Abstract
Outside Asia, Egypt is considered to be an influenza H5N1 epicentre and presents a far greater pandemic risk than other countries. The long-term endemicity of H5N1 and the recent emergence of H9N2 in poultry call attention to the need for unravelling the epidemiology, ecology and highly diverse gene pool of influenza A virus (IAV) in Egypt which is the aim of this review. Isolation of a considerable number of IAV subtypes from several avian and mammalian hosts was described. Co-infections of poultry with H5N1 and H9N2 and subclinical infections of pigs and humans with H1N1 and H5N1 may raise the potential for the reassortment of these viruses. Moreover, the adjustment of IAV genomes, particularly H5N1, to optimize their evolution toward efficient transmission in human is progressing in Egypt. Understanding the present situation of influenza viruses in Egypt will help in the control of the disease and can potentially prevent a possible pandemic.
Abbreviations
| ELISA | = | Enzyme linked immunosorbent assay |
| IAV | = | influenza A viruses |
| HA | = | hemagglutinin |
| HI | = | hemagglutination inhibition test |
| HPAIV | = | highly pathogenic avian influenza viruses |
| LBM | = | live bird markets |
| LPAIV | = | low pathogenic avian influenza viruses |
| M | = | matrix |
| NA | = | neuraminidase |
| NAMRU-3 | = | Naval Medical Research Unit–3 |
| NLQP | = | National Laboratory for Veterinary Quality Control on Poultry Production |
| NS | = | non-structural |
| PA | = | acidic polymerase |
| PB | = | basic polymerase |
| WHO | = | World Health Organization |
Introduction
Influenza A viruses (IAV) are segmented RNA viruses belonging to the family Orthomyxoviridae which infect a wide range of hosts including but not limited to 100 species in 12 orders of birds and several mammals.Citation1,2 The genome of IAV consists of 8 gene segments where each segment represents an independent replication unit encoding one or more proteins. According to the surface glycoproteins, the haemagglutinin (HA) and the neuraminidase (NA), 16 HA and 9 NA of IAV have been isolated from birds,Citation3 while H17N10 and H18N11 have been recently identified in bats.Citation4,5 The segmented nature of IAV allows the swapping of gene segments (reassortment or shift) between different influenza subtypes that infect the same cell. The resultant IAV reassortants differ compared to their parental viruses regarding virulence, adaptation and/or pathogenesis. Emergence of a novel reassortant virus in immune-naïve human populations may result in a pandemic with severe mortality.Citation6 Another feature for IAV is the constant minor changes due to errors induced by the viral RNA-dependent RNA polymerase during replication inside the infected cells. The gradual changes (antigenic drift) in the antigenic sites or in the receptor binding domain enable the virus to escape from the vaccine-induced immune response or to expand the host range, respectively.Citation7
A central dogma of influenza virus is that the wild birds are the reservoir for all IAV subtypes. The transmission of IAV from wild birds to domestic poultry occurs frequently,Citation3,8 whereas the spillover of IAV from domestic birds to humans is less reported.Citation9 In poultry, with the exception of some H5 and H7 viruses, all IAV subtypes are of low virulence. On the other hand, the severity of IAV in humans is not correlated with their virulence to poultry. Some highly pathogenic avian influenza viruses (HPAIV) induced up to 100% mortality in chickens, however it caused mild if any clinical signs in humans.Citation10 Conversely, the recent low pathogenic avian influenza viruses (LPAIV) H7N9 in China and Malaysia showed no clinical signs in poultry but it killed 112 out of 355 (∼32%) confirmed laboratory human cases since February 2013.Citation11 Exceptionally, the evolving HPAIV H5N1 since 1997 caused devastating outbreaks in poultry and wild birds in several countries and it was able to kill 393 out of 667 (∼59%) infected humans.Citation11 Although many countries successfully eradicated the HPAIV H5N1 from poultry, Egypt, China, Vietnam, Bangladesh, Cambodia and Indonesia were declared as H5N1-endemic countries.Citation12
Egypt is considered a hotspot for the evolution of a pandemic potential virus either via antigenic drift of the H5N1 to increase its adaptation to humansCitation13,14 or through reassortment with other IAV subtypes, especially H3N2 virusCitation15 or H9N2.Citation16 Egypt has experienced several waves of IAV since 1900s. Surveillance in the wild birds described the isolation of different IAV subtypes, some of these viruses found their way to infect domestic poultry as well as humans. In domestic poultry, H5N1, H9N2 and H7Nx viruses are circulating since few years. Other IAV subtypes have been reported from human, horses, donkeys, pigs and possibly cats and dogs. Therefore, this review highlights important aspects regarding the history, epidemiology, ecology and the gene pool of influenza viruses in animals and humans in Egypt.
Prevalence of IAV in Domestic Poultry
Highly pathogenic avian influenza
Fowl plague (H7N1)
The earliest quoted date for the beginning of the history of avian influenza in Egypt was as far back as 1912.Citation17 Instances were also mentioned as a part of the global outbreak of fowl plague in 1923.Citation18-20 From 1923, the disease was endemic in Egypt and nearly every poultry establishment was threatenedCitation17,21 and the virus was isolated from chickens, turkeys, waterfowls, peafowl, parrots and pheasants.Citation22 At that time, control of the disease was mainly based on nationwide vaccination with whole-virus inactivated vaccines developed from circulating field strains in 1945 which was effective to protect chickens and turkeys from fowl plague.Citation23 Between 1948 and 1950, the emergence of antigenically drift variants was described.Citation24 In the early 1950s, the development of attenuated living vaccine from peafowl-origin virus after 400 passages in pigeon embryos was successful to prevent clinical signs and/or mortality against the variant strains under experimental conditions.Citation25 By the 1960s, the disease was no longer mentioned and dramatically disappeared. Later the virus was classified as H7N1 (A/fowl/Egypt/45 or A/FPV/Egypt/45) with a typical HPAI multiple basic amino acid sequence at the HA cleavage site KKRRKR*GLF and intravenous pathogenicity index of 3.0.Citation26-28
H5N1
After the re-emergence of H5N1 virus in Southeast Asia in 2003, Egypt started a nationwide surveillance in domestic poultry and wild birds for a period of 3 y (2003–2006). A preparedness stated the intention to control the disease immediately by stamping out the infected poultry at the index farm and those located within a diameter of 3 km. It was further proposed that a buffer zone of 10 km diameter be created in which the movement of live poultry and eggs would be restricted. While no evidence of H5N1 was obtained during the surveillance, a few weeks before the end of the project, on February 10, 2006, the National Laboratory for Veterinary Quality Control on Poultry Production (NLQP) received chickens, ducks, geese and turkeys from 7 backyards and live bird markets (LBMs) with symptoms commonly seen in HPAIV infections in 3 different provinces in the Nile Delta (north of Cairo). Isolation and genotyping of HPAIV H5N1 were confirmed in cooperation between NLQP and the Naval Medical Research Unit–3 (NAMRU3), Cairo, Egypt. The isolation of the virus was officially announced to the public on February 16, 2006. The first introduction of the virus into Egypt was thought to be by legal or illegal trade of live poultry or imported feed components. This scenario was neither confirmed nor excluded, but later on introduction via wild birds Citation29 was reported to be the most likely scenario (see below). Although, depopulation of the first cases was done according to the prepared contingency plan, the virus spread quickly, over a wide area within few weeks. As a result, inactivated vaccines were introduced in a trial to mitigate the severe socioeconomic impact of the disease which became officially endemic from 2008.Citation30 Although the temporal pattern of the disease in the winter months was typical for the outbreaks in 2006–2008 Citation31,32; in 2009–2012 the disease was reported year round particularly in the backyard sector.Citation33 Since then, outbreaks have been reported nationwide and the disease is now concentrated in the Nile Delta region where high-density human and poultry populations exist.Citation33,34
As of the end of 2011, approximately most of the domesticated birds have been infected with the virus including chickens, ducks, geese, turkeys, quails, and ostrich as well as zoo birdsCitation30,35,36 and evidence for the persistence of the virus in native-ducks in the backyards in mid-summer 2011 was reported.Citation37 In the first outbreak in 2006 we could not detect the virus from pigeons,Citation30 however in 2009–2010 the viral RNA was detected in one pigeon and in 2010–2012 in 36 samples.Citation35,36 A recent study described an outbreak in a small backyard pigeons flock in 2011 with mortality up to 50%.Citation38 Whether the virus progressively adapted in pigeons since 2006 in Egypt or it was surveillance bias should be studied. Other potential reservoirs of the virus have not been fully identified. The virus has been reported from egrets and crows which are the most common feral birds seen in close contact with poultry in EgyptCitation30,39 as well as herons from 2 different locations.Citation40 So far the incidences of the virus in other feral birds – particularly sparrows, starling, eagles, owls, black kite and/or Hoopoe (Upupa epops) that access poultry farms, feed stores/mills and litter disposal points and are in close contact with wild migratory birds - have not been investigated. Intriguingly, the viral RNA has been detected in several types of fish in some regions in the Nile DeltaCitation40 and in litter beetles (Alphitobius diaperinus) that were found in a commercial farm previously experienced H5N1 outbreaks (Hussein A.H., personal communication). Nevertheless, the significance of these potential hosts in terms of the epidemiology of H5N1 in Egypt is still to be demonstrated.
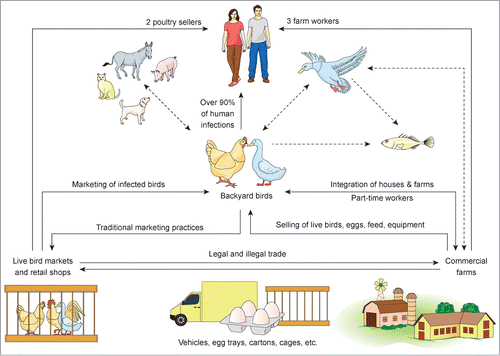
An important feature for the epidemiology of H5N1 in Egypt is the genetic variability of the virus: Two H5N1 viruses collected from birds in 2 neighboring backyards at the same day were genetically different.Citation41 A similar observation was reported by El-Zoghby et al.Citation34 who isolated 2 viruses from 2 adjacent poultry farms but belonging to 2 different genetic lineages whereas 2 viruses from LBM and commercial farms from 2 different provinces (about 800 km apart) clustered together. Another critical aspect in the epidemiology of H5N1 is LBM where the virus has been detected year-round.Citation32,42,43 It is worth mentioning with respect to this that in LBM (1) more than 70% of poultry are marketed alive, (2) there is no system for tracing back the infection and (3) veterinary inspection, cleaning and disinfection and disposal of offal are not adequately done. Also, commercial farms with low to null biosecurity measures are endemically infected with the virus. Birds in these farms have usually acquired the infection as a result of: part-time workers who keep infected backyard birds, contact with feral and wild birds, inappropriate cleaning and disinfection methods; and/or inadequate disposal of dead birds and/or infected litters.Citation12,44
A key feature of the dynamic of H5N1 in poultry in Egypt is the backyard birds that may play a significant role in human and poultry infections as summarized in (for more information the readers are referred toCitation44,45). Backyard birds are being infected mostly through (1) commercial farms through the selling of used equipment (i.e.,: hoppers, drinkers, feeders, etc.), feed, infected birds and eggs or via (2) the introduction of live birds from markets. Actually, the keeping of backyard birds and livestock in the countryside and suburbs is a complex cultural, social and ecological phenomenon which is rooted in Egypt's ancient past.Citation46 Backyard birds have been a primary economic support for many villagers for thousands of years. In recent times, while apparently healthy poultry are usually sold in traditional LBM's, 38% of households slaughter their birds once they become ill.Citation30,47 Since the problems arising out of keeping birds in backyards, the Egyptian authorities banned keeping or trading of live birds and provided vaccination free-of-charge twice a year until 2009.Citation48 H5N1-positive birds which were detected during the routine surveillance were culled but with very low, if any, compensationCitation30,49 and there was no voluntary culling of infected birds. This strategy was not successful to eradicate the disease in backyard birds in 2006–2009. Since then, an alternate approach focusing on improving biosecurity measures through the implementation of sustainable health education and awareness programs in rural communities was suggested, but not acted on.Citation50-52 More studies are required to identify the dynamic of H5N1 infections in poultry in Egypt including post-outbreak investigation in the commercial sector, period of persistence of H5N1 virus in LBM and transmission pathways from and to the backyard sector. The contribution of each sector of the poultry industry in Egypt to maintain and spread the virus merits further investigation.
Figure 1. Sites of interfaces of H5N1 virus of different hosts in Egypt. From the currently available data, backyard birds are an important source for the infection of birds in the commercial sector and LBM as well as for humans. Over 90% of infected humans probably acquired the infection through direct contact with backyard birds, whereas only 2 and 3 human-infections occurred due to contact with birds in the LBM or commercial sector, respectively. The confirmed transmission routes between different sectors and species are shown as straight lines while possible, but not yet confirmed-sources of infection are shown as dashed arrows. The virus was isolated from donkeys, feral and wild birds and the viral RNA was detected in fish. Only anti-H5 antibodies were detected in pigs, cats and dogs but no virus isolation or detection trials were reported.
Genetic analysis of the Egyptian strains and reverse genetic studies indicated several significant aspects due to the long-term evolution of the virus in poultry. During the last 8 years, viruses isolated from vaccinated birds clustered in clade 2.2.1.1 and 2.2.1.1aCitation53 have accumulated over 20 amino acid substitutions in the HA protein alone and several fixed mutations in other viral proteins.Citation33,54-56 A handful of mutations particularly in the HA-immunogenic epitopesCitation57 or glycosylation sites (Abdelwhab et al., submitted) of these viruses were enough for the escape of these antigenic-drift variants from humoral immune response in vitro which may explain the lack or insufficient protection in poultry afforded by some vaccines either in the field or under experimental conditions.32-4,Citation58,59 Presumably, the use of unsuitable H5 vaccine strains in Egypt might play a role in the evolution of immune-evasion viruses: Chinese Re-1/1996/H5N1 and Mexican H5N2 strains were used extensively until 2008, then local 2006 strains were used during 2009–2012, and updated to 2009 strain since 2012 onwards.Citation56,60
Moreover, it is thought that the recent Egyptian strains become less virulent in poultry than the parent 2006-virus.Citation61 A point mutation in the cleavage site resulted in a significant decrease in the virulence of the virus in chickens without affecting the transmission to contact birds.Citation62 Moreover, these antigenic-variant strains accumulated glycosylation sites in the HA protein approximately each year. The predecessor 2006-virus had 6 potential glycosylated sites, whereas the variant strains since 2009 possessed 9 glycosylation sites.Citation55 Recently, we found that this glycosylation pattern although did not significantly modulate the virulence or tissue tropism of the virus but it was necessary to increase and enhance virus replication, chicken-to-chicken transmission, virus excretion from infected birds, receptor binding affinity, neuraminidase activity and heat stability of the virus (Abdelwhab et al., submitted). Together, the virus continues to acquire genetic changes that are necessary to enhance the infectivity and transmission in poultry fostering its endemicity in Egypt.
Low pathogenic avian influenza
H9N2
Since the mid-1990s, H9N2 virus is endemic in poultry in the Middle East region,Citation63,64 however the first isolation reports of H9N2 virus in Egypt appeared from December 2010 to May 2011 and concerned chickens,Citation65,66 quailsCitation55,67 and turkeys (Arafa et al., unpublished data). To the best of our knowledge, no H9N2 infections were reported in waterfowls in Egypt. It is important to mention that the detection of the viral RNA from birds in LBM had been done in 2006, but the virus was not isolated, which may indicate the presence of the virus in poultry in Egypt long time ago but was not detected for instance because of the testing regimes in the laboratories that were focused toward H5N1. Also, serological investigation revealed that the H9N2 virus was wide-spread in commercial farm settings between February 2009 and April 2012.Citation68 About 50% (605/1225) of collected sera from 39 chicken flocks were tested positive for H9 subtype.Citation68 In another study, 165 commercial flocks and only 3 cases of backyards were found positive.Citation16 In 2011–2013, one study identified H9N2 in 24 out 60 broiler, one out of 5 layer and one out of 5 breeder flocksCitation69 and the second study isolated the virus from 22 broiler flocks.Citation70 While the majority of infected chickens showed respiratory distressCitation66,68 and there was a decrease in egg production in breeder and layer flocks,Citation68 infected quails and some broiler flocks were clinically healthy.Citation16,67,70 Importantly, co-infection or prior infection with H5N1 and H9N2 was also reported in many cases in poultry in Egypt.Citation16,36,66 Although predictable, the reassortment between H5N1 and H9N2 has not been yet reported.Citation70 The highest incidence of H9N2 infections was in January 2012 (6.6%, 49/736) and concentrated mainly in the Nile Delta.Citation16 The routes of the introduction of H9N2 into Egypt are not yet clear. The close genetic relatedness of the Egyptian H9N2 viruses to the viruses circulating in the Middle East region and the distribution of infected flocks along the main migration routes of birds in Egypt suggested that the virus was most likely introduced via wild birds.Citation65 Vaccination using inactivated local field strains of H9N2 viruses has been recently implemented. Bivalent or trivalent vaccines with H5, Newcastle disease virus and/or infectious bronchitis virus have been also used firstly in the breeder and layer flocks then in the broilers. Thus, the acceleration of antigenic drift of H9N2 virus in Egypt due to vaccination pressure is expected similar to what has been reported for H5N1 virus.Citation58,59,71
H6 and H7 subtypes
In the early 1990s, LPAIV H7N1 was isolated from turkeysCitation72 and in the early 2000s, serological evidence for H6 among broiler and layer breeders was reported.Citation73 In 2009–2010, antibodies against H7 virus were detected in 14/39 commercial chicken flocks. While the highest prevalence of positive samples was from layer flocks (9/18) followed by breeders (3/12) and broilers (2/9),Citation68 there is no report for virus isolation in commercial or backyard poultry to date.
Prevalence of IAV in Wild Birds
Egypt lies at the crossroads of 2 major spatially-overlapping migration flyways; the Black Sea-Mediterranean flyway and the East African-West Asian flyway linking Africa, Europe and Asia. There are 34 defined migratory wetlands and stopovers where 7 and 15 regions are located along the coastlines of the Mediterranean and Red seas, respectively. The Nile Delta is an important wintering stopover for millions of birds between Eurasia and Africa. Therefore, attention has been paid to the possible role of wild birds in the introduction of different pathogens including IAV.Citation29,34,Citation74-79 While a considerable number of IAV subtypes have been isolated from wild birds in Egypt through several collaborative research projects (); nevertheless infections of domestic poultry and/or humans with any of these viruses remain poorly defined.
Table 1. Infections of birds, animals and humans with different influenza A virus in Egypt from 1910s to 2014
A recent analysis of 15 IAV's representing 8 different HA subtypes isolated from wild birds in Egypt from 2003 to 2007 indicated a close relation to Eurasian, African and/or central Asian lineages.Citation78 Interestingly, it was found that viruses originated from 4 different flyways rather than the 2 flyways in Egypt.Citation78 The most infected wild birds in Egypt were the northern shoveler, common teal and Egyptian goose ().
H5 subtypes
While the isolation of H5N1 from wild birds in Egypt was not reported before or after 2005,Citation77,80,81 however, LPAIV H5N2 was isolated from shoveler in 2003Citation39 and from a common teal (Anas crecca) in October 2005.Citation29 The first and only report of isolation of H5N1 from wild birds was in December 2005Citation29; a few weeks before the epidemic in domestic poultry.Citation31 Out of 1304 sampled migratory birds, one swab from a common teal (Anas crecca) trapped in the Nile Delta was positive for HPAIV H5N1 (A/Teal/Egypt/14051-NAMRU3/2006). This report was published at the end of 2006 due to failure of the virus isolation in the original sample. The teal-virus was genetically close to H5N1 viruses isolated from domestic poultry and humans in Egypt suggesting that the introduction of the virus into domestic poultry was by wild birds.Citation29
H10 subtypes
H10 has been the most prevalent IAV subtype in wild birds in Egypt since 2003. It has been isolated in combination with N1, N4, N7 and N9 NA-subtypes (). Five H10N7 viruses were isolated from wild birds (teal ducks and shoveler) between 18 and 22 April 2004, in a market of hunted migratory birds.Citation82 Other H10N7 viruses were also isolated in 2007.Citation39
H7 subtypes
Since 2003, H7 subtype has been the second most prevalent subtype in wild birds in Egypt (). An H7N7 was isolated from a black kite “Milvus migrans” in migration-season in the 2005 from South Sinai.Citation83 The virus has been also isolated from shoveler, green-wing teal and Egyptian goose in the period 2004–2006.Citation39,84 Moreover, H7N1, H7N3 and H7N9 were identified between 2004 and 2007 ().
Other subtypes
From 1970s to 2007, IAV's of the subtypes H1, H2, H3, H4, H6, H9, H11 and H13 were identified in wild birds in Egypt as summarized in .Citation75,78,Citation84-88
Prevalence of IAV in Animals
Pigs
While pigs are frequently infected by H1 and H3 in combination with N1 or N2 subtypes however, they are susceptible to many other IAV subtypes Citation89 and hence they act as a “mixing vessel” for different influenza viruses.Citation90 Egypt was one of the first countries to domesticate and intensively produce pigs and has been doing so from at least the fourth millennium BC.Citation91 That said, the official number of pigs in Egypt did not exceed 40000 head in 2011.Citation92 In recent years, they have been found concentrated primarily in slums, rural and peri-urban areas with insufficient biosecurity measures or veterinary inspection. Pigs in Egypt are fed organic waste, including dead birds, and are in a regular contact with wild and feral birds.Citation44,93,94 Little is known about the prevalence of IAV in pigs in Egypt that may be related to the low number of pig populations in the country.
H1N1 and H3N2
In 1979–1980, a total number of 480 pig serum samples were tested for anti-influenza antibodies. Of these, about 52.5% were found positive against swine influenza H1N1 and 10.4% showed seroconversion to human H3N2.Citation95 Detection of the 2009 pandemic H1N1 (pH1N1) antibodies in pigs in Egypt was also reportedCitation96 but no further details are available.
H5N1
Generally, pigs have low susceptibility to H5N1 viruses with limited virus excretion from the respiratory tract upon artificial infection, therefore it is stated that pigs do not play a remarkable role in the epidemiology and evolution of H5N1.Citation97,98 In Egypt, 1.6% (4/250) and 4.4% (11/250) serum samples in 2008 were found positive when tested against H5N2 and local H5N1 antigens, respectively.Citation93 More recently, 4.3% (4/93) of tested pigs sera were found positive to H5N1.Citation99 The two independent studies showed higher rates of H5N1 infection of pigs in Egypt than those recorded in China and VietnamCitation100,101 that possess a very high number of pigs in comparison to Egypt. These findings raised an alarm on the potential role that might be played by pigs in the epidemiology and evolution of H5N1 virus in Egypt. The panic was aggravated after the emergence of pH1N1 in 2009 which harboured reassortant genes from IAV of human, avian and swine origins. To reduce the risk of reassortment between pH1N1 and H5N1, the Egyptian authority slaughtered over 300000 pigs and planned to transfer pig “farms” to locations outside densely populated areasCitation96; an action, which in retrospect, was considered a misguided attempt to control the disease.Citation102
Equine
Equine influenza is caused by 2 distinct IAV subtypes: H7N7 and H3N8. Both subtypes cause an acute respiratory tract infection of horses, donkeys and mules. Equine H7N7 subtype has not been isolated since 1978Citation103 but H3N8 virus is still evolving. It crossed the species barrier to dogsCitation104-108 and probably to humans as well.Citation109-111 Swapping of gene segments between equine H3N8, avian, swine and humans IAV has been reported.Citation111-113
Egypt is an important center for raising and marketing of the pure breed of Arabian horses.Citation114 The number of equine in 2011 exceeded 3.4 million head; 64000 horses, 3.4 million asses and 1160 mules.Citation92 Therefore, the contribution of equine influenza in the expansion of the gene pool of IAV in Egypt should not be neglected.
H3N8
So far, there have been 3 outbreaks of equine influenza H3N8 in horses, mules and donkeys in Egypt. The first outbreak was in October 1989 in Monofiya governorate in the Nile Delta.Citation115,116 This outbreak was thought to be caused by equine H7N7 virusCitation115 or H7N7 mixed with H3N8.Citation114 The second outbreak in 2000 was reported from the Nile DeltaCitation117 as well as from Upper Egypt.Citation118 The most recent and most severe outbreak, this time involving H3N8 was in 2008.Citation119 The infections spread to several provinces within short time.Citation114,120,121 The virus had 98% genetic identity with H3N8 viruses from USA and Japan.Citation114 Trials to develop inactivated vaccines for this virus were described.Citation122,123 It is notable that there are gaps in the epidemiology of equine influenza in Egypt particularly the source of infection and post-outbreak surveillance, although severe economic losses occurred due to suspension of horseracing or death of animals.Citation114,120
H5N1
In March 2009, H5N1 was isolated for the first time from donkeys suffering from respiratory distress and evidence of exposure to H5N1 was recorded in 27 out of 105 (25.71%) serologically tested donkeys. The epidemic occurred one week after infection of household poultry at the same village.Citation124 In another independent study, antibodies against H5N1 were detected in 23.8% (38/160) and 30.6% (11/36) of the tested horses and donkeys, respectively.Citation99 The equine isolate possessed viral genes that were closely related to other Egyptian avian HPAIV H5N1 indicating a common origin of these viruses. It had Q226 and G228 in the HA protein which are typical for avian and equine viruses but not for human ones.Citation125,126 It also possessed a deletion at the receptor binding domain (serine at position 145) which enhanced the affinity of the Egyptian H5N1 viruses to human-type receptorsCitation14 and lysine at position 627 of PB2 which is a marker for efficient replication of IAV in human.Citation127 Uniquely, it had 5 amino acids in PB2 (529M and 530A), PA(472S), NS2(66L) and M2(29T). Still needing an answer is the question of whether such peculiar amino acids play a role in the virulence and/or transmission of H5N1 virus to equine or not.Citation128
Other animals
H5N1
In 2013, few H5N1 positive samples from other animals in Egypt using hemagglutination inhibition test (HI) and enzyme linked immunosorbent assay (ELISA) were obtained.Citation99 Of those samples, about 28% (7/25) of cats and 12% (3/25) of dogs were found to have anti-H5 antibodies. Meanwhile no evidence for virus infections in cattle, buffaloes, sheep and goats was reported until now.Citation30,99 It is worth pointing out that the application of traditional serological tests (i.e.,: HI and ELISA) in different animal species particularly pigs, donkeys, cats and dogs has not be extensively validated for application in these species. Therefore, it is important to conduct surveillance based on virus isolation to avoid misinterpretation or overestimation of the biological role, if any, of these animals in the spread of the virus in Egypt. Moreover, under laboratory conditions ferrets survived the infection after eating of heavily-infected chicken meats with an Egyptian H5N1 virus showing mild meningoencephalitis without neurological signs or viral antigen detection in the brain. It was stated that the Egyptian virus was less lethal than the Asian H5N1 virus.Citation129 In mice, a virus isolated in 2006 was less virulent in comparison to generated mutants resembling the human-origin virus from subclade 2.2.1/C since 2009.Citation14 The latter was able to infect mice at lower titers inducing moderate to severe bronchiolar necrosis and pulmonary edema and inflammatory cell infiltrates indicating progressive adaptation on mammals.Citation14
IAV in Humans
Seasonal infection of humans occurs often by H1N1 and H3N2.Citation130 In the case of pandemics fatalities are estimated to be several millions of people. In the last century, 4 pandemics were documented: H1N1 in 1918, H2N2 in 1957, H3N2 in 1968 and H1N1 in 1977.Citation131 In 2009, pH1N1 emerged in Mexico then the USA and then worldwideCitation132 causing about 148000–249000 deaths.Citation133 So far, there is scanty information on the history of influenza in humans in Egypt and most of the available literature briefly described some epidemiological and/or immunological aspects.Citation134
Human IAV
Although it is difficult to confirm, the first reported epidemic of human influenza in Egypt took place sometime between 1650 BC – 1550 BC and resulted in thousands of deaths.Citation46 In the 19th century (1889–1892), 2 influenza epidemics were recorded in Egypt where fever, respiratory distress and renal disorders were described among infected patients.Citation135 There are no available data on the situation of IAV pandemics in Egypt during this period.Citation134 In the 1918–1919 pandemic, infections ranged from mild illness to 50% fatality in the British expeditionary forces stationed in the country.Citation136 More information was published on the seasonal influenza in human in some regions in Egypt in 1952–1953,Citation137 1963–1965,Citation138 1966–1972,Citation139 1973–1974.Citation140,141
In 1983, a novel H1N2 resulted from the reassortment between the contemporary circulating H3N2 and H1N1 in humans in China without further spread to other regions.Citation142 In 2001–2002, this H1N2 subtype was reported in over 41 countries in 4 continents including Egypt.Citation143 Infected persons in Egypt were from 5 to 15 year-old with classic upper respiratory tract infections.Citation144 Further, H3N2 was reported in Egypt in 2002–2007Citation145-147 but no specific details are available. The 2009 pH1N1 was first detected in Egypt in June and became the predominant influenza virus in human in mid-November of the same year.Citation148 To December 2010, less than 17,000 infected cases, among them 281 confirmed related fatalities, were officially reported.Citation149 From June 2009 to the end of 2011, the case fatality rate among 13782 children under 10 y was 0.5% (71 deaths) most of them were in 2009 (46 deaths) then 2010 (20 deaths) and 2011 (5 deaths).Citation150 In another study, 47.5% of 2–60 months old children (n = 1200) were positive in 2010–2011 in Egypt.Citation151 In addition to the flu-like illness induced by the virus,Citation152 ocular manifestations were also reported.Citation153
Zoonotic transmission of IAV in Egypt
Hitherto, 3 IAV sub-types have successfully jumped from animals to humans in Egypt; H1N1, H5N1 and H10N7 ().
H1N1
The only available report on transmission of IAV from animals other than birds in Egypt was from pigs in 1979–1980, since 20 out of 200 human sera (10%) reacted positive against the swine H1N1 virus.Citation95
H5N1
Egypt, after Indonesia, is the country worst affected by H5N1 and the country with the highest reported human infections since 2009. The first reported human infection was in March 2006, a few weeks after the suspected date of the infection of domestic poultry. As of August 02, 2014, the fatality rate of H5N1 in Egypt was 36% (63/173) compared to 59% (386/650) global fatality rate.Citation154 The low case-fatality rate in Egypt was probably due to (1) rapid identification of a large number of infected children who were successfully treated and recovered (2) infection of humans with low-virulent virus clade (3) pre-infection with H1N1 virus induced non-specific cross immunity to H5N1 (4) some deaths from H5N1 might not have been reported or (5) probably due to genetic traits of the population. To March 2009, only 1% (36/6355) of suspected cases of H5N1 in Egypt was positive for H5N1 virus and 3 family clusters were described.Citation155 In another surveillance study, 14% (42/299) of tested individuals were subclinically infected with H5N1 where the number of infected females was higher than males. Unexpectedly, 12% of Cairo residents investigated in that surveillance were positive while 8.4% of the farmers in the country side were found to be infected.Citation99 These findings might be explained by inadequate surveillance procedures.
To date, there have been 173 human infections reported to the WHO; over 90% were linked to close contact with backyard birds.Citation156 On the other hand, only 3 workers in the commercial sector were infected and they were reported to have recovered.Citation30,155 In addition, 2 poultry sellers acquired the infection from LBM and the source of infection in 2 other cases remained unknown.Citation30,155 Two important features in the epidemiology H5N1 infection in humans in Egypt have been found to be the sex and age of infected people. The prevalence of infection was remarkably high in women in the age group 20–39 y and children less than 10 y.Citation51,155,Citation157-160 Keeping, slaughtering, defeathering and evisceration of birds are the common sources of infection in women whereas close contact, cleaning of infected premises and playing with infected birds (or poultry wastes) are considered the main reasons for the disease in toddlers and children.Citation45,51,161
The most common symptoms in 81 confirmed H5N1 human cases in Egypt from March 2006 to June 2009 were fever, acute respiratory distress, shock, muscle and joint pain and to a lesser extent, sore throat and renal failure.Citation161 Nevertheless, there is an expectation that subclinical (or mild) infection of H5N1 is widespread in humans in Egypt and that the number of infected persons are actually higher than the detected prevalence.Citation162,163 Like the seasonal prevalence of H5N1 outbreaks in poultry, the increased infection rate in humans is associated with winter months where high humidity and low temperature probably favor the droplet transmission of the virus.Citation61,164,165 The infection is concentrated mainly in the Nile Delta, the influenza epicentre in Egypt.Citation15,155,166
Fortunately, the vast majority of the Egyptian strains are sensitive to the NA inhibitor oseltamivirCitation55,61,166,167 which may also explain the lower case-fatality rate of H5N1 in human in Egypt. Nevertheless, the continuous evolution of H5N1 in Egypt makes it a potential candidate for being a possible pandemic virus. The H5N1 virus of human-origin (2.2.1/C subclade) is dominant in backyard birds and has been also isolated in small-scale commercial poultry.Citation33,43,55 Interestingly, there is an assumption that selection pressure from vaccination of poultry in the commercial sector has created escape mutants that were unable to infect humans.Citation60 Two reverse genetic studies showed that the Egyptian viruses acquired 2 mutations in the HA protein (seen only in 2.2.1/C) which enhanced binding affinity to human-type receptors but retained its specificity to the avian-type receptors as well.Citation14,168 Also, these mutations increased the attachment of the virus to the epithelial cells of the lower respiratory tract of human.Citation14 Moreover, it is now known that only 5 mutations are required for the airborne transmission of H5N1 viruses in mammals.Citation169,170 Some Egyptian viruses encode 3 of these mutations: HA-220K, HA-N154D and PB2–627K which is a real matter of concern.Citation13 Additionally, the H9N2 virus in Egypt, although the prevalence in human is unknown, contains mutations that may enhance the affinity to human-type receptors as well as replication and virulence in mammals.Citation65,70 Long-term circulation of both pandemic-potential viruses with progressive adjustment to optimize their evolution toward efficient transmission in human is very critical in Egypt. Therefore, Egypt has been considered a far greater risk than H5N1 viruses circulating in other countries for the emergence of pandemic.Citation13
H10N7
In 2004, H10N7 was isolated from 2 one-year old infants with fever and cough. Epidemiological investigations indicated that a father of one of them was a poultry vendor. The origin of the human infection was not elucidated. No further public-health implications were describedCitation82 and the situation in domestic poultry is unknown.
Concluding Remarks and Outlook
This review has thrown the light on the history, prevalence and diversity of IAV in animals and humans in Egypt where many puzzles must be solved to reduce the potential of the emergence of pandemic influenza virus. Egypt is on 2 of the main flyways of migratory birds linking Eurasian and Africa, therefore influenza viruses in wild birds in Egypt merit more in-depth investigation. Wild birds were claimed to be the source for the infection of domestic poultry with HPAIV H5N1 and humans with H10N7 virus. In addition, the majority of IAV HA subtypes were identified from wild birds in Egypt. The endemic HPAIV H5N1 became established in birds and jumped from poultry to humans and some animals. Thus, surveillance to identify the potential reservoir hosts of HPAIV H5N1 in Egypt is important to gain a better understanding of the virus epidemiology and to develop effective control strategies. Target and active surveillance in poultry in the commercial sector should be rigoursly conducted because many factors foster the undetected spread of the virus such as (1) vaccination of commercial poultry in Egypt, in addition to masking the clinical disease, decreased the passive outbreaks reporting,Citation171 (2) the virus acquired mutations to reduce virulence but enhance transmission in poultry and (3) misdiagnosis due to mutations in the PCR primer sitesCitation172,173 may influence the overall incidence of the disease in different poultry sectors. Innovative approaches to eradicate the infection in backyard birds particularly in the Nile Delta should be developed and must consider the cultural, social and economic aspects of the household poultry. Egypt has many “Trojan horses” in the epidemiology of IAV including asymptomatic vaccinated birds, subclinically infected ducks, quails, equine, pigs and even humans. There are several scenarios for the potential reassortment of IAV in Egypt from animals and human sources () which must be extensively studied to predict the gene constellation of viruses with public and animal health implications. There are substantial gaps in the epidemiology, genetic information and pathobiology of IAV in human including the H5N1 virus. Nationwide surveillance to map the distribution of subclinical and mixed infections of IAV is highly desired. Monitoring of IAV in humans, birds, and different animal species should be conducted regularly. Continuous evolution, jumping from host to host and diverse gene pool of IAV in Egypt (resembling the situation in China) warrant more global attention to control the disease in animals before it becomes a serious worldwide public health problem.
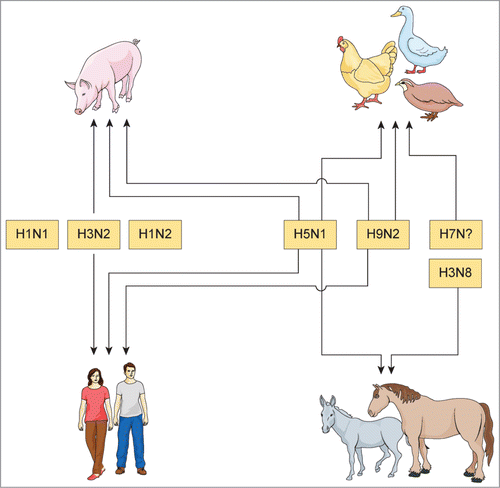
Figure 2. Scenarios for the potential reassortment of influenza A virus in animals and humans in Egypt. There are many hosts to be considered as “mixing vessels” for the potential reassortment of IAV in Egypt. The arrows refer to the possible co-infection of susceptible host(s) with different IAV subtypes in Egypt. Waterfowls, vaccinated chickens and quails are frequently infected without showing clinical signs where H5N1, H9N2 and/or H7Nx viruses may reassort. Infected donkeys and horses with H5N1 and equine H3N8 or pigs co-infected with human IAV subtypes, H5N1 and/or H9N2 viruses are possible sources for reassortment of IAV in Egypt. Reassortants can also emerge after infection with human (H1N1, H3N2 or H1N2) and avian (H5N1 and/or H9N2) IAV's subtypes in humans.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
The authors are grateful to Dr. Simon Combe, Graduate School of Language and Culture, Osaka University for language editing of the manuscript.
References
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev 1992; 56:152-79; PMID:1579108
- Yee KS, Carpenter TE, Cardona CJ. Epidemiology of H5N1 avian influenza. Comp Immunol Microbiol Infect Dis 2009; 32:325-40; PMID:18448168; http://dx.doi.org/10.1016/j.cimid.2008.01.005
- Alexander DJ. An overview of the epidemiology of avian influenza. Vaccine 2007; 25:5637-44; PMID:17126960; http://dx.doi.org/10.1016/j.vaccine.2006.10.051
- Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, et al. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A 2012; 109:4269-74; PMID:22371588; http://dx.doi.org/10.1073/pnas.1116200109
- Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, et al. New world bats harbor diverse influenza a viruses. PLoS Pathog 2013; 9:e1003657; PMID:24130481; http://dx.doi.org/10.1371/journal.ppat.1003657
- Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis 2006; 12:15-22; PMID:16494711; http://dx.doi.org/10.3201/eid1209.05-0979
- Bouvier NM, Palese P. The biology of influenza viruses. Vaccine 2008; 26:D49-53; PMID:19230160; http://dx.doi.org/10.1016/j.vaccine.2008.07.039
- Krauss S, Obert CA, Franks J, Walker D, Jones K, Seiler P, Niles L, Pryor SP, Obenauer JC, Naeve CW, et al. Influenza in migratory birds and evidence of limited intercontinental virus exchange. PLoS Pathog 2007; 3:e167; PMID:17997603; http://dx.doi.org/10.1371/journal.ppat.0030167
- Kalthoff D, Globig A, Beer M. (Highly pathogenic) avian influenza as a zoonotic agent. Vet Microbiol 2010; 140:237-45; PMID:19782482; http://dx.doi.org/10.1016/j.vetmic.2009.08.022
- Reperant LA, Kuiken T, Osterhaus AD. Influenza viruses: from birds to humans. Hum Vaccines Immunoth 2012; 8:7-16; PMID:22251997; http://dx.doi.org/10.4161/hv.8.1.18672
- WHO. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003-2014. Available online at: http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/ ( accessed 03 Oct, 2014). 2014.
- FAO. The Global Strategy for Prevention and Control of H5N1 Highly Pathogenic Avian Influenza. Rome, Italy, 2008; Available from: ftp://ftp.fao.org/docrep/fao/011/aj134e/aj134e00.pdf
- Neumann G, Macken CA, Karasin AI, Fouchier RA, Kawaoka Y. Egyptian H5N1 influenza viruses-cause for concern? PLoS Pathog 2012; 8:e1002932; PMID:23166487; http://dx.doi.org/10.1371/journal.ppat.1002932
- Watanabe Y, Ibrahim MS, Ellakany HF, Kawashita N, Mizuike R, Hiramatsu H, Sriwilaijaroen N, Takagi T, Suzuki Y, Ikuta K. Acquisition of human-type receptor binding specificity by new H5N1 influenza virus sublineages during their emergence in birds in Egypt. PLoS Pathog 2011; 7:e1002068; PMID:21637809; http://dx.doi.org/10.1371/journal.ppat.1002068
- Fuller TL, Gilbert M, Martin V, Cappelle J, Hosseini P, Njabo KY, Abdel Aziz S, Xiao X, Daszak P, Smith TB. Predicting hotspots for influenza virus reassortment. Emerg Infect Dis 2013; 19:581-8; PMID:23628436; http://dx.doi.org/10.3201/eid1904.120903
- Arafa AS, Hagag NM, Yehia N, Zanaty AM, Naguib MM, Nasef SA. Effect of cocirculation of highly pathogenic avian influenza H5N1 subtype with low pathogenic H9N2 subtype on the spread of infections. Avian Dis 2012; 56:849-57; PMID:23402103; http://dx.doi.org/10.1637/10152-040812-Reg.1
- Salem DF. Fowl plague in Egypt. Worlds Poult Sci J 1946; 2:69-70; http://dx.doi.org/10.1079/WPS19460024
- Lagrange E. Une nouvelle maladie des poules à virus filtrable observée en Egypte. Bull Soc Pathol Exot 1929; 22:64-8.
- Lagrange E. Études sur la Peste Aviaire d´Egypte. Annales de l´Institut Pasteur 1932; 32:208-67.
- Rashad AM. Fowl plague in Egypt. Tech Sci Serv Vet Bull Cairo Egypt 1934:140.
- El-Nassary BB, Eskarous JK. The distribution of fowl plague and Newcastle disease in upper Egypt. Arch Mikrobiol 1960; 36:147-50; PMID:14408436; http://dx.doi.org/10.1007/BF00412282
- Mickail IG. Immunization trials with a pigeon embryo adapted pea- fowl strain of fowl plague virus. Tech Scient Serv Vet Lab Bull 1969; 5:33.
- Daubney R, Mansi W, Zahran G. Vaccination against fowl plague. J Comp Path Ther 1949; 59:1-18; http://dx.doi.org/10.1016/S0368-1742(49)80001-4
- Daubney R, Mickail IG. Mutations in pathogenicity of some recently isolated strains of fowl-plague virus. J Comp Pathol 1953; 63:255-82; PMID:13109032; http://dx.doi.org/10.1016/S0368-1742(53)80028-7
- Mickail GI. A new living vaccine against fowl plague disease. Nature 1962; 195:1231-2; PMID:14473516; http://dx.doi.org/10.1038/1951231b0
- Wood GW, McCauley JW, Bashiruddin JB, Alexander DJ. Deduced amino acid sequences at the haemagglutinin cleavage site of avian influenza A viruses of H5 and H7 subtypes. Arch Virol 1993; 130:209-17; PMID:8503786; http://dx.doi.org/10.1007/BF01319010
- Banks J, Speidel EC, McCauley JW, Alexander DJ. Phylogenetic analysis of H7 haemagglutinin subtype influenza A viruses. Arch Virol 2000; 145:1047-58; PMID:10881690; http://dx.doi.org/10.1007/s007050050695
- Baigent SJ, McCauley JW. Glycosylation of haemagglutinin and stalk-length of neuraminidase combine to regulate the growth of avian influenza viruses in tissue culture. Virus Res 2001; 79:177-85; PMID:11551658; http://dx.doi.org/10.1016/S0168-1702(01)00272-6
- Saad MD, Ahmed LS, Gamal-Eldein MA, Fouda MK, Khalil F, Yingst SL, Parker MA, Montevillel MR. Possible avian influenza (H5N1) from migratory bird, Egypt. Emerg Infect Dis 2007; 13:1120-1; PMID:18214200; http://dx.doi.org/10.3201/eid1307.061222
- Abdelwhab EM, Hafez HM. An overview of the epidemic of highly pathogenic H5N1 avian influenza virus in Egypt: epidemiology and control challenges. Epidemiol Infect 2011; 139:647-57; PMID:21281550; http://dx.doi.org/10.1017/S0950268810003122
- Aly MM, Arafa A, Hassan MK. Epidemiological findings of outbreaks of disease caused by highly pathogenic H5N1 avian influenza virus in poultry in Egypt during 2006. Avian Dis 2008; 52:269-77; PMID:18646456; http://dx.doi.org/10.1637/8166-103007-Reg.1
- Hafez MH, Arafa A, Abdelwhab EM, Selim A, Khoulosy SG, Hassan MK, Aly MM. Avian influenza H5N1 virus infections in vaccinated commercial and backyard poultry in Egypt. Poult Sci 2010; 89:1609-13; PMID:20634514; http://dx.doi.org/10.3382/ps.2010-00708
- Arafa A, Suarez D, Kholosy SG, Hassan MK, Nasef S, Selim A, Dauphin G, Kim M, Yilma J, Swayne D, et al. Evolution of highly pathogenic avian influenza H5N1 viruses in Egypt indicating progressive adaptation. Arch Virol 2012; 157:1931-47; PMID:22760662; http://dx.doi.org/10.1007/s00705-012-1385-9
- El-Zoghby EF, Aly MM, Nasef SA, Hassan MK, Arafa AS, Selim AA, Kholousy SG, Kilany WH, Safwat M, Abdelwhab EM, et al. Surveillance on A/H5N1 virus in domestic poultry and wild birds in Egypt. Virol J 2013; 10:203; PMID:23799999; http://dx.doi.org/10.1186/1743-422X-10-203
- Kayali G, El-Shesheny R, Kutkat MA, Kandeil AM, Mostafa A, Ducatez MF, McKenzie PP, Govorkova EA, Nasraa MH, Webster RG, et al. Continuing threat of influenza (H5N1) virus circulation in Egypt. Emerg Infect Dis 2011; 17:2306-8; PMID:22172626; http://dx.doi.org/10.3201/eid1712.110683
- Kayali G, Kandeil A, El-Shesheny R, Kayed AS, Gomaa MM, Maatouq AM, Shehata MM, Moatasim Y, Bagato O, Cai Z, et al. Active surveillance for avian influenza virus, Egypt, 2010–2012. Emerg Infect Dis 2014; 20:542-51; PMID:24655395; http://dx.doi.org/10.3201/eid2004.131295
- Hassan MK, Jobre Y, Arafa A, Abdelwhab EM, Kilany WH, Khoulosy SG, Bakry NR, Baile E, Ali A, Ankers P, et al. Detection of A/H5N1 virus from asymptomatic native ducks in mid-summer in Egypt. Arch Virol 2013; 158:1361-5; PMID:23381391; http://dx.doi.org/10.1007/s00705-012-1599-x
- Mansour SM, ElBakrey RM, Ali H, Knudsen DE, Eid AA. Natural infection with highly pathogenic avian influenza virus H5N1 in domestic pigeons (Columba livia) in Egypt. Avian Pathol 2014:1-6.
- Soliman A, Saad M, Elassal E, Amir E, Plathonoff C, Bahgat V, El-Badry M, Ahmed LS, Fouda M, Gamaleldin M, et al. Surveillance of avian influenza viruses in migratory birds in Egypt, 2003-09. J Wildl Dis 2012; 48:669-75; PMID:22740532; http://dx.doi.org/10.7589/0090-3558-48.3.669
- Eissa AE, Hussein HA, Zaki MM. Detection of avian influenza (H5N1) In some fish and shellfish from different aquatic habitats across some Egyptian provinces. Life Sci J 2012; 9:2702-12.
- Abdel-Moneim AS, Shany SA, Fereidouni SR, Eid BT, el-Kady MF, Starick E, Harder T, Keil GM. Sequence diversity of the haemagglutinin open reading frame of recent highly pathogenic avian influenza H5N1 isolates from Egypt. Arch Virol 2009; 154:1559-62; PMID:19669616; http://dx.doi.org/10.1007/s00705-009-0461-2
- Abdelwhab EM, Selim AA, Arafa A, Galal S, Kilany WH, Hassan MK, Aly MM, Hafez MH. Circulation of avian influenza H5N1 in live bird markets in Egypt. Avian Dis 2010; 54:911-4; PMID:20608538; http://dx.doi.org/10.1637/9099-100809-RESNOTE.1
- El-Zoghby EF, Arafa AS, Kilany WH, Aly MM, Abdelwhab EM, Hafez HM. Isolation of avian influenza H5N1 virus from vaccinated commercial layer flock in Egypt. Virol J 2012; 9:294; PMID:23185975; http://dx.doi.org/10.1186/1743-422X-9-294
- FAO. Mapping Influenza A (H5N1) Virus Transmission Pathways and Critical Control Points in Egypt. Animal Production and Health Working Paper No 11. Rome, Italy: Food and Agriculture Organisation, 2013.
- Lohiniva AL, Dueger E, Talaat M, Refaey S, Zaki A, Chisholm Horton K, Kandeel A. Poultry rearing and slaughtering practices in rural Egypt: an exploration of risk factors for H5N1 virus human transmission. Influenza Other Respir Viruses 2013; 7:1251-9; PMID:23145955; http://dx.doi.org/10.1111/irv.12023
- Noble A. Plagues and epidemics. In: Noble B, ed. Encyclopedia of Ancient Egypt: Maps, Timeline, Information about the Dynasties, Pharaohs, Laws, Culture, Government, Military and More: MobileReference, 2008:1041.
- El Zanaty F, El Ghazaly N. Avian Influenza survey: Knowledge, attitudes and practices of the Egyptian public. Available online at: http://www.comminit.com/content/avian-influenza-survey-knowledge-attitudes-and-practices-egyptian-public ( Accessed 3 Oct, 2014). Egypt: UNICEF, 2007.
- El Masry I, Rijks J, Peyre M, Taylor N, Lubroth J, Jobre Y. Modelling influenza A H5N1 vaccination strategy scenarios in the household poultry sector in Egypt. Trop Anim Health Prod 2014; 46:57-63; PMID:23868547; http://dx.doi.org/10.1007/s11250-013-0446-8
- Albrechtsen L, Saade M, Riviere A, Rushton J. Pro-active engagement in compensation and rehabilitation policy formulation and implementation: the case of HPAI in Egypt. Worlds Poult Sci J 2009; 65:225-9; http://dx.doi.org/10.1017/S0043933909000178
- Ismail NA, Ahmed HA. Knowledge, attitudes and practices related to avian influenza among a rural community in Egypt. J Egypt Public Health Assoc 2010; 85:73-96; PMID:21073849
- Aly FA, Oueda MA, Helal HA, Madian AM. Study of avian flu preventive practices among home poultry breeders in rural Egypt. World Appl Sci J 2012; 17:1009.
- Fasina FO, Ali AM, Yilma JM, Thieme O, Ankers P. The cost-benefit of biosecurity measures on infectious diseases in the Egyptian household poultry. Prev Vet Med 2012; 103:178-91; PMID:21982688; http://dx.doi.org/10.1016/j.prevetmed.2011.09.016
- World Health Organization/World Organisation for Animal HF, Agriculture Organization HNEWG. Revised and updated nomenclature for highly pathogenic avian influenza A (H5N1) viruses. Influenza Other Respir Viruses 2014; 8:384-8; PMID:24483237; http://dx.doi.org/10.1111/irv.12230
- Abdel-Moneim AS, Afifi MA, El-Kady MF. Genetic drift evolution under vaccination pressure among H5N1 Egyptian isolates. Virol J 2011; 8:283; PMID:21651796; http://dx.doi.org/10.1186/1743-422X-8-283
- Abdelwhab EM, Arafa AS, Stech J, Grund C, Stech O, Graeber-Gerberding M, Beer M, Hassan MK, Aly MM, Harder TC, et al. Diversifying evolution of highly pathogenic H5N1 avian influenza virus in Egypt from 2006 to 2011. Virus Genes 2012; 45:14-23; PMID:22669540; http://dx.doi.org/10.1007/s11262-012-0758-1
- Cattoli G, Fusaro A, Monne I, Coven F, Joannis T, El-Hamid HS, Hussein AA, Cornelius C, Amarin NM, Mancin M, et al. Evidence for differing evolutionary dynamics of A/H5N1 viruses among countries applying or not applying avian influenza vaccination in poultry. Vaccine 2011; 29:9368-75; PMID:22001877; http://dx.doi.org/10.1016/j.vaccine.2011.09.127
- Cattoli G, Milani A, Temperton N, Zecchin B, Buratin A, Molesti E, Aly MM, Arafa A, Capua I. Antigenic drift in H5N1 avian influenza virus in poultry is driven by mutations in major antigenic sites of the hemagglutinin molecule analogous to those for human influenza virus. J Virol 2011; 85:8718-24; PMID:21734057; http://dx.doi.org/10.1128/JVI.02403-10
- Grund C, Abdelwhab EM, Arafa AS, Ziller M, Hassan MK, Aly MM, Hafez HM, Harder TC, Beer M. Highly pathogenic avian influenza virus H5N1 from Egypt escapes vaccine-induced immunity but confers clinical protection against a heterologous clade 2.2.1 Egyptian isolate. Vaccine 2011; 29:5567-73; PMID:21244859; http://dx.doi.org/10.1016/j.vaccine.2011.01.006
- Abdelwhab EM, Grund C, Aly MM, Beer M, Harder TC, Hafez HM. Multiple dose vaccination with heterologous H5N2 vaccine: immune response and protection against variant clade 2.2.1 highly pathogenic avian influenza H5N1 in broiler breeder chickens. Vaccine 2011; 29:6219-25; PMID:21745517; http://dx.doi.org/10.1016/j.vaccine.2011.06.090
- Wagh K, Bhatia A, Greenbaum BD, Bhanot G. Bird to Hhman transmission biases and vaccine escape mutants in H5N1 infections. PLoS One 2014; 9:e100754; PMID:24988306; http://dx.doi.org/10.1371/journal.pone.0100754
- Kayali G, Webby RJ, Ducatez MF, El Shesheny RA, Kandeil AM, Govorkova EA, Mostafa A, Ali MA. The epidemiological and molecular aspects of influenza H5N1 viruses at the human-animal interface in Egypt. PLoS One 2011; 6:e17730; PMID:21445292; http://dx.doi.org/10.1371/journal.pone.0017730
- Yoon SW, Kayali G, Ali MA, Webster RG, Webby RJ, Ducatez MF. A single amino acid at the hemagglutinin cleavage site contributes to the pathogenicity but not the transmission of Egyptian highly pathogenic H5N1 influenza virus in chickens. J Virol 2013; 87:4786-8; PMID:23408622; http://dx.doi.org/10.1128/JVI.03551-12
- Alexander DJ. Report on avian influenza in the Eastern Hemisphere during 1997-2002. Avian Dis 2003; 47:792-7; PMID:14575066; http://dx.doi.org/10.1637/0005-2086-47.s3.792
- Brown IH, Banks J, Manvell RJ, Essen SC, Shell W, Slomka M, Londt B, Alexander DJ. Recent epidemiology and ecology of influenza A viruses in avian species in Europe and the Middle East. Dev Biol 2006; 124:45-50.
- Abdel-Moneim AS, Afifi MA, El-Kady MF. Isolation and mutation trend analysis of influenza A virus subtype H9N2 in Egypt. Virol J 2012; 9:173; PMID:22925485; http://dx.doi.org/10.1186/1743-422X-9-173
- Monne I, Hussein HA, Fusaro A, Valastro V, Hamoud MM, Khalefa RA, Dardir SN, Radwan MI, Capua I, Cattoli G. H9N2 influenza A virus circulates in H5N1 endemically infected poultry population in Egypt. Influenza Other Respir Viruses 2013; 7:240-3; PMID:22747716; http://dx.doi.org/10.1111/j.1750-2659.2012.00399.x
- El-Zoghby EF, Arafa AS, Hassan MK, Aly MM, Selim A, Kilany WH, Selim U, Nasef S, Aggor MG, Abdelwhab EM, et al. Isolation of H9N2 avian influenza virus from bobwhite quail (Colinus virginianus) in Egypt. Arch Virol 2012; 157:1167-72; PMID:22426861; http://dx.doi.org/10.1007/s00705-012-1269-z
- Afifi MA, El-Kady MF, Zoelfakar SA, Abdel-Moneim AS. Serological surveillance reveals widespread influenza A H7 and H9 subtypes among chicken flocks in Egypt. Trop Anim Health Prod 2013; 45:687-90; PMID:22941731; http://dx.doi.org/10.1007/s11250-012-0243-9
- Awad AA, Arafa A, Hagag SY. Incidence of avian influenza among commercial and native breeds in west Delta region. Alex J Vet Sci 2013; 39:31-9.
- Kandeil A, El-Shesheny R, Maatouq AM, Moatasim Y, Shehata MM, Bagato O, Rubrum A, Shanmuganatham K, Webby RJ, Ali MA, et al. Genetic and antigenic evolution of H9N2 avian influenza viruses circulating in Egypt between 2011 and 2013. Arch Virol 2014; 159:2861-76; PMID:24990416; http://dx.doi.org/10.1007/s00705-014-2118-z
- Rauw F, Palya V, Van Borm S, Welby S, Tatar-Kis T, Gardin Y, Dorsey KM, Aly MM, Hassan MK, Soliman MA, et al. Further evidence of antigenic drift and protective efficacy afforded by a recombinant HVT-H5 vaccine against challenge with two antigenically divergent Egyptian clade 2.2.1 HPAI H5N1 strains. Vaccine 2011; 29:2590-600; PMID:21292007; http://dx.doi.org/10.1016/j.vaccine.2011.01.048
- Khafagy AK, Sheble A, Sarhan AA, Gough RE. Isolation of Influenza Virus (Subtype H7N1). Proceedings of the 2nd Congress of Faculty of Veterinary Medicine. Cairo, Egypt: Faculty of Veterinary Medicine, Cairo University, 1992:65-8.
- Hussien HA, El-Azab M. Evidence for the presence H6 and H9 among broiler and layer breeders in Egypt. In: Sami Ahmed AA, ed. Proceedings of the XII International Congress of the World Veterinary Poultry association. Cairo, Egypt: The Egyptian Veterinary Poultry Association, 2002:227.
- Ahmed AAS, Sabban MS, Ibrahim AMM, Amin A, Khafagi AR, Sheble A. Some properties of Newcastle-disease virus isolates recovered from migratory birds to Egypt. Zentralbl Veterinarmed [B] 1980; 27:313-9; PMID:7424293; http://dx.doi.org/10.1111/j.1439-0450.1980.tb01696.x
- Amin A, Shalaby MA, Imam IZ. Studies on influenza virus isolated from migrating birds in Egypt. Comp Immunol Microbiol Infect Dis 1980; 3:241-6; PMID:7471714; http://dx.doi.org/10.1016/0147-9571(80)90063-6
- Malkinson M, Banet C. The role of birds in the ecology of West Nile virus in Europe and Africa. Curr Top Microbiol Immunol 2002; 267:309-22; PMID:12082995
- El-Zoghby EF, Abdelwhab EM, Arafa A, Selim AA, Kholousy SG, Kilany WH, Hassan MK, El-Kanawati Z, Aly MM, Hafez HM. Active surveillance of avian influenza virus in backyard birds in Egypt. J Appl Poult Res 2011; 20:584-8; PMID:343; http://dx.doi.org/10.3382/japr.2011-00343
- Gerloff NA, Jones J, Simpson N, Balish A, Elbadry MA, Baghat V, Rusev I, de Mattos CC, de Mattos CA, Zonkle LE, et al. A high diversity of Eurasian lineage low pathogenicity avian influenza A viruses circulate among wild birds sampled in Egypt. PloS one 2013; 8:e68522; PMID:23874653; http://dx.doi.org/10.1371/journal.pone.0068522
- Sheta BM, Fuller TL, Larison B, Njabo KY, Ahmed AS, Harrigan R, Chasar A, Abdel Aziz S, Khidr AA, Elbokl MM, et al. Putative human and avian risk factors for avian influenza virus infections in backyard poultry in Egypt. Vet Microbiol 2014; 168:208-13; PMID:24315038; http://dx.doi.org/10.1016/j.vetmic.2013.11.010
- Gaidet N, Dodman T, Caron A, Balanca G, Desvaux S, Goutard F, Cattoli G, Lamarque F, Hagemeijer W, Monicat F. Avian influenza viruses in water birds, Africa. Emerg Infect Dis 2007; 13:626-9; PMID:17553284; http://dx.doi.org/10.3201/eid1304.061011
- Snoeck CJ, Adeyanju AT, De Landtsheer S, Ottosson U, Manu S, Hagemeijer W, Mundkur T, Muller CP. Reassortant low-pathogenic avian influenza H5N2 viruses in African wild birds. J Gen Virol 2011; 92:1172-83; PMID:21248176; http://dx.doi.org/10.1099/vir.0.029728-0
- PAHO. Pan American Health Organization: avian influenza virus A (H10N7) circulating among humans in Egypt. EID Weekly Updates 2:2. Available online at: http://www.paho.org/english/ad/dpc/cd/eid-eer-07-may-2004.htm ( Accessed 03 December 2013). 2004.
- Aly MM, Arafa A, Kilany WH, Sleim AA, Hassan MK. Isolation of a low pathogenic avian influenza virus (H7N7) from a black kite (Milvus migrans) in Egypt in 2005. Avian Dis 2010; 54:457-60; PMID:20521679; http://dx.doi.org/10.1637/8719-032109-ResNote.1
- Aly MM, Arafa A, Hassan MK, Saad FE. Epidemiological Investigation on Avian Influenza Viruses in Wild and Migratory Birds in Egypt. Proceedings of the 8th Scientific Conference of the Egyptian Veterinary Poultry Association. Cairo, Egypt: The Egyptian Veterinary Poultry Association, 2008:90-7.
- Hosny A, Imam ZE, Imam I, Sharaan N, Saleh S, Orchan M, Amin A, El-Senousy A. Isolation and identification of influenza virus A/Duck/England from migrating birds to Egypt. J Egypt Public Health Assoc 1980; 55:13-22; PMID:7341721
- Alexander DJ. Ecological aspects of influenza A viruses in animals and their relationship to human influenza: a review. J R Soc Med 1982; 75:799-811.
- Abla-El-Said MS, Magda MA, Afaf AA, El-Sisi MA, Amal AHT, Hamouda MSM. Isolation and Identification and Carcinogenicity of an Avian Influenza Virus From Ducks in Egypt. Proceedings of the 5th Scientific Conference of the Egyptian Veterinary Poultry Association. Cairo, Egypt: The Egyptian Veterinary Poultry Association, 1998:29-49.
- Lin B, Malanoski AP, Wang Z, Blaney KM, Long NC, Meador CE, Metzgar D, Myers CA, Yingst SL, Monteville MR, et al. Universal detection and identification of avian influenza virus by use of resequencing microarrays. J Clin Microbiol 2009; 47:988-93; PMID:19279171; http://dx.doi.org/10.1128/JCM.01346-08
- Kida H, Ito T, Yasuda J, Shimizu Y, Itakura C, Shortridge KF, Kawaoka Y, Webster RG. Potential for transmission of avian influenza viruses to pigs. J Gen Virol 1994; 75:2183-8; PMID:8077918; http://dx.doi.org/10.1099/0022-1317-75-9-2183
- Torremorell M, Allerson M, Corzo C, Diaz A, Gramer M. Transmission of influenza A virus in pigs. Transboundary Emerg Dis 2012; 59:68-84; PMID:22226050; http://dx.doi.org/10.1111/j.1865-1682.2011.01300.x
- Blench RM. A history of pigs in Africa. In: Blench RM, MacDonald KC, eds. The Origin and Development of African Livestock. London: University College Press, 2000:355-67.
- FAO. Food and agriculture organisation of the United Nations. FAOSTAT. Available online at: http://faostat3.fao.org/faostat-gateway/go/to/browse/Q/QA/E ( Accessed 03 Dec 2013). 2011.
- El-Sayed A, Awad W, Fayed A, Hamann HP, Zschock M. Avian influenza prevalence in pigs, Egypt. Emerg Infect Dis 2010; 16:726-7; PMID:20350404; http://dx.doi.org/10.3201/eid1604.091316
- Fahmi W, Sutton K. Cairo's contested garbage: Sustainable solid waste management and the Zabaleen's right to the city. Sustainability 2010; 2:1765-83; http://dx.doi.org/10.3390/su2061765
- Shalaby MA, Nafi BM, Saber MS, Hosny AH. Serological studies on swine influenza in Egypt. Int J Zoonoses 1981; 8:100-6; PMID:6282772
- Meseko C, Olaleye D, Capua I, Cattoli G. Swine influenza in Sub-Saharan Africa – current knowledge and emerging insights. Zoonoses Pub Health 2013; 61:229-37; PMID:23826898; http://dx.doi.org/10.1111/zph.12068
- Lipatov AS, Kwon YK, Sarmento LV, Lager KM, Spackman E, Suarez DL, Swayne DE. Domestic pigs have low susceptibility to H5N1 highly pathogenic avian influenza viruses. PLoS Pathog 2008; 4:e1000102; PMID:18617994; http://dx.doi.org/10.1371/journal.ppat.1000102
- Londt BZ, Brookes SM, Kelly MD, Nash BJ, Brown IH. Failure to infect pigs co-housed with ducks or chickens infected experimentally with A/turkey/Turkey/1/2005 (H5N1) highly pathogenic avian influenza virus. Vet Microbiol 2013; 162:944-8; PMID:23266109; http://dx.doi.org/10.1016/j.vetmic.2012.11.040
- El-Sayed A, Prince A, Fawzy A, Nadra-Elwgoud M, Abdou I, Omar L, Fayed A, Salem M. Sero-prevalence of avian influenza in animals and human in Egypt. Pak J Biol Sci 2013; 16:524-9; PMID:24498821; http://dx.doi.org/10.3923/pjbs.2013.524.529
- Choi YK, Nguyen TD, Ozaki H, Webby RJ, Puthavathana P, Buranathal C, Chaisingh A, Auewarakul P, Hanh NT, Ma SK, et al. Studies of H5N1 influenza virus infection of pigs by using viruses isolated in Vietnam and Thailand in 2004. J Virol 2005; 79:10821-5; PMID:16051873; http://dx.doi.org/10.1128/JVI.79.16.10821-10825.2005
- Cyranoski D. Bird flu spreads among Java's pigs. Nature 2005; 435:390-1; PMID:15917759; http://dx.doi.org/10.1038/435390a
- Seef S, Jeppsson A. Is it a policy crisis or it is a health crisis? The Egyptian context–analysis of the Egyptian health policy for the H1N1 flu pandemic control. Pan Afr Med J 2013; 14:59; PMID:23565306
- Webster RG. Are equine 1 influenza viruses still present in horses? Equine Vet J 1993; 25:537-8; PMID:8276003; http://dx.doi.org/10.1111/j.2042-3306.1993.tb03009.x
- Crawford PC, Dubovi EJ, Castleman WL, Stephenson I, Gibbs EP, Chen L, Smith C, Hill RC, Ferro P, Pompey J, et al. Transmission of equine influenza virus to dogs. Science 2005; 310:482-5; PMID:16186182; http://dx.doi.org/10.1126/science.1117950
- Yamanaka T, Nemoto M, Tsujimura K, Kondo T, Matsumura T. Interspecies transmission of equine influenza virus (H3N8) to dogs by close contact with experimentally infected horses. Vet Microbiol 2009; 139:351-5; PMID:19596528; http://dx.doi.org/10.1016/j.vetmic.2009.06.015
- Crispe E, Finlaison DS, Hurt AC, Kirkland PD. Infection of dogs with equine influenza virus: evidence for transmission from horses during the Australian outbreak. Aust Vet J 2011; 89 Suppl 1:27-8; PMID:21711279; http://dx.doi.org/10.1111/j.1751-0813.2011.00734.x
- Mancini DA, Mendonca RM, Pereira AS, Kawamoto AH, Vannucchi CI, Pinto JR, Mori E, Mancini Filho J. Influenza viruses in adult dogs raised in rural and urban areas in the state of Sao Paulo, Brazil. Rev Inst Med Trop Sao Paulo 2012; 54:311-4; PMID:23152313; http://dx.doi.org/10.1590/S0036-46652012000600004
- Ramirez-Martinez LA, Contreras-Luna M, De la Luz J, Manjarrez ME, Rosete DP, Rivera-Benitez JF, Saavedra-Montanez M, Ramirez-Mendoza H. Evidence of transmission and risk factors for influenza A virus in household dogs and their owners. Influenza Other Respir Viruses 2013; 7:1292-6; PMID:24034782; http://dx.doi.org/10.1111/irv.12162
- Minuse E, McQueen JL, Davenport FM, Francis T Jr. Studies of antibodies to 1956 and 1963 equine influenza viruses in horses and man. J Immunol 1965; 94:563-6; PMID:14299031
- Rose MA. Respiratory virus infections in man and animals. Equine influenza viruses isolated at Cambridge in 1963 and 1965. Proc R Soc Med 1966; 59:51-4; PMID:5948143
- Alexander DJ, Brown IH. Recent zoonoses caused by influenza A viruses. Rev Sci Tech 2000; 19:197-225; PMID:11189716
- Laver WG, Webster RG. Studies on the origin of pandemic influenza. 3. Evidence implicating duck and equine influenza viruses as possible progenitors of the Hong Kong strain of human influenza. Virology 1973; 51:383-91; PMID:4632653; http://dx.doi.org/10.1016/0042-6822(73)90437-6
- Spackman E, McCracken KG, Winker K, Swayne DE. H7N3 avian influenza virus found in a South American wild duck is related to the Chilean 2002 poultry outbreak, contains genes from equine and North American wild bird lineages, and is adapted to domestic turkeys. J Virol 2006; 80:7760-4; PMID:16840356; http://dx.doi.org/10.1128/JVI.00445-06
- Kalad MA, Ebied EM, Madkour NK, Warda SA, Saleh NS, El-Kabbany MMA, Soliman IMA, Saad MD, Shuck-Lee D, Younan MA, et al. Characterization of equine influenza A virus H3N8 isolated in Egypt in 2008. Ippologia 2011; 22:35-44.
- Ismail IM, Sami AM, Youssef HM, Abou Zaid HA. An outbreak of equine influenza type (1) in Egypt in 1989. Vet Med J Giza 1990; 38:195-206.
- Abdalla MA, Taleb ZA, Ebid MH. Characterization of serum lysosomal enzymatic activities. III. Effect of infectious influenza in Egyptian equines. Dtsch Tierarztl Wochenschr 1993; 100:147-8; PMID:8387424
- Hamoda FK, Magdi MI, Mahmoud MA, Magda AK. Some studies on an outbreak of equine influenza. J Egyp Vet Med Assoc 2001; 61:19-35.
- Abd El-Rahim IH, Hussein M. An epizootic of equine influenza in Upper Egypt in 2000. Rev Sci Tech 2004; 23:921-30; PMID:15861887
- Aly MM, Arafa A, Amal MAR, Salem SA, Hassan MK, Samaha H. Epizootiological and molecular characterization of equine influenza virus 2008 outbreak in Egypt. Vet Med J Giza 2009; 57:223-34.
- Abo-Hatab EM, Salem SAH, El-Monem HA, Kotb NS, Boseila AAH. Serological diagnosis of infected equine influenza virus (EIV) in Egypt 2008. Proceedings of the 3rd Scientific Conference of Animal Wealth Research in the Middle East and North Africa. Egypt, 2010:75-83.
- Mohamed AEA, Hatab EA, Kotb NS, Mottelib AA. Studies on an outbreak of equine influenza at the southern district of Egypt. In: Köfer J, Schobesberger H, eds. Animal Hygiene and Sustainable Livestock Production Proceedings of the XVth International Congress of the International Society for Animal Hygiene. Vienna, Austria, 2011:835-7.
- Ebied EM, Salem ZT, Al-Imam HS. Immune response of pregnant mares and their foals for inactivated equine influenza vaccine. Int J Virol 2011; 7:210-4; http://dx.doi.org/10.3923/ijv.2011.210.214
- Madkour NK, Warda SA, El-Kabbany MMA. Trials for preparation of tissue culture inactivated equine influenza vaccine. Proceedings of the 5th Scientific Conference of Animal Wealth Research in the Middle East and North Africa. Giza, Egypt: Faculty of Agriculture, Cairo University, 2012:144-56.
- Abdel-Moneim AS, Abdel-Ghany AE, Shany SA. Isolation and characterization of highly pathogenic avian influenza virus subtype H5N1 from donkeys. J Biomed Sci 2010; 17:25; PMID:20398268; http://dx.doi.org/10.1186/1423-0127-17-25
- Connor RJ, Kawaoka Y, Webster RG, Paulson JC. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology 1994; 205:17-23; PMID:7975212; http://dx.doi.org/10.1006/viro.1994.1615
- Suzuki Y, Ito T, Suzuki T, Holland RE, Chambers TM, Kiso M, Ishida H, Kawaoka Y. Sialic acid species as a determinant of the host range of influenza A viruses. J Virol 2000; 74:11825-31; PMID:11090182; http://dx.doi.org/10.1128/JVI.74.24.11825-11831.2000
- Steel J, Lowen AC, Mubareka S, Palese P. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog 2009; 5:e1000252; PMID:19119420; http://dx.doi.org/10.1371/journal.ppat.1000252
- Abdel-Moneim AS, Shehab GM, Abu-Elsaad AA. Molecular evolution of the six internal genes of H5N1 equine influenza A virus. Arch Virol 2011; 156:1257-62; PMID:21431346; http://dx.doi.org/10.1007/s00705-011-0966-3
- Bertran K, Swayne DE. High doses of highly pathogenic avian influenza virus in chicken meat are required to infect ferrets. Vet Res 2014; 45:60; PMID:24894438; http://dx.doi.org/10.1186/1297-9716-45-60
- Gasparini R, Amicizia D, Lai PL, Panatto D. Clinical and socioeconomic impact of seasonal and pandemic influenza in adults and the elderly. Hum Vaccines Immunoth 2012; 8:21-8; PMID:22252007; http://dx.doi.org/10.4161/hv.8.1.17622
- Taubenberger JK, Morens DM. Influenza: the once and future pandemic. Public Health Rep 2010; 125:16-26; PMID:20568566
- Girard MP, Tam JS, Assossou OM, Kieny MP. The 2009 A (H1N1) influenza virus pandemic: a review. Vaccine 2010; 28:4895-902; PMID:20553769; http://dx.doi.org/10.1016/j.vaccine.2010.05.031
- Simonsen L, Spreeuwenberg P, Lustig R, Taylor RJ, Fleming DM, Kroneman M, Van Kerkhove MD, Mounts AW, Paget WJ. Global mortality estimates for the 2009 influenza pandemic from the GLaMOR project: a modeling study. PLoS Med 2013; 10:e1001558; PMID:24302890; http://dx.doi.org/10.1371/journal.pmed.1001558
- Ikuta K, Ibrahim MS. Flu pandemic: the host or the hosting land? Future Virology 2008; 3:197-201; http://dx.doi.org/10.2217/17460794.3.3.197
- Anon. Epidemic influenza in Egypt. Br Med J 1894; 1:1369-70.
- Benjafield JD. Notes on the influenza epidemic in the Egyptian expeditionary force. Br Med J 1919; 2:167-9; PMID:20769574; http://dx.doi.org/10.1136/bmj.2.3058.167
- Zaghloul IS, Rizk F, Kader MA. An influenza epidemic in Egypt, 1952-3, with special reference to haemagglutination inhibition. Bull World Health Organ 1955; 13:289-97; PMID:13260888
- Hosny AH, Imam ZE, el-Rai F. Incidence of influenza in the seasons 1963-1964 and 1964-1965 in U.A.R. J Egypt Public Health Assoc 1966; 41:281-7; PMID:6002843
- Hosny AH, Imam IZ, Nour AA, el-Beltagy AM. Influenza in the Arab Republic of Egypt from 1966 to 1972. (With special reference to the “Hong Kong” epidemic of 1968). J Egypt Public Health Assoc 1972; 47:170-81; PMID:4638553
- Mazloum HM, Mourad AS, Awad AM, Ibrahim II. The role of influenza viruses in acute respiratory infections in children during the year 1974-1975 in Alexandria. J Egypt Public Health Assoc 1977; 52:344-55; PMID:617667
- Mazloum HM, El-Heneidy AR, Mourad AS, Awatef MA, Sorour S. The role of influenza viruses in acute respiratory infections in adults. J Egypt Public Health Assoc 1978; 53:53-66; PMID:739207
- Nishikawa F, Sugiyama T. Direct isolation of H1N2 recombinant virus from a throat swab of a patient simultaneously infected with H1N1 and H3N2 influenza A viruses. J Clin Microbiol 1983; 18:425-7; PMID:6619292
- Xu X, Smith CB, Mungall BA, Lindstrom SE, Hall HE, Subbarao K, Cox NJ, Klimov A. Intercontinental circulation of human influenza A(H1N2) reassortant viruses during the 2001-2002 influenza season. J Infect Dis 2002; 186:1490-3; PMID:12404167; http://dx.doi.org/10.1086/344738
- Gregory V, Bennett M, Orkhan MH, Al Hajjar S, Varsano N, Mendelson E, Zambon M, Ellis J, Hay A, Lin YP. Emergence of influenza A H1N2 reassortant viruses in the human population during 2001. Virology 2002; 300:1-7; PMID:12202200; http://dx.doi.org/10.1006/viro.2002.1513
- Ansaldi F, D'Agaro P, de Florentiis D, Lai P, Morelli P, Amicizia D, Righello O, Ferro G, Bacilieri S, Micillo D, et al. The emergence of a new influenza A/H3N2 virus variant in northern Italy: which impact on non- and vaccinated population? Int Congr Ser 2004; 1263:325-8; http://dx.doi.org/10.1016/j.ics.2004.02.165
- O'Brien K, Barr IG. Annual report of the national influenza surveillance scheme, 2006. Communicable Dis Intell Quart Rep 2007; 31:167-79; PMID:17724991
- Russell CA, Jones TC, Barr IG, Cox NJ, Garten RJ, Gregory V, Gust ID, Hampson AW, Hay AJ, Hurt AC, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science 2008; 320:340-6; PMID:18420927; http://dx.doi.org/10.1126/science.1154137
- Kandeel A, Deming M, Elkreem EA, El-Refay S, Afifi S, Abukela M, Earhart K, El-Sayed N, El-Gabay H. Pandemic (H1N1) 2009 and Hajj Pilgrims who received predeparture vaccination, Egypt. Emerg Infect Dis 2011; 17:1266-8.
- Anon. Egypt – Death # 143 for the 2010/11 season total cumulative case's (4078) of H1N1 Thru January 15th, 2011. Available online at: http://www.flutrackers.com/forum/showthread.php?t=156929 ( Accessed 08 December 2013). 2011.
- Ahmed MA, Awadalla NJ, Saleh AB. Clinical epidemiology comparison of H1N1 RT-PCR-positive and RT-PCR-negative pneumonia during the 2009-2010 pandemic in Mansoura University hospitals, Egypt. Influenza Other Respir Viruses 2011; 5:241-6; PMID:21651734; http://dx.doi.org/10.1111/j.1750-2659.2011.00203.x
- Khattab A, Shaheen M, Kamel T, El Faramay A, El Rahman SA, Nabil D, Gouda M. Burden of pediatric influenza A virus infection post swine-flu H1N1 pandemic in Egypt. Asian Pac J Trop Med 2013; 6:693-8; PMID:23827145; http://dx.doi.org/10.1016/S1995-7645(13)60120-0
- Amin NM, El Basha NR, El Rifai NM, El Baz MS, Draz IH, El Kholy AA, Sherif MM. Viral causes of acute respiratory infection among Egyptian children hospitalized with severe acute asthma exacerbation. J Egypt Public Health Assoc 2013; 88:52-6; PMID:23528533; http://dx.doi.org/10.1097/01.EPX.0000427636.90615.ad
- Mansour DE, El-Shazly AA, Elawamry AI, Ismail AT. Comparison of ocular findings in patients with H1N1 influenza infection versus patients receiving influenza vaccine during a pandemic. Ophthalmic Res 2012; 48:134-8; PMID:22572924; http://dx.doi.org/10.1159/000337138
- WHO. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003-2013. Available online at: http://www.who.int/influenza/human_animal_interface/EN_GIP_20131008CumulativeNumberH5N1cases.pdf ( accessed December 08, 2013). 2013.
- Kandeel A, Manoncourt S, Abd el Kareem E, Mohamed Ahmed AN, El-Refaie S, Essmat H, Tjaden J, de Mattos CC, Earhart KC, Marfin AA, et al. Zoonotic transmission of avian influenza virus (H5N1), Egypt, 2006-2009. Emerg Infect Dis 2010; 16:1101-7; PMID:20587181; http://dx.doi.org/10.3201/eid1607.091695
- WHO. H5N1 highly pathogenic avian influenza: Timeline of major events. Available: http://www.who.int/influenza/human_animal_interface/h5n1_avian_influenza_update20140714.pdf?ua=1 ( accessed 02 Aug 2014). 2014.
- Dudley JP. Age-specific infection and death rates for human A(H5N1) avian influenza in Egypt. Euro Surveill 2009; 14: pii: 19198; PMID:19422779
- Abdelwhab EM, Hafez HM, Grund C, Aly MM, Harder TC. Increasing prevalence of unique mutation patterns in H5N1 avian influenza virus HA and NA glycoproteins from human infections in Egypt. Sequencing 2010; 2010:1-3; http://dx.doi.org/10.1155/2010/450823
- Fasina FO, Ifende VI, Ajibade AA. Avian influenza A(H5N1) in humans: lessons from Egypt. Euro Surveill 2010; 15:19473; PMID:20122384
- Schroedl A. Age-based human influenza A virus (H5N1) infection patterns, Egypt. Emerg Infect Dis 2010; 16:161-2; PMID:20031073; http://dx.doi.org/10.3201/eid11601.090560
- Ashour MM, Khatab AM, El-Folly RF, Amer WA. Clinical features of avian influenza in Egyptian patients. J Egypt Soc Parasitol 2012; 42:385-96; PMID:23214216
- Tseng WC, Li HA, Huang WC, Liang LH. Potential number of human cases of H5N1 avian influenza in Egypt. Public Health 2010; 124:452-9; PMID:20633908; http://dx.doi.org/10.1016/j.puhe.2010.04.002
- Perovic VR, Muller CP, Niman HL, Veljkovic N, Dietrich U, Tosic DD, Glisic S, Veljkovic V. Novel phylogenetic algorithm to monitor human tropism in Egyptian H5N1-HPAIV reveals evolution toward efficient human-to-human transmission. PLoS One 2013; 8:e61572; PMID:23658611; http://dx.doi.org/10.1371/journal.pone.0061572
- Murray EJ, Morse SS. Seasonal oscillation of human infection with influenza A/H5N1 in Egypt and Indonesia. PLoS One 2011; 6:e24042; PMID:21909409; http://dx.doi.org/10.1371/journal.pone.0024042
- Rabinowitz PM, Galusha D, Vegso S, Michalove J, Rinne S, Scotch M, Kane M. Comparison of human and animal surveillance data for H5N1 influenza A in Egypt 2006-2011. PLoS One 2012; 7:e43851; PMID:23028474; http://dx.doi.org/10.1371/journal.pone.0043851
- Younan M, Poh MK, Elassal E, Davis T, Rivailler P, Balish AL, Simpson N, Jones J, Deyde V, Loughlin R, et al. Microevolution of highly pathogenic avian influenza A(H5N1) viruses isolated from humans, Egypt, 2007-2011. Emerg Infect Dis 2013; 19:43-50; PMID:23260983; http://dx.doi.org/10.3201/eid1901.121080
- Earhart KC, Elsayed NM, Saad MD, Gubareva LV, Nayel A, Deyde VM, Abdelsattar A, Abdelghani AS, Boynton BR, Mansour MM, et al. Oseltamivir resistance mutation N294S in human influenza A(H5N1) virus in Egypt. J Infect Public Health 2009; 2:74-80; PMID:20701864; http://dx.doi.org/10.1016/j.jiph.2009.04.004
- Xiong X, Xiao H, Martin SR, Coombs PJ, Liu J, Collins PJ, Vachieri SG, Walker PA, Lin YP, McCauley JW, et al. Enhanced human receptor binding by H5 haemagglutinins. Virology 2014; 456-457:179-87; PMID:24889237
- Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 2012; 486:420-8; PMID:22722205
- Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 2012; 336:1534-41; PMID:22723413; http://dx.doi.org/10.1126/science.1213362
- Vergne T, Grosbois V, Jobre Y, Saad A, El Nabi AA, Galal S, Kalifa M, El Kader SA, Dauphin G, Roger F, et al. Avian influenza vaccination of poultry and passive case reporting, Egypt. Emerg Infect Dis 2012; 18:2076-8; PMID:23171740; http://dx.doi.org/10.3201/eid1812.120616
- Abdelwhab el SM, Erfan AM, Grund C, Ziller M, Arafa AS, Beer M, Aly MM, Hafez HM, Harder TC. Simultaneous detection and differentiation by multiplex real time RT-PCR of highly pathogenic avian influenza subtype H5N1 classic (clade 2.2.1 proper) and escape mutant (clade 2.2.1 variant) lineages in Egypt. Virol J 2010; 7:260; PMID:20929539; http://dx.doi.org/10.1186/1743-422X-7-260
- Abdelwhab EM, Arafa AS, Erfan AM, Aly MM, Hafez HM. Modified H5 real-time reverse transcriptase-PCR oligonucleotides for detection of divergent avian influenza H5N1 viruses in Egypt. Avian Dis 2010; 54:1301-5; PMID:21313854; http://dx.doi.org/10.1637/9412-053110-ResNote.1
- Saad MD, Ahmed LS, Gamal-Eldein MA, Fouda MK, Khalil F, Yingst SL, Parker MA, Montevillel MR. Possible avian influenza (H5N1) from migratory bird, Egypt. Emerg Infect Dis 2007; 13:1120-1; PMID:18214200; http://dx.doi.org/10.3201/eid1307.061222
- Shalaby MA, Nafi BM, Saber MS, Hosny AH. Serological studies on swine influenza in Egypt. Int J Zoonoses 1981; 8:100-6; PMID:6282772
- Khattab A, Shaheen M, Kamel T, El Faramay A, El Rahman SA, Nabil D, Gouda M. Burden of pediatric influenza A virus infection post swine-flu H1N1 pandemic in Egypt. Asian Pac J Trop Med 2013; 6:693-8; PMID:23827145; http://dx.doi.org/10.1016/S1995-7645(13)60120-0
- Abla-El-Said MS, Magda MA, Afaf AA, El-Sisi MA, Amal AHT, Hamouda MSM. Isolation and Identification and Carcinogenicity of an Avian Influenza Virus From Ducks in Egypt. Proceeding of the 5th Scientific Conference of the Egyptian Veterinary Poultry Association. Cairo, Egypt: the Egyptian Veterinary Poultry Association, 1998:29-49.
- Abd El-Rahim IH, Hussein M. An epizootic of equine influenza in Upper Egypt in 2000. Rev Sci Tech 2004; 23:921-30; PMID:15861887
- Aly MM, Arafa A, Amal MAR, Salem SA, Hassan MK, Samaha H. Epizootiological and molecular characterization of equine influenza virus 2008 outbreak in Egypt. Vet Med J Giza 2009; 57:223-34.
- Amin A, Shalaby MA, Imam IZ. Studies on influenza virus isolated from migrating birds in Egypt. Comp Immunol Microbiol Infect Dis 1980; 3:241-6; PMID:7471714; http://dx.doi.org/10.1016/0147-9571(80)90063-6
- Aly MM, Arafa A, Hassan MK, Saad FE. Epidemiological investigation on avian influenza viruses in wild and migratory birds in Egypt. Proceeding of the 8th Scientific Conference of the Egyptian Veterinary Poultry Association. Cairo, Egypt: The Egyptian Veterinary Poultry Association, 2008.
- Abdel-Moneim AS, Afifi MA, El-Kady MF. Isolation and mutation trend analysis of influenza A virus subtype H9N2 in Egypt. Virol J 2012; 9:173; PMID:22925485; http://dx.doi.org/10.1186/1743-422X-9-173
- Arafa AS, Hagag NM, Yehia N, Zanaty AM, Naguib MM, Nasef SA. Effect of cocirculation of highly pathogenic avian influenza H5N1 subtype with low pathogenic H9N2 subtype on the spread of infections. Avian Dis 2012; 56:849-57; PMID:23402103; http://dx.doi.org/10.1637/10152-040812-Reg.1
- El-Zoghby EF, Arafa AS, Hassan MK, Aly MM, Selim A, Kilany WH, Selim U, Nasef S, Aggor MG, Abdelwhab EM, et al. Isolation of H9N2 avian influenza virus from bobwhite quail (Colinus virginianus) in Egypt. Arch Virol 2012; 157:1167-72; PMID:22426861; http://dx.doi.org/10.1007/s00705-012-1269-z
- Lin B, Malanoski AP, Wang Z, Blaney KM, Long NC, Meador CE, Metzgar D, Myers CA, Yingst SL, Monteville MR, et al. Universal detection and identification of avian influenza virus by use of resequencing microarrays. J Clin Microbiol 2009; 47:988-93; PMID:19279171; http://dx.doi.org/10.1128/JCM.01346-08
