Abstract
Shiga toxin 1 (Stx1) is a virulence factor of enterohaemorrhagic Escherichia coli strains such as O157:H7 and Shigella dysenteriae. To prevent entry of Stx1 from the mucosal surface, an immunoglobulin A (IgA) specific for Stx1 would be useful. Due to the difficulty of producing IgA monoclonal antibodies (mAb) against the binding subunit of Stx1 (Stx1B) in mice, we took advantage of recombinant technology that combines the heavy chain variable region from Stx1B-specific IgG1 mAb and the Fc region from IgA. The resulting hybrid IgG/IgA was stably expressed in Chinese hamster ovary cells as a dimeric hybrid IgG/IgA. We separated the dimeric hybrid IgG/IgA from the monomeric one by size-exclusion chromatography. The dimer fraction, confirmed by immunoblot analyses, was used for toxin neutralization assays. The dimeric IgG/IgA was shown to neutralize Stx1 toxicity toward Vero cells by assaying their viability. To compare the relative effectiveness of the dimeric hybrid IgG/IgA and parental IgG1 mAb, Stx1-induced apoptosis was examined using 2 different cell lines, Ramos and Vero cells. The hybrid IgG/IgA inhibited apoptosis more efficiently than the parental IgG1 mAb in both cases. The results indicated that the use of high affinity binding sites as variable regions of IgA would increase the utility of IgA specific for virulence factors.
Abbreviations
| CHO | = | Chinese hamster ovary |
| J chain | = | joining chain |
| SIgA | = | secretory IgA |
| Stx1 | = | Shiga toxin 1 |
| Stx1B | = | B subunit of Stx1 |
Introduction
Immunoglobulin A (IgA) is the major class of antibodies present on the mucosal surface as secretory IgA (SIgA). SIgA is believed to prevent pathogens and their virulence factors invading through the mucosal barrier.Citation1,2
As a virulence factor of enterohaemorrhagic Escherichia coli (EHEC) strains such as O157:H7 and Shigella dysenteriae,Citation3,4 we have been investigating Shiga toxin 1 (Stx1). Stx1 comprises one cytotoxic subunit and 5 binding subunits (Stx1B).Citation5 We produced recombinant Stx1B as an antigen to investigate the immune response leading to efficient IgA production.Citation6 The immunogenicity of Stx1B is not high enough to induce Stx1B-specific IgA responses efficiently in mice.Citation7 This may indicate a limitation of vaccination against Stx1. On the other hand, preformed IgA against Stx1B may be useful for passive immunity in the form of a therapeutic antibody.
However, production of IgA monoclonal antibodies (mAb) turned out not to be straightforward, especially in the case of antigens with weak immunogenicity like Stx1B. In our case, we succeeded in the production of an Stx1B-specific IgA mAb, G2G7.Citation8 However, this IgA mAb did not, but another IgG1 mAb, D11C6, did neutralize the toxicity of Stx1 holotoxin.Citation9 To obtain antibodies with useful variable regions and with the IgA constant region, we produced a recombinant hybrid IgG/IgA, in which variable regions were from IgG1 mAb, while the heavy chain constant region was from IgA mAb.Citation10 This hybrid IgG/IgA was shown to neutralize Stx1, of which the toxicity toward Vero cells was measured.Citation11
Through expression of immunoglobulin heavy and light chains together with joining (J) chains in Chinese hamster ovary (CHO) cells, we were able to produce a dimeric form of the hybrid IgG/IgA. The dimerization of IgA is known to be required for the formation of SIgA.Citation1,12 In CHO cells capable of stably expressing the dimeric IgG/IgA, both dimeric and monomeric forms were present. After separation by means of size-exclusion chromatography, we demonstrated the dimeric form was 10-fold more effective than the monomeric one as to toxin neutralization.Citation11 However, comparison of the dimeric IgG/IgA and parental IgG1 mAb in terms of toxin neutralization was not performed.
Stx1 is known to induce apoptosis of Burkitt's lymphoma cells and kidney-derived Vero cells.Citation13,14 In this study, we focused on comparison of the dimeric form of IgG/IgA and the parental IgG1 mAb as to toxin neutralization. We utilized 2 types of cells, Ramos cells (Burkitt's lymphoma) and Vero cells, using 2 different assays that reflect apoptosis induction by Stx1 holotoxin.
Results
Preparation of dimeric hybrid IgG/IgA by size-exclusion chromatography
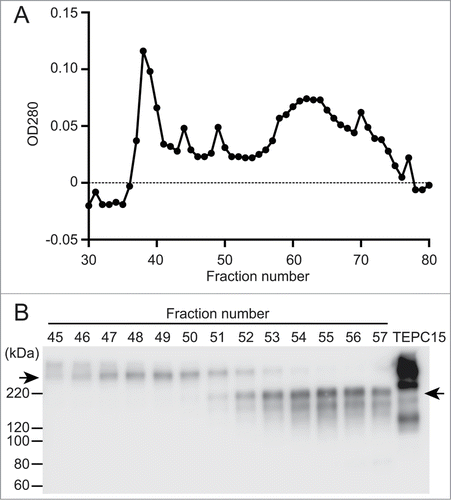
We previously established a CHO-K1 cell clone stably expressing dimeric hybrid IgG/IgA antibodies specific for Stx1B.Citation11 This clone expresses heavy, light and J chains. Because the heavy chain consists of VH, Cγ1, Cα2 and Cα3 domains, this antibody is able to dimerize through a J chain. A serum-free culture supernatant was prepared, concentrated and subjected to size-exclusion chromatography on Sephacryl S-300 (). Each fraction was examined by SDS-PAGE under non-reducing conditions, followed by immunoblotting with anti-IgA antibodies as a probe. The dimeric hybrid IgG/IgA (molecular mass higher than 220 kDa) was mainly separated in fractions 46 to 52. Some IgA molecules remain monomers in this clone. The monomeric hybrid IgG/IgA (molecular mass between 120 and 220 kDa) was found between fractions 52 to 57. To keep the incorporation of monomers as low as possible, we pooled fractions 47 to 50 in the present study. For biological assays, the antibody concentration in the pooled fraction was determined by sandwich ELISA.
Figure 1. Separation of dimeric and monomeric forms of a recombinant hybrid-IgG/IgA specific for Stx1B. A serum-free culture supernatant (60 ml) of CHO-K1 cells triple transfected with vector constructs for H, L and J chains of the hybrid-IgG/IgA was concentrated and separated on a column of Sephacryl S-300. Elution profile of proteins (A). SDS-PAGE (non-reducing conditions) and immunoblot analysis (probed with anti-α chain) of each fraction from the Sephacryl S-300 column (B). An aliquot (9 μl each) of each fraction (fractions 45 to 57) was analyzed. The positions of molecular weight standards are shown on the left. The left arrow indicates the dimeric form while the right arrow indicates the monomeric one. The result for TEPC 15 (8 ng) is shown as an IgA myeloma protein.
Preincubation with hybrid IgG/IgA dose-dependently neutralizes Stx1 toxicity toward Vero cells
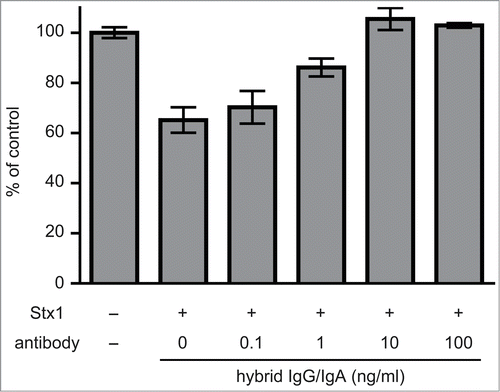
Vero cells are one of the cell lines sensitive to Stx1 toxicity. When Vero cells were cultured with 5 pg/ml of Stx1 holotoxin, cell viability decreased by 40%, as revealed by a cell viability assay (an MTT-like assay) that measures NAD(P)H-dependent cellular oxidoreductase activity by the reduction of water soluble tetrazorium salt (WST)-8. When Stx1 was treated with increasing concentrations of the dimeric fraction of hybrid IgG/IgA, the viability of Vero cells recovered (). Complete recovery was observed with more than 10 ng/ml of the hybrid IgG/IgA.
Figure 2. Toxin neutralization by the dimeric hybrid IgG/IgA. Stx1 (5 pg/ml) and varying concentrations (abscissa) of the dimeric hybrid IgG/IgA were incubated for 1 h at 37°C. Each mixture was added to Vero cells (2 × 104), followed by culture for 42 h. Cell viability was determined by an MTT-like assay that measures reducing power of cells with WST-8 as a substrate. The values are relative to that without Stx1 exposure. The bars represent the means ± SD of triplicate determinations.
Inhibition of Stx1-induced phosphatidylserine exposure on the Ramos cell surface by hybrid IgG/IgA
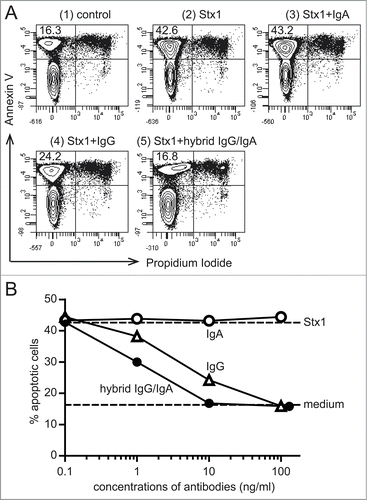
Ramos cells are one of the Stx1-sensitive cell types from Burkitt's lymphomas. Because of the nature of lymphoma cells, they are suitable for flow cytometry-based assays. Thus, cell surface exposure of phosphatidylserine was determined as an early event of apoptosis by flow cytometry. Apoptotic cells are recognized as cells that bind to annexin V but fail to incorporate propidium iodide (). When Stx1 was pre-incubated with the dimeric hybrid IgG/IgA, and then the mixture of Stx1 (5 pg/ml) and the antibody (10 ng/ml) was added to the Ramos cell culture, apoptosis was inhibited by 98%. At the same concentration, IgG1 mAb (D11C6) inhibited apoptosis by 70%, whereas IgA mAb (G2G7) did not inhibit it at all. Dose-response studies revealed that the Stx1-induced apoptosis was more efficiently inhibited by the hybrid IgG/IgA than the parental IgG1 mAb (). IgA mAb did not inhibit it up to 100 ng/ml.
Figure 3. Inhibition of Stx1-induced phosphatidylserine exposure on Ramos cells by the hybrid IgG/IgA. Ramos cells (1 × 106/ml) were incubated with Stx1 (5 pg/ml) in the presence of 10 ng/ml of IgA mAb, IgG1 mAb or the dimeric hybrid IgG/IgA for 5 h. As an early event of apoptosis, phosphatidylserine exposure was detected by annexin V binding (ordinate), while intact plasma membrane permeability was confirmed by the lack of propidium iodide incorporaton (abscissa) by flow cytometric analysis (A). The number in the upper left corner of each panel is the percentage of apoptotic cells. Dose-dependent inhibition of apoptosis (B). Effects of IgA mAb (open circles), IgG1 mAb (open triangles), and the hybrid IgG/IgA (filled circles) are shown.
Inhibition of Stx1-induced caspase-3 activation in Vero cells by hybrid IgG/IgA
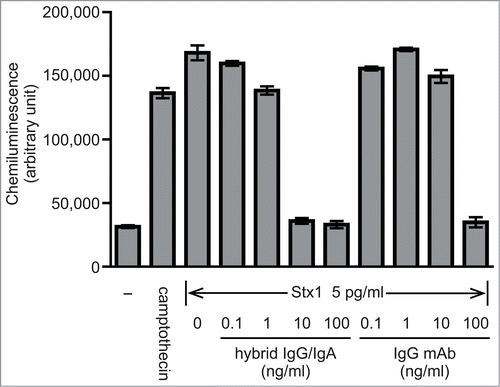
Vero cells were also used to examine the effect of antibody treatment on the Stx1-induced apoptosis. Incubation of Vero cells with 5 pg/ml Stx1 resulted in caspase-3 activation. This was shown by the luciferase reaction on a substrate that was made available through cleavage of the caspase recognition sequence on the substrate precursor. Stx1 caused caspase-3 activation to a similar level to that with 20 μM camptothecin. When Stx1 was treated with increasing concentrations of the hybrid-IgG/IgA, caspase-3 activation was dose-dependently inhibited (). At concentrations higher than 10 ng/ml of the hybrid IgG/IgA, complete inhibition was observed. When IgG1 mAb D11C6 was examined in parallel, caspase-3 inhibition was observed but 100 ng/ml was required for complete inhibition.
Figure 4. Inhibiton of Stx1-induced caspase-3 activation in Vero cells by the hybrid IgG/IgA. Stx1 (5 pg/ml) was incubated with varying concentrations of the dimeric hybrid IgG/IgA or IgG mAb (D11C6) for 1 h at 37°C. Each mixture was added to Vero cells (2 × 104), followed by culture for 24 h. Activation of caspase-3 was determined as cleavage of the caspase recognition sequence attached to a proluminescent substrate that emits a luminescent signal. Camptothecin was used as a positive control. Data are expressed as the means ± SD of triplicate determinations.
Discussion
Our previous studies demonstrated that the dimeric hybrid-IgG/IgA is more than 10-times more effective than the monomeric one as to toxin neutralization.Citation11 The dimeric hybrid IgG/IgA was prepared by size-exclusion chromatography from a culture supernatant of CHO cells that express H, L and J chains. Because the monomeric hybrid IgG/IgA preparation was derived from CHO cells expressing only H and L chains, this preparation could not contain the dimeric form.Citation1 That is, fair comparison was possible in terms of the antibody valence at the same constant region of IgA. However, it remains unknown whether or not the toxin neutralization activity was influenced by the difference in the Fc regions. Thus, we compared the parental IgG1 mAb and the hybrid IgG/IgA, which share the same variable region.
The results of our previous toxin neutralization assay involving Vero cell metabolic activity showed that more than 10 ng/ml of the dimeric form of hybrid IgG/IgA induced complete inhibition of the toxicity of Stx1.Citation11 As to the present dimer preparation, similar dose-dependent inhibition by the hybrid IgG/IgA was observed; that is 10 ng/ml of the hybrid IgG/IgA gave rise to complete neutralization in the MTT-like assay ().
To evaluate the neutralization activity of the hybrid IgG/IgA by assays other than the MTT-like assay, we examined the effect on the apoptosis induction by Stx1 holotoxin.Citation13,14 We used 2 different assays involving different cell lines. For Ramos cells, phosphatidylserine exposure was detected as an early event of apoptosis. For Vero cells, caspase-3 activation was examined as a process involved in the apoptotic pathway. In both assays, 10 ng/ml of the hybrid IgG/IgA was shown to be sufficient to inhibit apoptosis induction by Stx1 completely. This is consistent with the results of the MTT-like assay. In contrast, this concentration is not sufficient for the parental IgG1 in either the assay involving Ramos or that involving Vero cells.
In the Ramos cell assay, no inhibition of apoptosis by IgA mAb was observed up to 100 ng/ml. The lack of toxin neutralization by IgA mAb is consistent with our previous findings.Citation9 The lack of inhibitory activity of IgA mAb is not due to the absence of the dimeric form.Citation9 The efficient inhibition by the hybrid IgG/IgA indicated that weak ability of toxin neutralization is not intrinsic to the IgA class. If appropriate variable regions are linked to IgA constant regions, efficient toxin neutralization can be attained.
To determine the relative efficacy of antibodies, caspase-3 activation in Vero cells was measured with a more sensitive assay. Camptothecin was used as a positive control showing the activation of caspase-3. Stx1 induced caspase-3 activation to a similar level to that with camptothecin. In this system, the hybrid IgG/IgA completely inhibited caspase-3 activation at more than 10 ng/ml, whereas 100 ng/ml is needed for the parental IgG1 mAb to obtain the same level of inhibition. Thus, the dimeric hybrid IgG/IgA was 10-fold more efficient than the parental IgG1 mAb as to inhibition of caspase-3 activation in Vero cells. This is in parallel with our previous finding that the dimeric hybrid IgG/IgA was at least 10-fold more efficient than the monomeric form as to recovery of the Vero cell viability decrease induced by Stx1. The increased valence of antibodies seems to contribute to the lattice formation with antigens.
In conclusion, the dimeric form of hybrid IgG/IgA specific for Stx1B inhibited Stx1-induced apoptosis in 2 different cell lines, Ramos and Vero cells. The dimeric form of hybrid IgG/IgA inhibited apoptosis more effectively than the parental IgG1 mAb did. The results suggested that the utilization of high affinity binding sites as the variable regions for IgA might contribute to expansion of the IgA repertoire. This includes ones that are difficult to obtain by active immunization in vivo. The potential application of the hybrid IgG/IgA is to the neutralization of toxins on the mucosal surface of the intestine through oral administration. It should be noted that an appropriate choice of the variable regions would expand the range of application of the hybrid IgG/IgA to virulence factors of various pathogens.
Materials and Methods
Reagents
Shiga toxin 1 (Stx1) and the purified recombinant B subunit of Stx1 (Stx1B) were prepared as described previously.Citation6,9 Mouse mAbs specific for Stx1B of IgA class (G2G7) and of IgG1 subclass (D11C6) were prepared as described previously.Citation8,9 The expression of dimeric hybrid IgG/IgA specific for Stx1B in CHO-K1 cells was performed as described previously.Citation10,11 A culture supernatant containing hybrid IgG/IgA was prepared in CD CHO-A medium (Life Technologies). Mouse myeloma proteins TEPC 15 (IgA, κ) and MOPC 21 (IgG1, κ), bovine serum albumin (BSA; Fraction V), and camptothecin were purchased from Sigma; purified goat anti-mouse κ chain and horseradish peroxidase (HRP)-goat anti-mouse IgA (α chain-specific) from Southern Biotech; and purified rabbit anti-mouse IgG (γ chain-specific), HRP-goat anti-mouse IgG (γ chain-specific) and Medium 199 (M199) were purchased from Life Technologies; RPMI 1640 and kanamycin sulfate from Wako Pure Chemicals (Osaka, Japan); fetal bovine serum (FBS) from Hyclone; and N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) and a Cell Counting Kit-8 from DOJONDO (Kumamoto, Japan). The Cell Counting Kit-8 utilizes WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium) as a substrate. Sephacryl S-300 High Resolution was purchased from GE Healthcare; and Vivaspin 20–30 K and Vivaspin 500–30 K from Sartorius. Caspase-Glo® 3/7 Assay was purchased from Promega; and an FITC-Annexin V Apoptosis Detection Kit from BD PharMingen.
Cells
African green monkey kidney-derived Vero cells (American Type Culture Collection, ATCC) were cultured in M199 supplemented with 10 mM HEPES (pH 7.2), 10% FBS and 60 μg/ml kanamycin (10% FBS-M199). Burkitt's lymphoma Ramos cells (ATCC) were cultured in RPMI 1640 supplemented with 10 mM HEPES (pH 7.2), 10% FBS and 60 μg/ml kanamycin (10% FBS-RPMI 1640). Cell cultures were performed at 37 °C under a humidified atmosphere of 5% CO2/95% air.
Separation of dimeric hybrid IgG/IgA
A serum-free culture supernatant of CHO-K1 cells producing dimeric hybrid IgG/IgA was concentrated by means of Vivaspin 20–30 K and Vivaspin 500–30 K, and the buffer was changed to 160 μl of phosphate-buffered saline containing 0.02% NaN3 (PBS-NaN3). The concentrated supernatant was separated on a column of Sephacryl S-300 (1.6 cm × 60 cm) equilibrated with PBS-NaN3. Fractions (1 ml each) were collected and OD280 nm was measured. To detect hybrid IgG/IgA in each fraction, 9 μl aliquots were subjected to SDS-PAGE (7.5% non-reducing Laemmli's conditions; Mini-PROTEAN TXG Precast Gel, BIO-RAD) with MagicMark™ XP Western Protein Standards (Life Technologies) as molecular weight standards. After blotting onto a PVDF membrane (Millipore), the hybrid IgG/IgA was detected with HRP-goat anti-mouse IgA (1:1,000) using a chemiluminescence reagent (West Pico; Thermo Scientific Pierce) with a luminescent image analyzer, LAS-3000 (FUJI Photo Film, Tokyo, Japan).Citation15 Fractions containing dimeric hybrid IgG/IgA were pooled and concentrated, and the buffer was changed to sterile PBS by means of a Vivaspin to remove NaN3, followed by sterilization by membrane filtration.
ELISA
To quantify assembled hybrid IgG/IgA and IgA mAb, a sandwich ELISA was employed. Thus, immobilized goat anti-mouse κ chain (1:1,000) was used as a capture antibody and HRP-goat anti-mouse IgA (1:1,000) for detection.Citation10 BSA was used for blocking of nonspecific binding sites on the wells of an ELISA plate (Costar 9018, Corning).Citation8 IgA myeloma TEPC 15 was used as a standard. For IgG1 mAbs, immobilized rabbit anti-mouse IgG (1:1,000) was used as a capture antibody and HRP-goat anti-mouse IgG (1:1,000) for detection.Citation9 IgG1 myeloma MOPC 21 was used as a standard.
Cell viability detection by MTT-like assay
Vero cells were plated at 2 × 104 cells/100 μl of 10% FBS-M199 into the wells of a 96-well plate (Falcon® 353072, Corning), and then cultured for 16 h. Stx1 (5 pg) and an antibody (varying amount) were mixed in 1 ml of 10% FBS-M199, followed by incubation for 1 h at 37°C. After replacing the medium with a 100-μl aliquot of the mixture of Stx1 and an antibody, the Vero cells were further cultured for 42 h. After the medium had been removed, 100 μl of M199 and 10 μl of Cell Counting Kit-8 (containing WST-8) were added to each well, and the cell culture was continued for 4 h. Cell viability was colorimetrically measured as described.Citation9 Absorbance readings were made with a microplate reader (SUNRISE Rainbow RC-R; Tecan, Salzburg, Austria) at 450 nm with reference at 650 nm. Viability was expressed as the percentage of the control level (without toxin exposure).
Annexin V binding assay
Stx1 (1 pg) and varying amounts of an antibody (0.02 to 20 ng) were mixed and incubated for 1 h at 37 °C in 40 μl of 10% FBS-RPMI 1640. The mixture of Stx1 and an antibody was added to 160 μl of a Ramos cell suspension (1.25 × 106 cells/ml) in 10% FBS-RPMI 1640. Thus, the final concentrations were 1 × 106 cells/ml, 5 pg/ml of Stx1, and 0.1 to 100 ng/ml of antibodies, respectively. The cells were cultured for 5 h at 37 °C, and then washed twice in PBS by centrifugation (200 × g for 5 min). The cells were then incubated with FITC-annexin V and propidium iodide for 15 min according to the manufacturer's instructions. The cells were analyzed for the binding of annexin V and the incorporation of propidium iodide with a flow cytometer (FACS Canto II; BD Biosciences, San Jose, CA, USA).
Caspase-3 activation
Vero cells were plated at 1 × 104 cells/100 μl of 10% FBS-M199 into the wells of an opaque white 96-well plate (Falcon® 353296, Corning), and then cultured for 16 h. Stx1 (5 pg) and an antibody (varying amount) were mixed in 1 ml of 10% FBS-M199, followed by incubation for 1 h at 37°C. After replacing the medium with a 50-μl aliquot of the mixture of Stx1 and an antibody, the Vero cells were further cultured for 24 h. The Caspase-Glo reagent (50 μl) was added to each well according to the manufacturer's instructions for the detection of activated caspase-3. Cells were incubated for 1 h at 37°C, and then signals were measured with a luminometer (Wallac 1420 ARVOsx; PerkinElmer Inc., Waltham, MA, USA). As a positive control, Vero cells were incubated with 20 μM camptothecin.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was partly supported by a Grant-in-Aid for Scientific Research (C) (16590055), a Grant-in-Aid for Challenging Exploratory Research (23659067, 25670063) to YI; Grant-in-Aid for JSPS Fellows (25·10915) to KN; and by research funding for the Global COE Program from the Japan Society for the Promotion of Science.
References
- Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol 2006; 208:270-82; PMID:16362985; http://dx.doi.org/10.1002/path.1877
- Brandtzaeg P. Function of mucosa-associated lymphoid tissue in antibody formation. Immunol Invest 2010; 39:303-55; PMID:20450282; http://dx.doi.org/10.3109/08820131003680369
- Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol 2004; 2:123-40; PMID:15040260; http://dx.doi.org/10.1038/nrmicro818
- Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ 1999; 77:651-66; PMID:10516787
- Paton JC, Paton AW. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev 1998; 11:450-79; PMID:9665978
- Miyashita S, Matsuura Y, Miyamoto D, Suzuki Y, Imai Y. Development of recombinant B subunit of Shiga-like toxin 1 as a probe to detect carbohydrate ligands in immunochemical and flowcytometric application. Glycoconj J 1999; 16:697-705; PMID:11003554; http://dx.doi.org/10.1023/A:1007107425891
- Imai Y, Nagai R, Ono Y, Ishikawa T, Nakagami H, Tanikawa T, Kurohane K. Production of secretory immunoglobulin A against Shiga toxin-binding subunits in mice by mucosal immunization. Infect Immun 2004; 72:889-95; PMID:14742533; http://dx.doi.org/10.1128/IAI.72.2.889-895.2004
- Imai Y, Ishikawa T, Tanikawa T, Nakagami H, Maekawa T, Kurohane K. Production of IgA monoclonal antibody against Shiga toxin binding subunits employing nasal-associated lymphoid tissue. J Immunol Methods 2005; 302:125-35; PMID:15992815; http://dx.doi.org/10.1016/j.jim.2005.05.007
- Tanikawa T, Ishikawa T, Maekawa T, Kurohane K, Imai Y. Characterization of monoclonal immunoglobulin A and G against Shiga toxin binding subunits produced by intranasal immunization. Scand J Immunol 2008; 68:414-22; PMID:18782271; http://dx.doi.org/10.1111/j.1365-3083.2008.02153.x
- Tobisawa Y, Maruyama T, Tanikawa T, Nakanishi K, Kurohane K, Imai Y. Establishment of recombinant hybrid-IgGIgA immunoglobulin specific for Shiga toxin. Scand J Immunol 2011; 74:574-84; PMID:21883352; http://dx.doi.org/10.1111/j.1365-3083.2011.02617.x
- Iwata K, Kurohane K, Nakanishi K, Miyake M, Imai Y. Stable expression and characterization of monomeric and dimeric recombinant hybrid-IgGIgA immunoglobulins specific for Shiga toxin. Biol Pharm Bull 2014; 37:1510-5; PMID:24989136; http://dx.doi.org/10.1248/bpb.b14-00323
- Johansen FE, Braathen R, Brandtzaeg P. The J chain is essential for polymeric Ig receptor-mediated epithelial transport of IgA. J Immunol 2001; 167:5185-92; PMID:11673531; http://dx.doi.org/10.4049/jimmunol.167.9.5185
- Mangeney M, Lingwood CA, Taga S, Caillou B, Tursz T, Wiels J. Apoptosis induced in Burkitt's lymphoma cells via Gb3/DC77, a glycolipid antigen. Cancer Res 1993; 53:5314-9; PMID:8221667
- Inward CD, Williams J, Chant I, Crocker J, Milford D V, Rose PE, Taylor CM. Verocytotoxin-1 induces apoptosis in Vero cells. J Infect 1995; 30:213-8; PMID:7673744; http://dx.doi.org/10.1016/S0163-4453(95)90693-2
- Nakanishi K, Narimatsu S, Ichikawa S, Tobisawa Y, Kurohane K, Niwa Y, Kobayashi H, Imai Y. Production of hybrid-IgG/IgA plantibodies with neutralizing activity against Shiga toxin 1. PLoS One 2013; 8:e80712; PMID:24312238; http://dx.doi.org/10.1371/journal.pone.0080712
