Abstract
In this study, we describe the isolation and characterization of epithelial stem-like cells from the swine umbilical cord and their susceptibility to influenza virus infection. Swine umbilical cord epithelial stem cells (SUCECs) expressed stem cell and pluripotency associated markers such as SSEA-1, SSEA-4, TRA 1–60 and TRA 1–81 and Oct4. Morphologically, cells displayed polygonal morphology and were found to express epithelial markers; pancytokeratin, cytokeratin-18 and occludin; mesenchymal cell markers CD44, CD90 and haematopoietic cell marker CD45 were not detected on these cells. The cells had extensive proliferation and self- renewal properties. The cells also possessed immunomodulatory activity and inhibited the proliferation of T cells. Also, higher levels of anti-inflammatory cytokine IL-10 were detected in SUCEC-T cell co-cultures. The cells were multipotent and differentiated into lung epithelial cells when cultured in epithelial differentiation media. We also examined if SUCECs are susceptible to infection with influenza virus. SUCECs expressed sialic acid receptors, used by influenza virus for binding to cells. The 2009 pandemic influenza virus and swine influenza virus replicated in these cells. SUCECs due to their differentiation and immunoregulatory properties will be useful as cellular therapy in a pig model for human diseases. Additionally, our data indicate that influenza virus can infect SUCECs and may transmit influenza virus from mother to fetus through umbilical cord and transplantation of influenza virus-infected stem cells may transmit infection to recipients. Therefore, we propose that umbilical cord cells, in addition to other agents, should also be tested for influenza virus before cryopreservation for future use as a cell therapy for disease conditions.
Introduction
Stem cells have been shown to possess extensive self-renewal, differentiation and immunoregulatory properties thus are ideal source for cell based therapies for several autoimmune, inflammatory and degenerative diseases. Stem cell-based therapy is being explored for treatment of several human diseases especially for those diseases that have limited or no other treatment options. Bone marrow contains multipotent stem cells and these cells are extensively studied in animal models and human clinical trials. However, isolation of stem cells from bone marrow requires painful invasive procedure. Moreover, available data indicate that the number of isolated cells from bone marrow and their differentiation potential decreases with age.Citation1,2
Lately, umbilical cord which is the extension of fetal amnion is being evaluated as a source of stem cells. Two types of stem cells: epithelial and mesenchymal stem cells (MSCs) have been isolated and characterized from human umbilical cord.Citation3-5 Umbilical cord stem cells are multipotent, possess high proliferation and immunoregulatory properties.Citation6 Moreover, their collection is relatively easy and does not involve any invasive interventions.Citation7,8 Due to these properties, umbilical cord stem cells are being actively tested in human clinical trials as an alternative source of stem cells.Citation9,10
Influenza A and B viruses cause seasonal annual epidemics resulting in 3–5 million clinical cases and between 250,000–500,000 deaths annually.Citation11 During the last century, novel influenza viruses of the H1N1, H2N2 and H3N2 subtypes against which general population had no prior immunity caused pandemics of 1918, 1957 and 1968; respectively. A novel H1N1 virus of swine origin caused the 2009 human pandemic. In addition to these viruses, certain types of influenza viruses of H5N1 and H7N7 directly transmit from avian species to humans. Most recently new avian H7N9 influenza virus transmitted from poultry to humans and according to latest WHO report (June 27, 2014), 450 laboratory confirmed human cases of infection with 165 deaths, mainly in China, have been reported, thus, posing a real threat for new influenza pandemic (http://www.who.int/influenza/human_animal_interface/influenza_h7n9/riskassessment_h7n9_27june14.pdf).
Influenza viruses often cause severe infections in young children, elderly, pregnant women and immunocompromised individuals. Available data indicate that several influenza viruses including the highly pathogenic avian influenza H5N1 and 2009 H1N1 influenza virus infected pregnant women resulting in either their death, abortion or affected the fetal development.Citation12-15 Although not fully established, a few studies in humans and pigs speculated the vertical transmission of influenza virus from mother to fetus.Citation16-18
Pig's anatomy, physiology and immunology closely resemble to humans; therefore, pigs are useful large animal model for evaluating stem cell therapy in a preclinical model.Citation19-23 Additionally, pigs are natural host for influenza virus and clinical signs of influenza and disease pathogenesis in pigs closely resemble that observed in humans.Citation24 In this study, for the first time, we isolated epithelial stem cells from the umbilical cord of pigs (referred as SUCECs in the text). The cells expressed pluripotency, stem and epithelial cell markers. The cells also differentiated into lung epithelial cells and inhibited the proliferation of T cells. These cells should be useful as preclinical cellular therapy studies for human diseases in large animal models such as pigs. Additionally, we have demonstrated that these cells are susceptible to influenza virus indicating the potential of these cells to transmit infection from mother to fetus. Currently, human umbilical cord stem cells are being investigated as a cellular therapy in human clinical trials, our data demonstrating the replication of influenza in SUCECs suggests that umbilical cord stem cells isolated from influenza-virus infected humans may transmit infection to the recipients and virus infection may also alter the differentiation and immunomodulatory properties of these cells.
Results
Proliferation and self-renewal potential of SUCECs
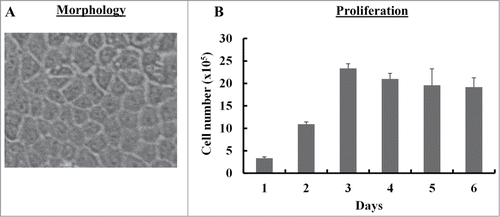
We isolated single cell suspension from UC of pigs by enzymatic treatment (n = 3). The cell suspensions were cultured on collagen-coated tissue culture flasks. Epithelial cell-like colonies exhibiting typical cobblestone morphology were observed in 7–8 d culture (). The epithelial colony cells grew slowly initially, however, after 2 passages cells displayed extensive proliferation potential in culture. As shown in , cells proliferated extensively in culture. Cell number increased more than 10 fold as compared to starting cell number by day 3. The epithelial colony cells were also examined for their ability to form colonies in vitro by colony forming unit (CFU) assay. Single cells were able to form colonies (>50 cells), suggesting that these cells possess self-renewal potential (data not shown).
Figure 1. Morphology and proliferation of SUCECs: SUCECs were isolated from the umbilical cords of near term pigs (n = 3) by collagenase treatment. (A) SUCECs displayed characteristic epithelial cell like cobblestone morphology. A representative epithelial colony observed 7–8 d after initial plating of umbilical cord cells is shown. (B) SUCECs (2 × 105/well) were plated in a 6-well plate and their proliferation was measured over a 6 day period by counting the viable cells by trypan blue dye. Data are expressed as mean from triplicates ± SD.
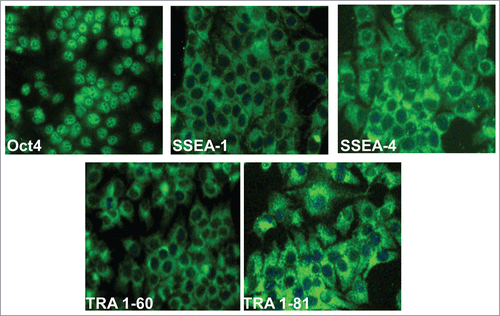
Stem and epithelial cell markers expression on SUCECs
The isolated epithelial cells showed self-renewal and extensive proliferation potential. Next, we examined the expression of stem cell and pluripotency markers on these cells. The cells expressed Oct4, and SSEA-1, SSEA-4, TRA 1–60 and TRA 1–81 markers (). The Oct4 was mainly detected in the nuclei of almost all the cells. SSEA-1 and 4, embryonic stem cell markers, were mainly localized on the surface, in the cytoplasm and in perinuclear region of epithelial cells. TRA 1–60 and 1–81 were also detected on the surface and cytoplasm of SUCECs ().
Phenotypic characteristics of SUCECs
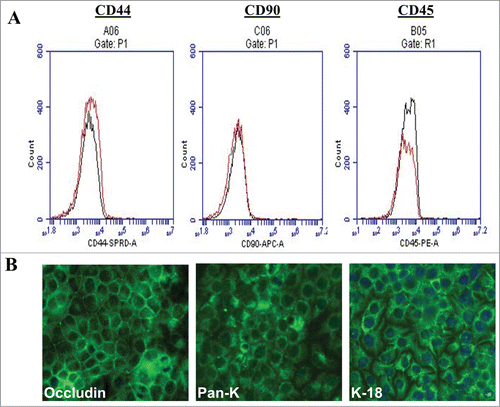
The expression of mesenchymal and haematopoietic markers on SUCECs was examined by flow cytometry. The cells were negative for the expression of mesenchymal (CD44, CD90) and haematopoietic marker (CD45). However, cells showed bright staining for epithelial markers; pancytokeratin (Pan-CK), cytokeratin-18 (CK-18) and occludin, when examined by IFA confirming the epithelial phenotype of these cells ().
Figure 3. Phenotypic characteristic of SUCECs: (A) SUCECs (n = 3) were analyzed by flow cytometry and (B) IFA for the expression of mesenchymal (CD44 and CD90), haematopoietic (CD45) and epithelial cell markers (Pan-CK, CK-18 and Occludin). Solid black line: isotype control; red line: specific antibody. Mesenchymal and haematopoietic markers were not detected on SUCECs, whereas epithelial markers were strongly expressed on these cells.
Differentiation of SUCECs into lung epithelial cells
SUCECs showed stem cell characteristics such as self-renewal and expression of pluripotency and stem cell markers. Therefore, we were interested to see if these cells also have differentiation potential. These cells were examined for differentiation into lung epithelial cell types.
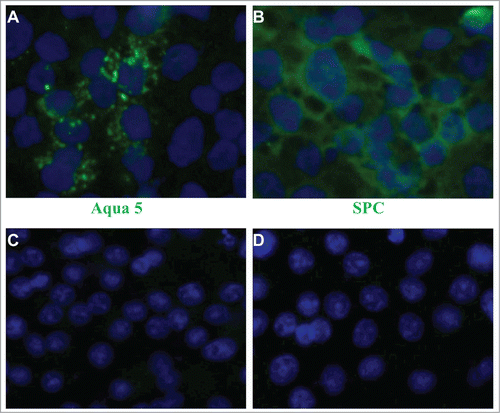
For inducing differentiation of SUCECs into type I and II lung epithelial cells, individual colonies of SUCECs were cultured in collagen I-coated tissue culture plates. The cells were cultured in epithelial differentiation medium that contained 50% epithelial growth media supplemented with bovine pituitary extract (70 μg/ml), human epidermal growth factor (5 ng/ml), insulin (5 μg/ml), and hydrocortisone (0.5 μg/ml) (MEBM, Lonza) and 50% lung MSC-CM medium for 6 days. After the incubation, expression of Aquaporin 5 (Aqua5) and pro surfactant protein C (SPC), markers for type I and II pneumocytes, respectively was examined on differentiated cells. SUCECs cultured in epithelial differentiation medium were flattened and larger in size as compared to parent epithelial colony cells. Also, expression of Aqua5 and SPC proteins was detected on differentiated cells ().
Figure 4. Differentiation potential of SUCECs into type (I)and II pneumocytes: SUCECs were cultured on collagen 1-coated culture dishes in epithelial differentiation medium for 6 d After the incubation, the cells were examined for the expression of (A) Aqua 5 and (B) SPC, specific markers for type I and II pneumocytes, respectively by IFA. Expression of Aqua 5 and SPC was not observed on SUCECs cultured in DMEM (C and D). Cell nuclei were stained by DAPI.
SUCECs inhibit T cell proliferation
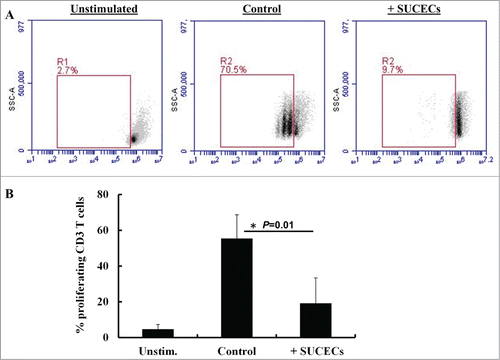
Stem cells possess potent immunoregulatory properties and are under evaluation as cell therapy for autoimmune and inflammatory diseases. In this study, we examined if SUCECs also have immunoregulatory activity. SUCECs were co-cultured with peripheral blood mononuclear cells (PBMCs) at 1:10 ratio and stimulated with phytohemagglutinin (PHA). Presence of SUCECs in cultures significantly suppressed (P < 0 .05) proliferation of T cells. In presence of SUCECs, there was 66% suppression in the number of proliferating T cells (). Additionally, levels of anti-inflammatory cytokine, IL-10 were also higher when SUCECs were present in co-cultures with PBMCs (data not shown). Thus, SUCECs possess immunomodulatory activity and may serve as useful tool to study their immunomodulatory and epithelial repair mechanisms in a pig model of acute inflammatory diseases.
Figure 5. SUCECs inhibit proliferation of (T)cells: SUCECs were cultured with CFSE labeled PBMCs at 1:10 ratio in 24-well plates. The cultures were stimulated with PHA (5 μg/ml) for 72 h. Proliferation of T cells cultured with or without SUCECs was examined by flow cyometry. (A) Representative dot plots of proliferating T cells are shown. (B) The data were expressed as mean ± SD of% proliferating T cells. The experiments were performed with 2 different SUCECs and 3 different PBMCs.
Detection of influenza virus receptors on SUCECs
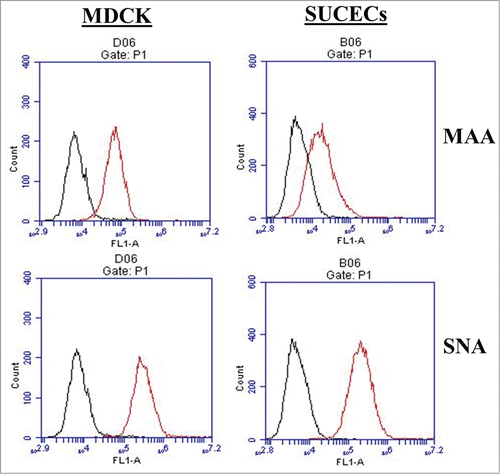
In earlier studies influenza viral genome was detected in placenta and/or fetuses/infants whose mothers were infected with influenza virus.Citation12,17 In this study, we wanted to see if SUCECs support influenza virus replication. First, we examined these cells for the expression of influenza virus receptors by flow cytometry. Madin-Darby canine kidney (MDCK) cells that support avian and mammalian influenza virus infection were included as positive controls. SUCECs expressed α-2,3- and α-2,6-linked sialic acid residues, receptors for avian and mammalian influenza viruses, respectively, on their surface. As shown in , the expression of both types of receptors on SUCECs was comparable to expression levels on MDCK cells. Nearly all MDCK cells (99%) and majority of SUCECs (95%) expressed α-2,6-linked sialic acid receptors whereas 55% of MDCK and 35% of SUCECs expressed α-2,3-linked sialic acid receptors.
Figure 6. SUCECs express influenza virus receptors: SUCECs (n = 3) and MDCK cells (positive control) were stained with fluorescein isothiocyanate (FITC)-labeled Maackia amurensis lectin II (MAA) and Sambucus niagra agglutinin (SNA) which detect α-2,3-linked sialic acid receptors (avian influenza virus) and α-2,6-linked sialic acid receptors (mammalian influenza virus) respectively. Expression of these lectins was examined by flow cytometry. Specific staining is indicated by red line; black line indicates unstained cell controls.
Replication of influenza viruses in SUCECs
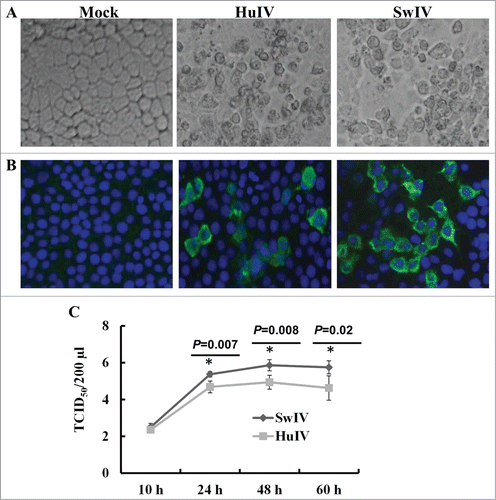
We examined the susceptibility of these cells to Swine/TX/1998, H3N2 (SwIV), and pandemic human influenza virus Human/CA/09, H1N1 (HuIV) viruses. Consistent with the expression of sialic acid receptors on SUCECs, influenza viruses replicated in SUCECs when infected at MOI of 1 even in the absence of TPCK trypsin and caused cytopathological changes in SUCECs (). Influenza virus NP was also detected in virus-infected cells by IFA at 24 h after infection (). While examining the kinetics of virus replication in SUCECs, the infectious viruses were detected in virus-infected culture supernatants starting from 10 h after infection. The virus titers increased steadily over time and reached peak at 48 h after infection. SwIV virus replicated to higher titers as compared to HuIV in these cells ().
Figure 7. Replication of influenza virus in SUCECs: SUCECs were infected with SwIV or HuIV at a MOI of 1. (A) Both SwIV (Sw/TX/98) and HuIV (Hu/CA/09) viruses replicated and induced cytopathology in SUCECs. Representative photographs of control, SwIV and HuIV-infected SUCECs at 48 h after infection are shown. (B) Influenza virus NP was detected in SwIV and HuIV-infected SUCECs at 24 h after infection by IFA. (C) Released virus quantification in SwIV and HuIV-infected culture supernatants at indicated time points was analyzed by titration in MDCK cells. Virus titers are expressed as mean ± SD from 3 independent experiments.
Discussion
Umbilical cord, which is normally discarded at birth, contains multipotent stem cells. Umbilical cord stem cells are under investigation for numerous human conditions under clinical settings. In this study, we report on isolation and characterization of porcine epithelial stem cell-like cells from umbilical cord and susceptibility of these cells to influenza virus. The cells displayed cuboidal morphology and expressed epithelial specific markers whereas mesenchymal and haematopoietic markers were not detected on these cells. The isolated cells expressed embryonic stem cell and pluripotency associated markers. These cells possessed immunomodulatory properties and inhibited the proliferation of T cells and caused the production of anti-inflammatory cytokine, IL-10. Upon culture in induction medium, cells differentiated into lung type I and II pneumocytes. The cells also expressed influenza virus receptors and supported the replication of influenza viruses including the 2009 pandemic H1N1 influenza virus.
Umbilical cord is the extension of placental amnion and is source of both epithelial and mesenchymal stem cells. Human UCECs have been isolated and characterized.Citation8,25,26 Similar to human UCECs, SUCECs were highly proliferative and expressed embryonic stem cell and pluripotency markers including SSEA-1, SSEA-4, TRA-1–60, TRA-1–81, and Oct-4 and showed in vitro differentiation potential.Citation25,26 The expression of these factors indicated that these cells possess many of the properties of embryonic cells. Consistent with the expression of stem cells markers, SUCECs possessed self-renewal and differentiation potential. In this study we tested the differentiation of these cells into lung epithelial cells. SUCECs readily differentiated into aqua 5 expressing type I and SPC expressing type II cells. Previously, human UCECs were shown to differentiate into several types of epithelial cells including skin and lung epithelial cells [ref.Citation25; http://www.cellresearchcorp.com/index.cfm?GPID=37].
Consistent with the previous reports suggesting the presence of both epithelial and mesenchymal stem cells in umbilical cords,Citation3-5 we also observed mesenchymal and epithelial cell colonies in our starting cultures of collagenase treated umbilical cord cell suspension. Mesenchymal colonies appeared earlier around 3–4 days after culture whereas epithelial colonies were evident in 7–8 days. Similar to these observations, we previously isolated progenitor epithelial cells from the lungs of pigs that were also surrounded by mesenchymal cells.Citation27 Not much is known about the exact relationship between co-existing epithelial and mesenchymal cells in this culture system. Recent publications demonstrated that MSCs can differentiate into epithelial cell phenotypes in vitro and in vivo.Citation28-Citation30 In vitro culture of MSCs in epithelial differentiation medium resulted in the expression of lung epithelial cell markers such as CCSP, SPC, Aqua5, and CFTR protein.Citation29 Similarly, differentiation of MSCs into lung epithelium was observed in vivo, cord blood MSCs when inoculated in SCID mice, engrafted and showed the expression of epithelial cell marker cytokeratin and CFTR. These observations provide evidence that mesenchymal cells may serve as precursors of progenitor epithelial cells. Further, we and others have shown previously that MSCs secreted factors may regulate the differentiation and self-renewal of progenitor epithelial cells. In our earlier study, we have shown that pig lung progenitor epithelial cells differentiated into type I and II pneumocytes upon culture in epithelial differentiation medium containing 50% conditioned medium from lung MSCs. In this study, using the same media composition, we were able to induce the differentiation of SUCECs into type I and II lung epithelial cells. Similarly, MSC-CM supported the proliferation and differentiation of neuronal and retinal stem cells and their differentiation into neurons.Citation31,32 These data clearly indicate that probably soluble factors released by MSCs may regulate the self-renewal and differentiation of progenitor epithelial cells. Studies are underway to identify secreted factors that may be involved in regulating the differentiation potential of epithelial progenitor/stem cells. Our preliminary studies indicate that porcine lung MSCs secrete keratinocyte growth factor (KGF), a well-known soluble factor that regulates epithelial cell proliferation and differentiation.
Stem cells have immunoregulatory properties, inhibit inflammation and are low immunogenic, therefore, these cells are being evaluated as cellular therapy for acute inflammatory and autoimmune diseases in autologous and allogenic settings. In this study, we demonstrated that similar to human UCECs,Citation8 SUCECs also possess immunoregulatory properties. SUCECs significantly suppressed the proliferation of T cells in vitro in response to mitogen, suggesting that SUCECs will be valuable cell source to examine the role of these cells in a pig model of human inflammatory diseases.
Influenza virus mainly replicates in the respiratory tract. However, virus infection has been detected in extra-pulmonary tissues.Citation33-36 Influenza virus genome and viral antigens were also detected in reproductive organs and fetal tissues and the virus has been associated with causing abortions and fetal abnormalities.Citation12,Citation37-39 However, it is not yet identified whether influenza virus replicates in umbilical cord cells. In this study, we examined the susceptibility of isolated SUCECs to HuIV and SwIV. Our data provide clear evidence that SUCECs express receptors required for influenza virus binding and actively support virus replication. Both HuIV and SwIV replicated in primary SUCECs without the addition of exogenous trypsin. Earlier reports have shown that primary cells isolated from human adenoids and respiratory epithelium and several cell lines including HepG2 and MDCK can support influenza virus replication without the addition of trypsin.Citation40-44 It is likely that SUCECs cells may release endogenous protease (s) which is able to cleave precursor hemagglutinin (HA0) into active hemagglutinin (HA1 and HA2), the initial step required for the efficient replication of influenza viruses in cells.Citation45 These findings have implication for stem cell therapy where umbilical cord stem cells are used for transplantation. Donor umbilical cord cells infected with influenza virus may transmit virus to recipients. Earlier studies indicated that stem cell derived from hepatitis B and C-, cytomegalovirus (CMV)-, human herpesvirus 6-, and parvovirus-infected donors transmitted these infections in the recipients.Citation46-51 Since stem cells possess immunomodulatory and differentiation properties and are being tested to treat autoimmune and inflammatory diseases, influenza virus infection of these cells may affect their engraftment in recipients and alter their differentiation and immunomodulatory properties and thereby, may adversely influence the outcome of the transplantation. Currently, the data addressing these questions are not available. In one earlier report, Carpell and co-workersCitation52 demonstrated that the engraftment of CMV-infected MSCs in fetal tissues was lower as compared to non-infected MSCs when MSCs were inoculated in utero in fetal sheep, demonstrating that CMV infection affected the engraftment of stem cells. Future in vivo studies will be conducted in pigs to examine the transplacental transmission of influenza and the replication of influenza in umbilical cord epithelial, mesenchymal and hematopoetic cells and effect of virus infection on the differentiation and immunoregulatory properties of these cells.
In conclusion, we isolated progenitor epithelial-like cells from umbilical cord of pigs. These cells differentiated into type I and II lung epithelial cells and also had immunomodulatory properties. Therefore, these cells should serve as useful tool to study beneficial effects of stem cell therapy in a pig model of important human diseases. We also provided evidence that these cells are susceptible to influenza virus infection. Therefore, umbilical cord stem cells should be tested for influenza virus before transplantation to prevent virus transmission to recipients.
Materials and Methods
Isolation of SUCECs
We collected swine UCs (n = 3) after cesarean section from full-term births. We isolated epithelial cells from the umbilical cord using essentially the same protocol used for the isolation of human umbilical cord epithelial cells.Citation10,25,53 Briefly, after harvesting, we washed the cords extensively with phosphate-buffered saline (PBS) containing 1% antibiotics (Life Technologies) and cut into small pieces. We then removed the blood vessels and treated the cord fragments with collagenase II (0.5 mg/ml) in a 6-well plate at 37°C for 2 hours (h). After digestion, single cell suspension was obtained by filtering through a 70 μm cell strainer. The cells were washed 2–3 times with Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). We then, cultured SUCECs in collagen-I coated culture plates. The epithelial colonies were visible in 7–8 d after seeding. Epithelial colonies were expanded in culture and passaged at 90% confluency. Cells passaged between 3 and 8 times were used for the following experiments.
Proliferation of SUCECs
The proliferation of SUCECs was determined by cell counting the cells for 6 d at 24 h intervals. A total of 2 × 105 cells were seeded in a 6-well plate. At 24 h intervals, the cells were detached by trypsin and counted by trypan blue dye exclusion method.
Detection of stem cell and epithelial cell markers
We detected the expression of stem and epithelial cell markers on SUCECs by immunofluorescence assay (IFA) using swine specific or cross-reactive antibodies as described.Citation54,55 Briefly, SUCECs were fixed in methanol:acetone (1:1) for 5 minutes (min) and incubated with 3% bovine serum albumin (BSA) for 30 min. We then incubated the cells overnight at 4°C with following primary antibodies: rabbit anti-human Oct4 (Santa Cruz Biotechnology), mouse anti-human SSEA-1 and 4, mouse anti- human TRA-1–60 and -1–81 (Millipore), mouse anti-human Pan-CK (Sigma), mouse anti-human CK-18 (Sigma), rabbit anti-human occludin (Life Technologies). Following incubation, we washed the cells and incubated at room temperature for 1 h with the following FITC-labeled secondary antibodies: goat anti-rabbit IgG and goat anti-mouse IgG (Sigma). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
Phenotyping of cells by flow cytometry
To study the phenotypic characteristics of SUCECs, we stained SUCECs with the following primary antibodies: mouse anti pig-CD90 (IgG1), mouse anti pig-CD44 (IgG2b, VMRD), and mouse anti pig-CD45 (IgM, VMRD), for 20 min at 4°C. Rat anti-mouse IgG1-APC (BD Biosciences), goat anti-mouse IgG2b- Spectral Red (SPRD; Southern Biotech), rat anti-mouse IgM-PE (BD Biosciences) were used as secondary antibodies. Relevant isotype and secondary antibodies were used as controls for non-specific binding. Stained cells were acquired by C6 flow cytometer (BD Accuri Cytometers) and analyzed using CFlow® plus Software (Accuri).Citation27
Preparation of lung MSC-CM
We collected CM from porcine lung MSCs cultures as described previously.Citation54 Briefly, porcine lung-MSCs were grown and expanded in DMEM containing 10% FBS. When MSCs reached at about 80% confluence, we removed the culture medium, washed the cells with phosphate-buffered saline and cultured in serum-free DMEM medium for 24 h. Then, we collected the CM, removed cellular debris by centrifugation and used in epithelial differentiation assays.
Differentiation of SUCECs into type I and type II pneumocytes
We examined the differentiation potential of isolated SUCECs. The cells were tested for differentiation into type I and II pneumocytes. For this, we seeded the cells in culture dishes pre-coated with collagen I in epithelial cell differentiation medium for 6 d After the incubation, we examined the cell morphology and for the expression of Aqua5 (type I pneumocytes) and SPC (type II pneumocytes) was examined by IFA. Goat anti-human Aqua5 (Santa Cruz Biotechnology) and rabbit anti-human SPC (Millipore) were used as primary antibodies.Citation54,55
T cell proliferation
To study the effect of SUCECs on T cell proliferation, we stained PBMCs with carboxyfluorescein diacetate succinimidyl ester (CFSE) dye. PBMCs were isolated by centrifugation over Ficoll-Hypaque gradients.Citation27 PBMCs (106/ml in PBS) were stained with 5 μM CFSE for 5 min at room temperature. After washing, we suspended the cells in RPMI 1640 medium supplemented with 10% FBS and 1% antibiotics (Gibco) and seeded in a 24-well plate in the presence or absence of SUCECs (SUCEC to PBMC ratio 1:10). The cultures were stimulated with PHA; 5 μg/ml and incubated for 72 h at 37°C. Cell proliferation was analyzed by flow cytometry (Accuri).
Detection of sialic acid receptors on SUCECs
We examined the expression of influenza virus receptors on progenitor epithelial cells by flow cytometry.Citation54,56 MDCK cells which express both α-2,3-linked sialic acid receptors and α-2,6-linked sialic acid receptors; known receptors for avian and mammalian influenza viruses, respectively; were used as positive controls. SUCECs and MDCK cells were incubated with FITC-labeled Maackia amurensis lectin II (MAA) or Sambucus niagra agglutinin (SNA) (EY Laboratories) at 4°C for 30 min in dark; washed three times with FACS buffer and acquired by Accuri C6 flow cytometer (BD Accuri) and analyzed by Flow plus software (Accuri).
Influenza viruses and infection of progenitor epithelial cells
Virus stocks of SwIV; Swine/TX/98, H3N2, and pandemic HuIV; Human/CA/09, H1N1 (kindly provided by Dr. Sagar M. Goyal, Department of Veterinary Population Medicine, University of Minnesota, Saint Paul, MN) were prepared in MDCK cells. SUCECs were infected with SwIV or HuIV at a multiplicity of infection (MOI) of 1. At indicated time points after infection, culture supernatants were collected and released virus was quantified by titration in MDCK cellsCitation27 and expression of influenza virus nucleoprotein (NP) in infected cells at 24 h after infection was detected by IFA.Citation54
Statistical analysis
Data are expressed as mean ± standard deviation (SD) and data were analyzed by using Student's t test. P value of less than 0.05 was considered statistical significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Dr. Khatri's research program is supported by grants from National Pork Board, Biotechnology Research and Development Corporation, USDA, and Pfizer Animal Health Inc., now Zoetis.
References
- Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev 2001; 122:713-34; PMID:11322994; http://dx.doi.org/10.1016/S0047-6374(01)00224-X
- Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev 2008; 129:163-73; PMID:18241911; http://dx.doi.org/10.1016/j.mad.2007.12.002
- Kita K, Gauglitz GG, Phan TT, Herndon DN, Jeschke MG. Isolation and characterization of mesenchymal stem cells from the sub-amniotic human umbilical cord lining membrane. Stem Cells Dev 2010; 19:491-502; PMID:19635009; http://dx.doi.org/10.1089/scd.2009.0192
- Mizoguchi M, Suga Y, Sanmano B, Ikeda S, Ogawa H. Organotypic culture and surface plantation using umbilical cord epithelial cells: morphogenesis and expression of differentiation markers mimicking cutaneous epidermis. J Dermatol Sci 2004; 35:199-206; PMID:15381241; http://dx.doi.org/10.1016/j.jdermsci.2004.06.003
- Ruetze M, Gallinat S, Lim IJ, Chow E, Phan TT, Staeb F, Wenck H, Deppert W, Knott A. Common features of umbilical cord epithelial cells and epidermal keratinocytes. J Dermatol Sci 2008; 50:227-31; PMID:18242061; http://dx.doi.org/10.1016/j.jdermsci.2007.12.006
- Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells 2003; 21:105-10; PMID:12529557; http://dx.doi.org/10.1634/stemcells.21-1-105
- Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007; 25:1384-92; PMID:17332507; http://dx.doi.org/10.1634/stemcells.2006-0709
- Zhou Y, Gan SU, Lin G, Lim YT, Masilamani J, Mustafa FB, Phua ML, Rivino L, Phan TT, Lee KO, et al. Characterization of human umbilical cord lining-derived epithelial cells and transplantation potential. Cell Transplant 2011; 20:1827-41; PMID:21439131; http://dx.doi.org/10.3727/096368910X564085
- Wang S, Cheng H, Dai G, Wang X, Hua R, Liu X, Wang P, Chen G, Yue W, An Y. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res 2013; 1532:76-84; PMID:23942181; http://dx.doi.org/10.1016/j.brainres.2013.08.001
- Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR. Immune properties of human umbilical cord Wharton's jelly-derived cells. Stem Cells 2008; 26:2865-74; PMID:18703664; http://dx.doi.org/10.1634/stemcells.2007-1028
- Kuiken T, Riteau B, Fouchier RA, Rimmelzwaan GF. Pathogenesis of influenza virus infections: the good, the bad and the ugly. Curr Opin Virol 2012; 2:276-86; PMID:22709515; http://dx.doi.org/10.1016/j.coviro.2012.02.013
- Gu J, Xie Z, Gao Z, Liu J, Korteweg C, Ye J, Lau LT, Lu J, Zhang B, McNutt MA, et al. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet 2007; 370:1137-45; PMID:17905166; http://dx.doi.org/10.1016/S0140-6736(07)61515-3
- Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis 2006; 12:1638-43; PMID:17283611; http://dx.doi.org/10.3201/eid1211.060152
- Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis 2008; 14:95-100; PMID:18258087; http://dx.doi.org/10.3201/eid1401.070667
- Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, Louie J, Doyle TJ, Crockett M, Lynfield R, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010; 303:1517-25; PMID:20407061; http://dx.doi.org/10.1001/jama.2010.479
- Cetinkaya M, Ozkan H, Celebi S, Koksal N, Hacimustafaoglu M. Human 2009 influenza A (H1N1) virus infection in a premature infant born to an H1N1-infected mother: placental transmission? Turk J Pediatr 2011; 53:441-4; PMID:21980848
- Dulyachai W, Makkoch J, Rianthavorn P, Changpinyo M, Prayangprecha S, Payungporn S, Tantilertcharoen R, Kitikoon P, Poovorawan Y. Perinatal pandemic (H1N1) 2009 infection, Thailand. Emerg Infect Dis 2010; 16:343-4; PMID:20113578; http://dx.doi.org/10.3201/eid1602.091733
- Wallace GD, Elm JL, Jr. Transplacental transmission and neonatal infection with swine influenza virus (Hsw1N1) in swine. Am J Vet Res 1979; 40:1169-72; PMID:575027
- Chang C, Niu D, Zhou H, Zhang Y, Li F, Gong F. Mesenchymal stroma cells improve hyperglycemia and insulin deficiency in the diabetic porcine pancreatic microenvironment. Cytotherapy 2008; 10:796-805; PMID:18979304; http://dx.doi.org/10.1080/14653240802461924
- Lim JH, Piedrahita JA, Jackson L, Ghashghaei T, Olby NJ. Development of a model of sacrocaudal spinal cord injury in cloned Yucatan minipigs for cellular transplantation research. Cell Reprogram 2010; 12:689-97; PMID:21108536; http://dx.doi.org/10.1089/cell.2010.0039
- Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg 2002; 73:1919-25; discussion 26; PMID:12078791; http://dx.doi.org/10.1016/S0003-4975(02)03517-8
- Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 2006; 1:e79; PMID:17183711; http://dx.doi.org/10.1371/journal.pone.0000079
- Zhou L, Wang W, Liu Y, Fernandez de Castro J, Ezashi T, Telugu BP, Roberts RM, Kaplan HJ, Dean DC. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem Cells 2011; 29:972-80; PMID:21491544; http://dx.doi.org/10.1002/stem.637
- Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine 2008; 26 Suppl 4:D59-66; PMID:19230162; http://dx.doi.org/10.1016/j.vaccine.2008.07.025
- Huang L, Wong YP, Gu H, Cai YJ, Ho Y, Wang CC, Leung TY, Burd A. Stem cell-like properties of human umbilical cord lining epithelial cells and the potential for epidermal reconstitution. Cytotherapy 2011; 13:145-55; PMID:20735166; http://dx.doi.org/10.3109/14653249.2010.509578
- Reza HM, Ng BY, Phan TT, Tan DT, Beuerman RW, Ang LP. Characterization of a novel umbilical cord lining cell with CD227 positivity and unique pattern of P63 expression and function. Stem Cell Rev 2011; 7:624-38; PMID:21181306; http://dx.doi.org/10.1007/s12015-010-9214-6
- Khatri M, Dwivedi V, Krakowka S, Manickam C, Ali A, Wang L, Qin Z, Renukaradhya GJ, Lee CW. Swine influenza H1N1 virus induces acute inflammatory immune responses in pig lungs: a potential animal model for human H1N1 influenza virus. J Virol 2010; 84:11210-8; PMID:20719941; http://dx.doi.org/10.1128/JVI.01211-10
- Karoubi G, Cortes-Dericks L, Breyer I, Schmid RA, Dutly AE. Identification of mesenchymal stromal cells in human lung parenchyma capable of differentiating into aquaporin 5-expressing cells. Lab Invest 2009; 89:1100-14; PMID:19652646; http://dx.doi.org/10.1038/labinvest.2009.73
- Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med 2008; 177:701-11; PMID:18063840; http://dx.doi.org/10.1164/rccm.200706-859OC
- Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA, Jr., Spees JL, Bertucci D, Peister A, Weiss DJ, et al. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci U S A 2005; 102:186-91; PMID:15615854; http://dx.doi.org/10.1073/pnas.0406266102
- Croft AP, Przyborski SA. Mesenchymal stem cells expressing neural antigens instruct a neurogenic cell fate on neural stem cells. Exp Neurol 2009; 216:329-41; PMID:19159625; http://dx.doi.org/10.1016/j.expneurol.2008.12.010
- Xia J, Luo M, Ni N, Chen J, Hu Y, Deng Y, Ji J, Zhou J, Fan X, Gu P. Bone marrow mesenchymal stem cells stimulate proliferation and neuronal differentiation of retinal progenitor cells. PLoS One 2013; 8:e76157; PMID:24098776; http://dx.doi.org/10.1371/journal.pone.0076157
- Ito Y, Ichiyama T, Kimura H, Shibata M, Ishiwada N, Kuroki H, Furukawa S, Morishima T. Detection of influenza virus RNA by reverse transcription-PCR and proinflammatory cytokines in influenza-virus-associated encephalopathy. J Med Virol 1999; 58:420-5; PMID:10421411; http://dx.doi.org/10.1002/(SICI)1096-9071(199908)58:4%3c420::AID-JMV16%3e3.0.CO;2-T
- Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, Muramoto Y, Tamura D, Sakai-Tagawa Y, Noda T, et al. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 2009; 460:1021-5; PMID:19672242
- Kaji M, Oseasohn R, Jordan WS, Jr., Dingle JH. Isolation of Asian virus from extrapulmonary tissues in fatal human influenza. Proc Soc Exp Biol Med 1959; 100:272-5; PMID:13634109; http://dx.doi.org/10.3181/00379727-100-24597
- Zhang Z, Zhang J, Huang K, Li KS, Yuen KY, Guan Y, Chen H, Ng WF. Systemic infection of avian influenza A virus H5N1 subtype in humans. Hum Pathol 2009; 40:735-9; PMID:19121843; http://dx.doi.org/10.1016/j.humpath.2008.08.015
- Acs N, Banhidy F, Puho E, Czeizel AE. Pregnancy complications and delivery outcomes of pregnant women with influenza. J Matern Fetal Neonatal Med 2006; 19:135-40; PMID:16690505; http://dx.doi.org/10.1080/14767050500381180
- Coffey VP, Jessop WJ. Maternal influenza and congenital deformities. A follow-up study. Lancet 1963; 1:748-51; PMID:14021958; http://dx.doi.org/10.1016/S0140-6736(63)91567-8
- Takahashi M, Yamada T. A possible role of influenza A virus infection for Parkinson's disease. Adv Neurol 2001; 86:91-104; PMID:11554013
- Ilyushina NA, Ikizler MR, Kawaoka Y, Rudenko LG, Treanor JJ, Subbarao K, Wright PF. Comparative study of influenza virus replication in MDCK cells and in primary cells derived from adenoids and airway epithelium. J Virol 2012; 86:11725-34; PMID:22915797; http://dx.doi.org/10.1128/JVI.01477-12
- Lee CW, Jung K, Jadhao SJ, Suarez DL. Evaluation of chicken-origin (DF-1) and quail-origin (QT-6) fibroblast cell lines for replication of avian influenza viruses. J Virol Methods 2008; 153:22-8; PMID:18638503; http://dx.doi.org/10.1016/j.jviromet.2008.06.019
- Lugovtsev VY, Melnyk D, Weir JP. Heterogeneity of the MDCK cell line and its applicability for influenza virus research. PLoS One 2013; 8:e75014; PMID:24058646; http://dx.doi.org/10.1371/journal.pone.0075014
- Ollier L, Caramella A, Giordanengo V, Lefebvre JC. High permissivity of human HepG2 hepatoma cells for influenza viruses. J Clin Microbiol 2004; 42:5861-5; PMID:15583326; http://dx.doi.org/10.1128/JCM.42.12.5861-5865.2004
- Smith KA, Colvin CJ, Weber PS, Spatz SJ, Coussens PM. High titer growth of human and avian influenza viruses in an immortalized chick embryo cell line without the need for exogenous proteases. Vaccine 2008; 26:3778-82; PMID:18524432; http://dx.doi.org/10.1016/j.vaccine.2008.04.048
- Tobita K, Sugiura A, Enomote C, Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol 1975; 162:9-14; PMID:1214709; http://dx.doi.org/10.1007/BF02123572
- Arnold DM, Neame PB, Meyer RM, Soamboonsrup P, Luinstra KE, O'Hoski P, Garner J, Foley R. Autologous peripheral blood progenitor cells are a potential source of parvovirus B19 infection. Transfusion 2005; 45:394-8; PMID:15752157; http://dx.doi.org/10.1111/j.1537-2995.2005.04267.x
- Blaha M, Mericka P, Zak P, Stepanova V, Vavra L, Maly J, Tousovska K. The risk of infection transmission from blood progenitor cell concentrates. J Hematother Stem Cell Res 2003; 12:161-4; PMID:12804175; http://dx.doi.org/10.1089/152581603321628304
- Jeulin H, Salmon A, Gautheret-Dejean A, Agut H, Bordigoni P, Fortier B, Venard V. Contribution of human herpesvirus 6 (HHV-6) viral load in whole blood and serum to investigate integrated HHV-6 transmission after haematopoietic stem cell transplantation. J Clin Virol 2009; 45:43-6; PMID:19321385; http://dx.doi.org/10.1016/j.jcv.2009.02.006
- Locasciulli A, Alberti A, Bandini G, Polchi P, Arcese W, Alessandrino P, Bosi A, Testa M, Bacigalupo A. Allogeneic bone marrow transplantation from HBsAg+ donors: a multicenter study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Blood 1995; 86:3236-40; PMID:7579420
- Locasciulli A, Testa M, Valsecchi MG, Bacigalupo A, Solinas S, Tomas JF, Ljungman P, Alberti A. The role of hepatitis C and B virus infections as risk factors for severe liver complications following allogeneic BMT: a prospective study by the Infectious Disease Working Party of the European Blood and Marrow Transplantation Group. Transplantation 1999; 68:1486-91; PMID:10589944; http://dx.doi.org/10.1097/00007890-199911270-00010
- Shuhart MC, Myerson D, Childs BH, Fingeroth JD, Perry JJ, Snyder DS, Spurgeon CL, Bevan CA, McDonald GB. Marrow transplantation from hepatitis C virus seropositive donors: transmission rate and clinical course. Blood 1994; 84:3229-35; PMID:7949194
- Crapnell KB, Almeida-Porada G, Khaiboullina S, St Jeor SC, Zanjani ED. Human haematopoietic stem cells that mediate long-term in vivo engraftment are not susceptible to infection by human cytomegalovirus. Br J Haematol 2004; 124:676-84; PMID:14871256; http://dx.doi.org/10.1111/j.1365-2141.2004.04827.x
- Cho PS, Messina DJ, Hirsh EL, Chi N, Goldman SN, Lo DP, Harris IR, Popma SH, Sachs DH, Huang CA. Immunogenicity of umbilical cord tissue derived cells. Blood 2008; 111:430-8; PMID:17909081; http://dx.doi.org/10.1182/blood-2007-03-078774
- Khatri M, Goyal SM, Saif YM. Oct4+ stem/progenitor swine lung epithelial cells are targets for influenza virus replication. J Virol 2012; 86:6427-33; PMID:22491467; http://dx.doi.org/10.1128/JVI.00341-12
- Khatri M, Saif YM. Epithelial cells derived from swine bone marrow express stem cell markers and support influenza virus replication in vitro. PLoS One 2011; 6:e29567; PMID:22216319; http://dx.doi.org/10.1371/journal.pone.0029567
- Zeng H, Goldsmith C, Thawatsupha P, Chittaganpitch M, Waicharoen S, Zaki S, Tumpey TM, Katz JM. Highly pathogenic avian influenza H5N1 viruses elicit an attenuated type i interferon response in polarized human bronchial epithelial cells. J Virol 2007; 81:12439-49; PMID:17855549; http://dx.doi.org/10.1128/JVI.01134-07