286:851-58; http://dx.doi.org/ 10.176305.[pii]
- Yasuda S, Tanaka H, Sugiura H, Okamura K, Sakaguchi T, Tran U, Takemiya T, Mizoguchi A, Yagita Y, Sakurai T, et al. Activity-induced protocadherin arcadlin regulates dendritic spine number by triggering N-cadherin endocytosis via TAO2beta and p38 MAP kinases. Neuron 2007; 56:456-71; http://dx.doi.org/ S0896-6273(07)00663-0 [pii] 10.1016/j.neuron.2007.08.020
- Taylor AM, Wu J, Tai HC, Schuman EM. Axonal translation of beta-catenin regulates synaptic vesicle dynamics. J Neurosci 2013; 33:5584-89; http://dx.doi.org/10.1523/JNEUROSCI.2944-12.2013 33/13/5584 [pii]
- Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, Reichardt LF. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron 2003; 40:719-31; http://dx.doi.org/ S0896627303007189 [pii]
- Sun Y, Aiga M, Yoshida E, Humbert PO, Bamji SX. Scribble interacts with beta-catenin to localize synaptic vesicles to synapses. Mol Biol Cell 2009; 20:3390-3400; http://dx.doi.org/10.1091/mbc.E08-12-1172 E08-12-1172 [pii]
- Okuda T, Yu LM, Cingolani LA, Kemler R, Goda Y. beta-Catenin regulates excitatory postsynaptic strength at hippocampal synapses. Proc Natl Acad Sci U S A 2007; 104:13479-13484; http://dx.doi.org/ 0702334104 [pii] 10.1073/pnas.0702334104
- Abe K, Chisaka O, Van Roy F, Takeichi M. Stability of dendritic spines and synaptic contacts is controlled by alpha N-catenin. Nat Neurosci 2004; 7, 357-363; http://dx.doi.org/10.1038/nn1212 nn1212 [pii]
- Silverman JB, Restituito S, Lu W, Lee-Edwards L, Khatri L, Ziff EB. Synaptic anchorage of AMPA receptors by cadherins through neural plakophilin-related arm protein AMPA receptor-binding protein complexes. J Neurosci 2007; 27:8505-8516; http://dx.doi.org/ 27/32/8505 [pii] 10.1523/JNEUROSCI.1395-07.2007
- Ochiishi T, Futai K, Okamoto K, Kameyama K, Kosik KS. Regulation of AMPA receptor trafficking by delta-catenin. Mol Cell Neurosci 2008; 39:499-507; http://dx.doi.org/10.1016/j.mcn.2008.06.002 S1044-7431(08)00155-3 [pii]
- Jungling, K. Eulenburg V, Moore R, Kemler R, Lessmann V, Gottmann K. N-cadherin transsynaptically regulates short-term plasticity at glutamatergic synapses in embryonic stem cell-derived neurons. J Neurosci 2006; 26:6968-78; http://dx.doi.org/ 26/26/6968 [pii] 10.1523/JNEUROSCI.1013-06.2006
- Okamura, K. Tanaka H, Yagita Y, Saeki Y, Taguchi A, Hiraoka Y, Zeng LH, Colman DR, Miki N. Cadherin activity is required for activity-induced spine remodeling. J Cell Biol 2004; 167:961-72; http://dx.doi.org/ jcb.200406030 [pii] 10.1083/jcb.200406030
- Bozdagi O, Wang XB, Nikitczuk JS, Anderson TR, Bloss EB, Radice GL, Zhou Q, Benson DL, Huntley GW. Persistence of coordinated long-term potentiation and dendritic spine enlargement at mature hippocampal CA1 synapses requires N-cadherin. J Neurosci 2010; 30:9984-89; http://dx.doi.org/10.1523/JNEUROSCI.1223-10.2010 30/30/9984 [pii]
- Tai CY, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron 2007; 54:771-785; http://dx.doi.org/ S0896-6273(07)00347-9 [pii] 10.1016/j.neuron.2007.05.013
- Tanaka H, Shan W, Phillips GR, Arndt K, Bozdagi O, Shapiro L, Huntley GW, Benson DL, Colman DR. Molecular modification of N-cadherin in response to synaptic activity. Neuron 2000; 25:93-107; http://dx.doi.org/ S0896-6273(00)80874-0 [pii]
- Murase S, Mosser E, Schuman EM. Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron 2002; 35:91-105; http://dx.doi.org/ S089662730200764X [pii]
- Brigidi GS, Sun Y, Beccano-Kelly D, Pitman K, Mobasser M, Borgland SL, Milnerwood AJ, Bamji SX. Palmitoylation of delta-catenin by DHHC5 mediates activity-induced synapse plasticity. Nat Neurosci 2014; 17:522-32; http://dx.doi.org/10.1038/nn.3657 nn.3657 [pii]
- Israely, I. Costa RM, Xie CW, Silva AJ, Kosik KS, Liu X, Deletion of the neuron-specific protein delta-catenin leads to severe cognitive and synaptic dysfunction. Curr Biol 2004; 14:1657-63; http://dx.doi.org/10.1016/j.cub.2004.08.065 S0960982204006761 [pii]
- Mills F, Bartlett TE, Dissing-Olesen L, Wisniewska MB, Kuznicki J, Macvicar BA, Wang YT, Bamji SX. Cognitive flexibility and long-term depression (LTD) are impaired following beta-catenin stabilization in vivo. Proc Natl Acad Sci U S A 2014; 111:8631-36; http://dx.doi.org/10.1073/pnas.1404670111 14046-70111 [pii]
- Drachman DA, Do we have brain to spare? Neurology 2005; 64:2004-05; http://dx.doi.org/ 64/12/2004 [pii] 10.1212/01.WNL.0000166914.38327.BB
- Medina M, Marinescu RC, Overhauser J, Kosik KS. Hemizygosity of delta-catenin (CTNND2) is associated with severe mental retardation in cri-du-chat syndrome. Genomics 2000; 63:157-64; http://dx.doi.org/10.1006/geno.1999.6090 S0888-7543(99)96090-1 [pii]
- Bacchelli, E, Ceroni F, Pinto D, Lomartire S, Giannandrea M, D'Adamo P, Bonora E, Parchi P, Tancredi R, Battaglia A, et al. A CTNNA3 compound heterozygous deletion implicates a role for alphaT-catenin in susceptibility to autism spectrum disorder. J Neurodev Disord 2014; 6, 17; http://dx.doi.org/10.1186/1866-1955-6-17 1866-1955-6-17 [pii]
- Tucci V, Kleefstra T, Hardy A, Heise I, Maggi S, Willemsen MH, Hilton H, Esapa C, Simon M, Buenavista MT, et al. Dominant beta-catenin mutations cause intellectual disability with recognizable syndromic features. J Clin Invest 2014; 124:1468-82; PMID:24614104; http://dx.doi.org/10.1172/JCI70372 70372 [pii]
- Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, Salyakina D, Imielinski M, Bradfield JP, Sleiman PM, et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature 2009; 459:528-33; PMID:19404256; http://dx.doi.org/10.1038/nature07999 nature07999 [pii]
- Pagnamenta AT, Khan H, Walker S, Gerrelli D, Wing K, Bonaglia MC, Giorda R, Berney T, Mani E, Molteni M, et al. Rare familial 16q21 microdeletions under a linkage peak implicate cadherin 8 (CDH8) in susceptibility to autism and learning disability. J Med Genet 2011; 48:48-54; PMID:20972252; http://dx.doi.org/10.1136/jmg.20.10.079426 jmg.20 10.079426.[pii]
- Singh SM, Castellani C, O'Reilly R. Autism meets schizophrenia via cadherin pathway. Schizophr Res 2010; 116:293-94; http://dx.doi.org/10.1016/j.schres.2009.09.031 S0920-9964(09)00475-7 [pii]
- Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, Nimgaonkar VL, McQueen MB, Faraone SV, Kirby A, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry 2008; 13:558-569; http://dx.doi.org/10.1038/sj.mp.4002151 4002151 [pii]
- Lydall GJ, Bass NJ, McQuillin A, Lawrence J, Anjorin A, Kandaswamy R, Pereira A, Guerrini I, Curtis D, Vine AE, et al. Confirmation of prior evidence of genetic susceptibility to alcoholism in a genome-wide association study of comorbid alcoholism and bipolar disorder. Psychiatr Genet 2011; 21:294-306; http://dx.doi.org/10.1097/YPG.0b013e32834915c2
- Morrow EM, Genomic copy number variation in disorders of cognitive development. J Am Acad Child Adolesc Psychiatry 2010; 49:1091-1104; http://dx.doi.org/10.1016/j.jaac.20.10.08.009 S0890-8567(10)00601-5 [pii]
- Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, Yamrom B, Lee YH, Narzisi G, Leotta A, et al. De novo gene disruptions in children on the autistic spectrum. Neuron 2012; 74:285-99; http://dx.doi.org/10.1016/j.neuron.2012.04.009 S0896-6273(12)00340-6 [pii]
- Anitha A, Thanseem I, Nakamura K, Yamada K, Iwayama Y, Toyota T, Iwata Y, Suzuki K, Sugiyama T, Tsujii M, et al. Protocadherin alpha (PCDHA) as a novel susceptibility gene for autism. J Psychiatry Neurosci 2013; 38:192-98; http://dx.doi.org/10.1503/jpn.120058.10.1503/jpn.120058. [pii]
- Blair IP, Chetcuti AF, Badenhop RF, Scimone A, Moses MJ, Adams LJ, Craddock N, Green E, Kirov G, Owen MJ, et al. Positional cloning, association analysis and expression studies provide convergent evidence that the cadherin gene FAT contains a bipolar disorder susceptibility allele. Mol Psychiatry 2006; 11:372-83; http://dx.doi.org/ 4001784 [pii] 10.1038/sj.mp.4001784
- Neale BM, Medland S, Ripke S, Anney RJ, Asherson P, Buitelaar J, Franke B, Gill M, Kent L, Holmans P, et al. Case-control genome-wide association study of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2010; 49:906-20; http://dx.doi.org/10.1016/j.jaac.20.10.06.007 S0890-8567(10)00482-X [pii]
- Chu TT, Liu Y. An integrated genomic analysis of gene-function correlation on schizophrenia susceptibility genes. J Hum Genet 2010; 55:285-92; http://dx.doi.org/10.1038/jhg.20.10.24 jhg201024 [pii]
- Bhalla K, Luo Y, Buchan T, Beachem MA, Guzauskas GF, Ladd S, Bratcher SJ, Schroer RJ, Balsamo J, DuPont BR, et al. Alterations in CDH15 and KIRREL3 in patients with mild to severe intellectual disability. Am J Hum Genet 2008; 83:703-13; http://dx.doi.org/10.1016/j.ajhg.2008.10.020 S0002-9297(08)00555-7 [pii]
- Jun G, Moncaster JA, Koutras C, Seshadri S, Buros J, McKee AC, Levesque G, Wolf PA, St George-Hyslop P, Goldstein LE, et al. delta-Catenin is genetically and biologically associated with cortical cataract and future Alzheimer-related structural and functional brain changes. PLoS One 2012; 7, e43728; http://dx.doi.org/10.1371/journal.pone.0043728 PONE-D-12-15379 [pii]
- Asada-Utsugi M, Uemura K, Noda Y, Kuzuya A, Maesako M, Ando K, Kubota M, Watanabe K, Takahashi M, Kihara T, et al. N-cadherin enhances APP dimerization at the extracellular domain and modulates Abeta production. J Neurochem 2011; 119:354-63; PMID:21699541; http://dx.doi.org/10.1111/j.1471-4159.2011.07364.x
- Andreyeva A, Nieweg K, Horstmann K, Klapper S, Müller-Schiffmann A, Korth C, Gottmann K. C-terminal fragment of N-cadherin accelerates synapse destabilization by amyloid-beta. Brain 2012; 135:2140-54; PMID:22637581; http://dx.doi.org/10.1093/brain/aws120 aws120 [pii]
- Depienne C, LeGuern E. PCDH19-related infantile epileptic encephalopathy: an unusual X-linked inheritance disorder. Hum Mutat 2012; 33:627-34; PMID:22267240; http://dx.doi.org/10.1002/humu.22029
- Dibbens LM, Kneen R, Bayly MA, Heron SE, Arsov T, Damiano JA, Desai T, Gibbs J, McKenzie F, Mulley JC, et al. Recurrence risk of epilepsy and mental retardation in females due to parental mosaicism of PCDH19 mutations. Neurology 2011; 76:1514-19; PMID:21519002; http://dx.doi.org/10.1212/WNL.0b013e318217e7b6 76/17/1514 [pii]
- Nagaoka T, Ohashi R, Inutsuka A, Sakai S, Fujisawa N, Yokoyama M, Huang YH, Igarashi M, Kishi M. The Wnt/planar cell polarity pathway component Vangl2 induces synapse formation through direct control of N-cadherin. Cell Rep 2014; 6, 916-27; PMID:24582966; http://dx.doi.org/10.1016/j.celrep.2014.01.044 S2211-1247(14)00078-3 [pii]
- Tanaka H, Takafuji K, Taguchi A, Wiriyasermkul P, Ohgaki R, Nagamori S, Suh PG, Kanai Y. Linkage of N-cadherin to multiple cytoskeletal elements revealed by a proteomic approach in hippocampal neurons. Neurochem Int 2012; 61:240-50; PMID:22609377; http://dx.doi.org/10.1016/j.neuint.2012.05.008 S0197-0186(12)00168-4 [pii]
