Abstract
Targeted therapies of malignancies currently consist of therapeutic monoclonal antibodies and small molecule kinase inhibitors. The combination of these novel agents raises the issue of potential antagonisms. We evaluated the potential effect of 4 kinase inhibitors, including the Bruton tyrosine kinase inhibitor ibrutinib, and 3 PI3K inhibitors idelalisib, NVP-BEZ235 and LY294002, on the effects of the 3 monoclonal antibodies, rituximab and obinutuzumab (directed against CD20) and trastuzumab (directed against HER2). We found that ibrutinib potently inhibits antibody-dependent cell-mediated cytotoxicity exerted by all antibodies, with a 50% inhibitory concentration of 0.2 microM for trastuzumab, 0.5 microM for rituximab and 2 microM for obinutuzumab, suggesting a lesser effect in combination with obinutuzumab than with rituximab. The 4 kinase inhibitors were found to inhibit phagocytosis by fresh human neutrophils, as well as antibody-dependent cellular phagocytosis induced by the 3 antibodies. Conversely co-administration of ibrutinib with rituximab, obinutuzumab or trastuzumab did not demonstrate any inhibitory effect of ibrutinib in vivo in murine xenograft models. In conclusion, some kinase inhibitors, in particular, ibrutinib, are likely to exert inhibitory effects on innate immune cells. However, these effects do not compromise the antitumor activity of monoclonal antibodies in vivo in the models that were evaluated.
Abbreviations
| ADCC | = | antibody-dependent cell-mediated cytotoxicity |
| ADCP | = | antibody-dependent cellular phagocytosis |
| NHL | = | non-Hodgkin's lymphoma |
| NK | = | natural killer |
Introduction
Targeted therapies of malignancies aim to exploit molecular specificities of tumor cells and spare normal tissues. Targets typically include cell surface antigens for immunotherapeutic approaches and intracellular proteins for small molecule inhibitors. The number of approved targeted therapies is increasing rapidly, and novel candidates in clinical trials are one of the fastest growing segments in pharmacology.Citation1 In spite of this progress targeted therapy is generally used in combination with other agents, including conventional chemotherapeutics. Improving therapeutic efficacy while improving tolerance represents a strong incentive to combine targeted agents, with reduced use of cytotoxic agents.Citation2,Citation3 These novel approaches raise the issue of the additivity, synergism or antagonism of combined targeted therapies. An example of antagonism between 2 biotherapeutic proteins, erythropoietin and trastuzumab, that was caused by conflicting effects on signalization pathways was reported by Liang et al.Citation4
Lymphoid malignancies represent a field of choice to explore this hypothesis given the availability of targeted therapies with different mechanisms of action in these diseases. Recent literature data has raised the issue that small molecule targeted therapies such as Bruton tyrosine kinase inhibitors may be antagonistic with therapeutic monoclonal antibodies. Kohrt et al. studied the effect of ibrutinib on natural killer (NK) cell cytokine secretion, degranulation and cytotoxicity in antibody-dependent cell-mediated cytotoxicity (ADCC) assays in CD20 and HER2 positive models.Citation5 Ibrutinib was found to be inhibitory in vitro at low concentrations (0.1 and 1 microM), and to inhibit the antitumor activity of rituximab and trastuzumab on xenografts in vivo when administered concurrently with the antibodies.
In order to investigate the potential synergism or antagonism of targeted therapies, we evaluated the potential effects of 4 kinase inhibitors, ibrutinib (PCI-32765; Bruton tyrosine kinase inhibitor),Citation6 and the PI3-kinase inhibitors: idelalisib (CAL-101; PI3Kdelta selective inhibitor),Citation7,Citation8 NVP-BEZ235 (dual pan PI3K/mTOR competitive inhibitor)Citation9 and LY294002 (pan PI3K inhibitor), on the biological properties of 3 monoclonal antibodies, trastuzumab, which targets HER2, and rituximab and obinutuzumab, which target CD20.Citation10
Results
Effect of kinase inhibitors on ADCC
The 4 kinase inhibitors ibrutinib (PCI-32765), idelalisib (CAL-101), NVP-BEZ235 and LY294002 were first tested at a concentration of 10 microM in in vitro ADCC assays involving trastuzumab, rituximab and obinutuzumab. As shown in , at 10 microM, ibrutinib had the strongest inhibitory effect on the 3 antibodies, while idelalisib and NVP-BEZ235 had a less pronounced effect and LY294002 had no effect. The inhibitory effects of ibrutinib, idelalisib and NVP-BEZ235 were confirmed using peripheral blood mononuclear cells or freshly isolated NK cells obtained from healthy donors (Fig. S1). A dose response study () confirmed a strong inhibitory effect of ibrutinib at high concentrations, with a 50% inhibitory effect of 0.2 microM for trastuzumab, 0.5 microM for rituximab and 2 microM for obinutuzumab. The inhibitory effect of ibrutinib was thus more pronounced with rituximab than with obinutuzumab. Idelalisib, NVP-BEZ235 and LY294002 also displayed a dose-dependent inhibition of ADCC for trastuzumab, with the half maximal inhibitory concentrations (IC50) of 5 microM, 25 microM and 70 microM, respectively (Fig. S2). Therefore, all kinase inhibitors tested showed an inhibition of ADCC, with ibrutinib displaying the strongest inhibitory effect. Furthermore the effect of ibrutinib was similar under hypoxic or normoxic conditions (Fig. S3).
Figure 1. Effect of kinase inhibitors ibrutinib, idelalisib, NVP-BEZ235, and LY294002 on the ADCC effect of trastuzumab (A), rituximab (B) and obinutuzumab (C). ADCC was performed using NK-92-CD16 cells as effectors and BT474 cells (trastuzumab) or RL cells (rituximab and obinutuzumab) as target cells, with the corresponding antibody at 1 μg/mL final. The effector : target (E:T) ratio = 5:1 for trastuzumab or 2:1 for rituximab and obinutuzumab. Ibrutinib, idelalisib, NVP-BEZ235 or LY294002 were added simultaneously at 10 μM final. Means ± SD of 3 independent experiments are shown. *P < 0.05; **P < 0,01.
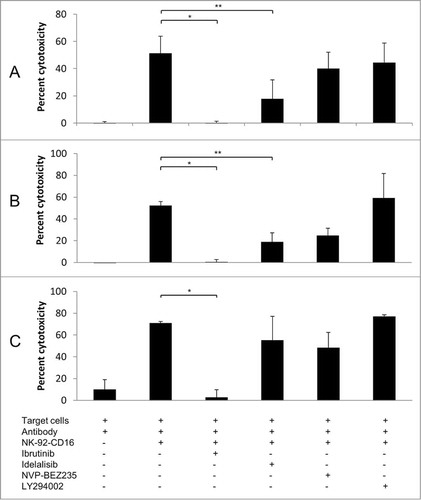
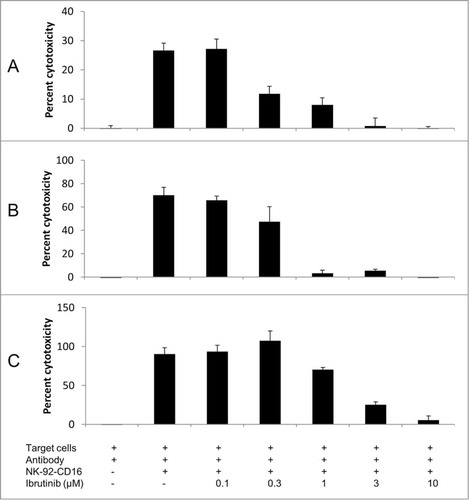
Figure 2. Dose response effect of ibrutinib on ADCC effect of trastuzumab (A), rituximab (B) and obinutuzumab (C). ADCC was performed using NK-92-CD16 cells as effectors and BT474 cells (trastuzumab) or RL cells (rituximab and obinutuzumab) as target cells, with the corresponding antibody at 1 μg/mL final, in the presence of indicated concentrations of ibrutinib. E:T = 5:1 for trastuzumab and E:T = 2:1 for rituximab and obinutuzumab. A representative experiment is shown.
Effect of kinase inhibitors on ADCP and phagocytic properties
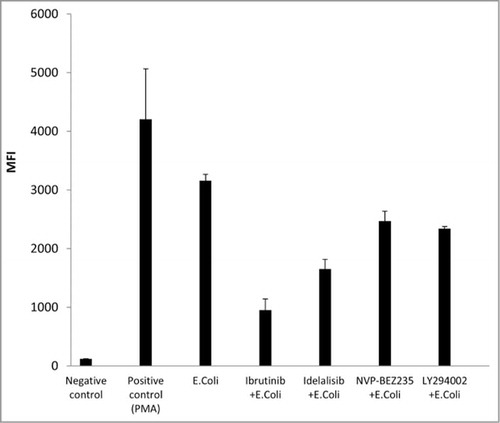
Fresh human neutrophils were tested for their ability to perform antibody-dependent cellular phagocytosis (ADCP) against BT474 or RL targets in the presence of trastuzumab and rituximab or obinutuzumab, respectively. As shown in , all of the kinase inhibitors tested inhibited ADCP to some degree. The most powerful inhibition was observed with idelalisib in the case of trastuzumab, and with ibrutinib in the case of rituximab and obinutuzumab. Preincubation experiments performed with ibrutinib showed that inhibition of ADCP could be observed both when target BT474 cells or neutrophils were preincubated with ibrutinib (Fig. S4). Evaluation of the effect of kinase inhibitors on the phagocytic activity of normal human neutrophils found a significant effect for all compounds tested, the most potent being ibrutinib in this setting ().
Figure 3. Effect of tyrosine kinase inhibitors ibrutinib, idelalisib, NVP-BEZ235, and LY294002 on the ADCP effect of trastuzumab (A), rituximab (B) and obinutuzumab (C). ADCP was performed using neutrophils as effectors and BT474 cells (trastuzumab) or RL cells (rituximab and obinutuzumab) as target cells, with the corresponding antibody at 1 μg/mL final. The effector : target (E:T) ratio = 5:1 for trastuzumab or 2:1 for rituximab and obinutuzumab. Ibrutinib, idelalisib, NVP-BEZ235 or LY294002 were used at 10 μM final.
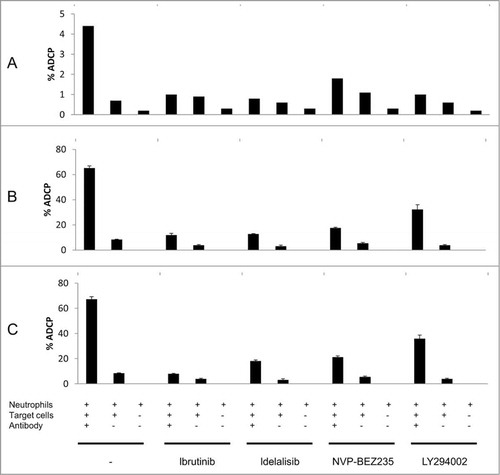
Figure 4. Effect of tyrosine kinase inhibitors ibrutinib, idelalisib, NVP-BEZ235, and LY294002 on phagocytic activity of normal human neutrophils. Phagocytic activity was evaluated using the FagoFlowEx® Kit after the stimulation of neutrophils with E. coli bacteria, in the presence of 10 μM of ibrutinib, idelalisib, NVP-BEZ235 or LY294002. Phorbol 12-myristate 13-acetate (PMA) was used as positive control. Median fluorescence intensity (MFI) is reported. Means ± SD of 2 independent experiments are shown.
Lack of effect in the in vivo setting
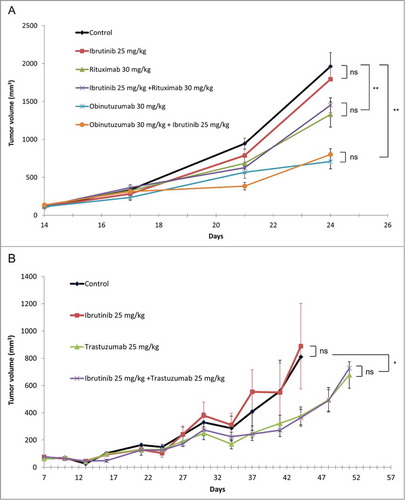
Immunodeficient SCID mice bearing established RL lymphoma xenografts were treated with either rituximab alone or obinutuzumab alone or in combination with ibrutinib. SCID mice bearing established BT474 breast carcinoma xenografts were treated with trastuzumab alone or in combination with ibrutinib. As shown in , ibrutinib itself had no inhibitory effect per se, and did not modify the antitumor activity of the antibodies in these models. Obinutuzumab had a more potent antitumor activity than rituximab, as previously described.Citation11
Figure 5. In vivo effect of combinations of antibodies with ibrutinib: (A) combination of rituximab and obinutuzumab with ibrutinib; (B) combination of trastuzumab with ibrutinib. The human follicular line RL () and the human breast cancer line BT474 () were grown as subcutaneous xenografts in SCID mice. Treatment with antibodies (rituximab 30 mg/kg/week, obinutuzumab 30 mg/kg/week, trastuzumab 25 mg/kg/week) and ibrutinib (25 mg/kg/day, 5 d a week) was initiated when tumor volume reached 100 mm3. Medians ± SEM are shown (n = 10 mice/group). *P < 0.05; **P < 0.01.
Discussion
Combining different targeted agents to increase antitumor efficacy is currently being explored in multiple clinical trials. In this study, we examined the potential impact of ibrutinib, a recently approved Bruton tyrosine kinase inhibitor, and 3 PI3K inhibitors, idelalisib, NVP-BEZ235, and LY294002 on the effect of antibodies targeted against HER2 (trastuzumab) and CD20 (rituximab and obinutuzumab). Our results showed that ibrutinib demonstrated strong inhibitory potency in in vitro ADCC assays with all 3 antibodies, which is coherent with the previous findings by Kohrt et al.Citation 5 We also showed that PI3K inhibitors idelalisib, NVP-BEZ-235 and LY294002 could potentially inhibit in vitro ADCC for anti-HER2 and anti-CD20 antibodies, but at higher concentrations than ibrutinib. The relative lack of effect of LY294002 in the inhibition of ADCC may be due to its lower inhibitory potency, which was reported to be much less than NVP-BEZ-235.Citation12 As antibody-mediated cellular destruction has been suggested to be a major mechanism of action of several therapeutic monoclonal antibodies in the clinic, this observation raises the issue of potential antagonism between these 2 types of targeted therapies. Conversely, in vivo studies did not show any negative impact of ibrutinib on the effect of rituximab or obinutuzumab in a human NHL xenograft model or of trastuzumab in a human breast cancer model. This apparently contradictory observation may be due to several factors, such as 1) the concentrations of ibrutinib obtained in vivo are too low to inhibit ADCC and ADCP, 2) murine effectors may be less sensitive to ibrutinib than human effectors, or 3) antibody-mediated apoptotic signaling remains unaffected or is enhanced by ibrutinib. It is worth noting that in the studies by Kohrt et al.,Citation 5 ibrutinib was administered twice daily, while in our settings, ibrutinib was only administered once daily with the same dose per injection. Therefore, the plasma concentrations of ibrutinib in mice in Kohrt's study were likely to be maintained at higher levels than those in our studies. This could explain the different effects of ibrutinib in vivo observed in Kohrt's study and in our study. Of note, it has been reported that rituximab induces tumor cell apoptosis through inhibition or BCR signaling, which might contribute to an additive effect of rituximab and ibrutinib on lymphoma cells.Citation13
Ibrutinib has been evaluated as a single agent for the treatment of different lymphoid malignancies.Citation14-Citation16 In a Phase 2 trial, Wang et al. showed that ibrutinib has durable single-agent efficacy in relapsed or refractory mantle-cell lymphoma.Citation14 In a Phase 1b/2 study, Byrd et al. treated 85 patients having relapsed chronic lymphocytic leukemia with a once daily dose of 420 or 840 mg and observed a 75% progression-free survival rate at 26 months with predominantly grade 1 and 2 toxicities and notably minimal hematological toxicity.Citation15 A recent Phase 3 study of 391 patients chronic lymphoid leukemia indicated that ibrutinib significantly improved progression-free survival, overall survival and response rate compared with ofatumumab.Citation16 Several trials are currently evaluating the combination of ibrutinib and rituximab. The Phase 3 HELIOS trial (trial registration: EudraCT No. 2012-000600-15; UTN No. U1111-1135-3745) will determine whether adding ibrutinib to a bendamustine/rituximab combination is beneficial in relapsed/refractory patients with chronic lymphocytic leukemia or small lymphocytic leukemia.Citation17 Likewise, idelalisib has been evaluated in a Phase 3 trial for its efficacy and safety when used in combination with rituximab in patients with relapsed chronic lymphocytic leukemia. Furman et al. recently reported that the combination of idelalisib and rituximab significantly improved progression-free survival, response rate, and overall survival compared with placebo and rituximab.Citation18
An important question in studies such as ours concerns the relevance of the concentrations used for in vitro studies. Of interest, after a single exposure to 12.5 mg/kg of ibrutinib, Cmax values in patients have been reported to be in the order of 383 ng/mL (870 nanoM). After repeated dosing at 840 mg/day, the Cmax value observed on day 8 was 221 ng/mL (500 nanoM) (205552Orig1s000ClinPharmR.pdf). Advani et al. reported peak plasma concentrations in the 250–300 nanoM range in patients receiving 560 mg.Citation19 Kohrt et al. studied concentrations of ibrutinib up to 1 microM in vitro and observed a significant dose-response effect. The effects of ibrutinib that we have reported are mostly observed at the relatively high concentration of 10 microM, although some degree of inhibition is observed at the lower concentration of 1 microM. Thus, the relevance of these observations in terms of concentrations that can be obtained in patients receiving ibrutinib requires confirmation, in particular given the prolonged exposure expected in patients. Furthermore, ibrutinib is highly protein bound (>97% in man, >99% in mice) and the effect observed in vitro in 10% serum may not be representative of the situation in vivo.
Another finding in our studies is that all of the kinase inhibitors tested in this study inhibited the phagocytic activity of fresh human neutrophils, as well as antibody-dependent cellular phagocytosis induced by monoclonal antibodies to some degree. Chang et al. previously reported that ibrutinib inhibited monocytes, macrophages and mast cells.Citation20 While the clinical relevance of this observation remains to be determined, these results suggest that targeted therapies may have an effect on innate immune cells. Granulocytes have been shown to be involved in the antitumor activity of antibodies.Citation21,Citation22 As these novel therapies are oral agents likely to be administered over prolonged periods, it will be important to determine their potential impact on normal leukocytes, with possible consequences on the phagocytosis of microorganisms or senescent cells. In conclusion, our findings raise the possibility that ibrutinib and possibly other kinase inhibitors may have unexpected effects on innate immune cells. Given the numerous roles of these cells on local immune control, it should prove to be fascinating to dissect the complex effects of kinase inhibitors on microenvironmental control of neoplasia.
Materials and Methods
Reagents and cell lines
RL, a human non-Hodgkin's lymphoma line derived from a patient with follicular lymphoma, and BT474, a human HER2+ breast adenocarcinoma line, were obtained from ATCC. Cells were cultured in complete RPMI medium (RPMI supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin). Ibrutinib (PCI-32765) was purchased from Euromedex (ref S2680), idelalisib (CAL-101) from Santa Cruz Biotechnology (ref sc-364453), NVP-BEZ235 from Interchim (ref LZR660) and LY294002 from Sigma-Aldrich (ref L9908). Antibodies were provided by Roche/Glycart.
Retroviral transduction of NK-92 cells
NK-92, the human NK cell line generously provided by Conkwest, was grown in complete RPMI culture medium. NK-92-CD16 cells were obtained by transduction of pMX/CD16 plasmidCitation23 using retroviral supernatant. Transient retroviral supernatants were produced by CaCl2 precipitation (Invitrogen) with 15 μg pMX/CD16 plasmid using Phoenix-Ampho cells. The conditioned medium was collected 24–48 h post-transfection, filtered through 0.45-μm pore-size filters and kept at −80°C until use. The viral titer was determined by the transduction of Jurkat T cells (10Citation6 cells per well in 6-well plates) with serial dilutions. Retroviral supernatant titers were typically 1–5 × 10Citation5 IU (Infectious Units)/mL. The NK-92 cell line was seeded at 10Citation6 cells into 6-well plates and exposed to 2 × 2 mL of retroviral supernatant by spinoculation (2400 g, 1.5 h, 32°C) in the presence of 4 μg/mL polybrene (Sigma-Aldrich) to generate the NK-92-CD16 cells. The culture medium was changed 24 h post-infection. Transduction efficiencies were assessed 5 d later by flow cytometry after staining with the CD16 PE-conjugated mouse anti-CD16 antibody (clone 3G8) (Beckman Coulter). NK-92-CD16 cells were selected for higher CD16 expression by cell sorter flow cytometry using anti-CD16 antibody (Beckman Coulter). Purity was >95%.
Purification of neutrophils and NK cells
Effector neutrophils were obtained from the bloods of healthy donors obtained through the local blood bank with written consent. Neutrophils were separated from peripheral blood mononuclear cells by Ficoll (Pancoll human, P04-60500, PAN Biotech) followed by gradient separation on dextran (8906, Sigma-Aldrich) and red blood cell lysis (BD Pharm lyse, 555899, BD Biosciences). NK cells were purified with NK cell isolation kit (130-092-657, Miltenyi) according to the manufacturer's instructions.
Antibody-dependent cell-mediated cytotoxicity
Target cells (RL or BT474) were labeled with 12.5 μM calcein-AM (Sigma-Aldrich) for 30 min at 37°C and added at 105 cells/0.5 mL/well. NK-92-CD16 cells were added at 5 × 105 cells/0.5 mL/well (effector : target (E:T) ratio = 5:1) for BT474 and 2 × 105 cells/0.5 mL/well (E:T ratio = 2:1) for RL. All antibodies were at a final concentration of 1 μg/mL final. After 4 h of incubation at 37°C, the supernatants in each well were harvested and measured for the fluorescence signals at 485/535 nm. Triton X100 (Sigma-Aldrich) was added in control wells to estimate total cell lysis. Cytotoxicity was calculated as percent cytotoxicity = (experimental lysis – spontaneous lysis) / (maximal lysis – spontaneous lysis) × 100.
Antibody-dependent cellular phagocytosis
Antibody-dependent cellular phagocytosis mediated by neutrophils is evaluated through fluorescence transfer from a target cell to an effector cells. Effector neutrophils were obtained from the blood of healthy donors as described in the Supplement. Briefly, target cells were stained with PKH67 (Sigma-Aldrich) as recommended by the manufacturer and co-incubated at an E:T ratio of 5:1 for BT474 and 2:1 for RL cells overnight at 37°C in the presence or absence of monoclonal antibodies. After incubation, acquisition was performed on a LSRII flow cytometer, and evaluation of phagocytosis was performed through PKH67 fluorescence transfer from target cells to effector cells. Gating of phagocytosing neutrophils was performed using anti-CD45 APC (340910, BD Biosciences) or anti-CD15 APC antibodies (551376, BD PharMingen).
Phagocytosis studies
Phagocytic activity of normal human peripheral blood leukocytes was assessed by measuring production of ROS with FagoFlowEx® Kit (Exbio Diagnostics) as recommended by the provider.
In vivo studies
All animal procedures were performed in accordance with the European Union Directive (86/609/EEC). Experiments were performed under individual permit and in animal care facilities accredited by the French Ministry of Research and Education. The study was approved by the local animal ethical committee. This study was conducted using SCID CB17 mice bearing established RL lymphoma and BT474 breast cancer subcutaneous xenografts. Three million tumor cells from exponentially growing in vitro cultures were injected subcutaneously in each mouse. Treatments were initiated when the tumor volume reached 100 mm3, with intraperitoneal administration of antibodies (rituximab or obinutuzumab 30 mg/kg, once weekly for 2 weeks; or trastuzumab 25 mg/kg, once weekly for 4 weeks), and daily oral administration of ibrutinib (25 mg/kg, 5 days/week, for 2 weeks when combined with rituximab or obinutuzumab; or for 3 weeks when combined with trastuzumab). Growth of tumors was directly measured twice weekly using a caliper.
Statistical analysis
All experiments were performed at least 2 or 3 times. Means ± SD or representative experiments are shown. Statistical significance was evaluated using paired Student t test for in vitro experiments and unpaired Student t test for in vivo experiments. p values below 0.05 (*); 0.01 (**) were deemed as significant while “ns” stands for not significant.
Disclosure of Potential Conflicts of Interest
CD is a recipient of research grants from Roche. Other authors declare no conflict of interest.
Supplemental_Data.pdf
Download PDF (390.3 KB)Acknowledgments
This work was supported by the Lyric Grant INCa-DGOS-4664. MND received a financial support from the Ligue Nationale Contre le Cancer.
Supplemental Material
Supplemental data for this article can be accessed on thepublisher's website.
References
- Reichert JM. Antibodies to watch in 2014: Mid-year update. mAbs 2014; 6:799-802; PMID:24846335; http://dx.doi.org/10.4161/mabs.29282
- Huang X, Wang S, Lee C-K, Yang X, Liu B. HDAC inhibitor SNDX-275 enhances efficacy of trastuzumab in erbB2-overexpressing breast cancer cells and exhibits potential to overcome trastuzumab resistance. Cancer Lett 2011; 307:72-9; PMID:21497990; http://dx.doi.org/10.1016/j.canlet.2011.03.019
- Gayle SS, Castellino RC, Buss MC, Nahta R. MEK inhibition increases lapatinib sensitivity via modulation of FOXM1. Curr Med Chem 2013; 20:2486-99; PMID:23531216; http://dx.doi.org/10.2174/0929867311320190008
- Liang K, Esteva FJ, Albarracin C, Stemke-Hale K, Lu Y, Bianchini G, Yang C-Y, Li Y, Li X, Chen C-T, et al. Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivation. Cancer Cell 2010; 18:423-35; PMID:21075308; http://dx.doi.org/10.1016/j.ccr.2010.10.025
- Kohrt HE, Sagiv-Barfi I, Rafiq S, Herman SEM, Butchar JP, Cheney C, Zhang X, Buggy JJ, Muthusamy N, Levy R, et al. Ibrutinib antagonizes rituximab-dependent NK cell-mediated cytotoxicity. Blood 2014; 123:1957-60; PMID:24652965; http://dx.doi.org/10.1182/blood-2014-01-547869
- Wiestner A. Targeting B-Cell receptor signaling for anticancer therapy: the Bruton's tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol 2013; 31:128-30; PMID:23045586; http://dx.doi.org/10.1200/JCO.2012.44.4281
- Lannutti BJ, Meadows SA, Herman SEM, Kashishian A, Steiner B, Johnson AJ, Byrd JC, Tyner JW, Loriaux MM, Deininger M, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 2011; 117:591-4; PMID:20959606; http://dx.doi.org/10.1182/blood-2010-03-275305
- Maira S-M, Stauffer F, Schnell C, García-Echeverría C. PI3K inhibitors for cancer treatment: where do we stand? Biochem Soc Trans 2009; 37:265-72; PMID:19143644; http://dx.doi.org/10.1042/BST0370265
- Bhende PM, Park SI, Lim MS, Dittmer DP, Damania B. The dual PI3K/mTOR inhibitor, NVP-BEZ235, is efficacious against follicular lymphoma. Leukemia 2010; 24:1781-4; PMID:20703254; http://dx.doi.org/10.1038/leu.2010.154
- Uddin S, Hussain AR, Siraj AK, Manogaran PS, Al-Jomah NA, Moorji A, Atizado V, Al-Dayel F, Belgaumi A, El-Solh H, et al. Role of phosphatidylinositol 3’-kinase/AKT pathway in diffuse large B-cell lymphoma survival. Blood 2006; 108:4178-86; PMID:16946303; http://dx.doi.org/10.1182/blood-2006-04-016907
- Dalle S, Reslan L, Besseyre de Horts T, Herveau S, Herting F, Plesa A, Friess T, Umana P, Klein C, Dumontet C. Preclinical studies on the mechanism of action and the anti-lymphoma activity of the novel anti-CD20 antibody GA101. Mol Cancer Ther 2011; 10:178-85; PMID:21220500; http://dx.doi.org/10.1158/1535-7163.MCT-10-0385
- Maira S-M, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker K, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther 2008; 7:1851-63; PMID:18606717; http://dx.doi.org/10.1158/1535-7163.MCT-08-0017
- Kheirallah S, Caron P, Gross E, Quillet-Mary A, Bertrand-Michel J, Fournié J-J, Laurent G, Bezombes C. Rituximab inhibits B-cell receptor signaling. Blood 2010; 115:985-94; PMID:19965664; http://dx.doi.org/10.1182/blood-2009-08-237537
- Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013; 369:507-16; PMID:23782157; http://dx.doi.org/10.1056/NEJMoa1306220
- Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369:32-42; PMID:23782158; http://dx.doi.org/10.1056/NEJMoa1215637
- Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, Coutre S, Tam CS, Mulligan SP, Jaeger U, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014; 371:213-23; PMID:24881631; http://dx.doi.org/10.1056/NEJMoa1400376
- Hallek M, Kay NE, Osterborg A, Chanan-Khan AA, Mahler M, Salman M, Wan Y, Sun S, Zhuang SH, Howes A. The HELIOS trial protocol: a Phase III study of ibrutinib in combination with bendamustine and rituximab in relapsed/refractory chronic lymphocytic leukemia. Future Oncol Lond Engl 2014; 1-9; PMID:24901734; http://dx.doi.org/10.2217/fon.14.119
- Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn I, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014; 370:997-1007; PMID:24450857; http://dx.doi.org/10.1056/NEJMoa1315226
- Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, Kolibaba KS, Furman RR, Rodriguez S, Chang BY, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 2013; 31:88-94; PMID:23045577; http://dx.doi.org/10.1200/JCO.2012.42.7906
- Chang BY, Huang MM, Francesco M, Chen J, Sokolove J, Magadala P, Robinson WH, Buggy JJ. The Bruton tyrosine kinase inhibitor PCI-32765 ameliorates autoimmune arthritis by inhibition of multiple effector cells. Arthritis Res Ther 2011; 13:R115; PMID:21752263; http://dx.doi.org/10.1186/ar3400
- Hernandez-Ilizaliturri FJ, Jupudy V, Ostberg J, Oflazoglu E, Huberman A, Repasky E, Czuczman MS. Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin's lymphoma severe combined immunodeficiency mouse model. Clin Cancer Res 2003; 9:5866-73; PMID:14676108
- Cartron G, Zhao-Yang L, Baudard M, Kanouni T, Rouillé V, Quittet P, Klein B, Rossi J-F. Granulocyte-macrophage colony-stimulating factor potentiates rituximab in patients with relapsed follicular lymphoma: results of a phase II study. J Clin Oncol 2008; 26:2725-31; PMID:18427151; http://dx.doi.org/10.1200/JCO.2007.13.7729
- Clémenceau B, Congy-Jolivet N, Gallot G, Vivien R, Gaschet J, Thibault G, Vié H. Antibody-dependent cellular cytotoxicity (ADCC) is mediated by genetically modified antigen-specific human T lymphocytes. Blood 2006; 107:4669-77; PMID:16514054; http://dx.doi.org/10.1182/blood-2005-09-3775
