Abstract
The tumor immune environment has been linked to prognosis in patients with a range of malignancies. Recently, we demonstrated in pre-clinical models that modifying the tumor immune environment using a small-molecule inhibitor of TGFb significantly improved outcome to subsequent radiation therapy. These data suggest that this and other immunotherapies may be used to remodel the tumor before conventional cancer therapies to improve outcomes.
Recent studies have explored the link between tumor immune cell infiltrate and overall survival, reporting that decreased T cell infiltrate and increased macrophage infiltrate correlate with decreased survival.Citation1,2. These data are particularly dramatic in colorectal cancer, where there is an international effort underway to evaluate immune infiltrates, or “immune score” in tumors as a prognostic tool for patients.Citation1 For those patients identified with poor immune scores, the question remains as to whether the tumor immune status can be improved, and whether that will increase the efficacy of treatment.
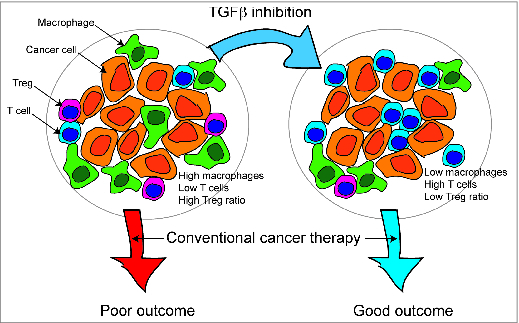
Figure 1. Immunotherapy to improve pre-treatment tumor immune environment to improve outcome with conventional cancer therapy. Patients bearing tumors with high macrophage infiltrates, low T cells infiltrates and high Treg:CD8+ ratios have been shown to have poor prognosis when treated with conventional therapies. Immunotherapy can change the infiltrates in tumors, and our studies with TGF® inhibition suggest this can improve outcome.
We hypothesized that an improved immune environment at the time of treatment would increase the efficacy of radiation (). To improve the immune environment of established tumors, we used targeted inhibition of TGFβ. During carcinogenesis, TGFβ can act as a tumor suppressor; though once invasive carcinoma has developed, TGFβ is an important force that sustains the invasive and immune suppressive phenotype of tumors. The small molecule Alk5 inhibitor SM16 is readily bioavailable and chronic administration of dietary SM16 re-polarizes tumor-infiltrating myeloid cells,Citation3,4 and dramatically improves the function of T cell targeted immunotherapy.Citation3 Our experiments demonstrate that pretreatment with SM16 improved the immune environment of tumors in mice, and significantly improved the efficacy of subsequent radiation therapy.Citation5 We demonstrated that this effect was entirely dependent on CD8+ T cells and generated long-term tumor-specific protection.Citation5 In addition, using a concomitant tumor model we found that SM16 administration followed by radiation to one tumor resulted in growth delay of distant tumors that was not seen with either modality alone.
The mechanisms why improving the pre-treatment tumor environment permits CD8+ T cell control of tumors remains to be determined. This may relate to an improved context of antigen presentation following radiation-induced antigen release, or since we see fewer T regulatory cells in the tumor, this may relate to decreased T regulatory cell-mediated suppression of immune responses that are initiated by radiation therapy. Since TGFβ suppresses the effector function of T cells in tumors,Citation3,6 it is likely that TGFβ inhibition improves the effector phase, though in our studies the short in vivo half-life of SM16 means that inhibition is not sustained through any effector phase initiated by radiation therapy. Similarly, while myeloid responses to radiation therapy may suppress adaptive immune responses at later times following radiation,Citation7 these later time points will not be affected by pretreatment with SM16. Since we see SM16-mediated early improvements in CD4+ differentiation in the tumor draining lymph node at early time points following radiation, we suspect that TGFβ inhibition permits CD4+ T cells to differentiate toward more helpful phenotypes that support rather than suppress effector CD8+ function.
TGFβ inhibition combined with radiation has a great deal of potential for many other reasons. TGFβ inhibition has been shown to reduce the severity of lung fibrosisCitation8 and rectal fibrosisCitation9 in murine models. In addition, TGFβ inhibition at the time of radiation increases radiosensitivity in vitro and in vivo.Citation10 In our studies, we did not observe changes in epithelial-to-mesenchymal transition in the tumor or changes to tumor vasculature,Citation5 perhaps due to the short duration of inhibition or due to the Alk5 specificity of the inhibitor. However, it may also be prudent to be cautious of extended TGFβ inhibition in the setting of radiation therapy, particularly in patients who have a “field cancerization effect," since there is potential for second malignancy.
Based on these studies, we conclude that there are significant opportunities to manipulate the immune environment of tumors for therapeutic gain. Since the immune environment of colorectal tumors is so important to outcome through conventional cancer therapies,Citation1 it is heartening to see that there is potential to improve outcomes for those with poor immune infiltrates through upfront immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-64; PMID:17008531; http://dx.doi.org/10.1126/science.1129139
- DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Disc 2011; 1:54-67; PMID:22039576; http://dx.doi.org/10.1158/2159-8274.CD-10-0028
- Garrison K, Hahn T, Lee WC, Ling LE, Weinberg AD, Akporiaye ET. The small molecule TGF-beta signaling inhibitor SM16 synergizes with agonistic OX40 antibody to suppress established mammary tumors and reduce spontaneous metastasis. Cancer Immunol, Immunother: CII 2012; 61:511-21; PMID:21971588; http://dx.doi.org/10.1007/s00262-011-1119-y
- Rausch MP, Hahn T, Ramanathapuram L, Bradley-Dunlop D, Mahadevan D, Mercado-Pimentel ME, Runyan RB, Besselsen DG, Zhang X, Cheung HK, et al. An orally active small molecule TGF-beta receptor I antagonist inhibits the growth of metastatic murine breast cancer. Anticancer Res 2009; 29:2099-2109 ; PMID:19528470
- Young KH, Newell P, Cottam B, Friedman D, Savage T, Baird JR, Akporiaye E, Gough MJ, Crittenden M. TGFbeta inhibition prior to hypofractionated radiation enhances efficacy in preclinical models. Cancer Immunol Res 2014; 2(10): 1011-22; PMID:25047233
- Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med 2001; 7:1118-22; PMID:PMID:11590434; http://dx.doi.org/10.1038/nm1001-1118
- Gough MJ, Young K, Crittenden M. The impact of the myeloid response to radiation therapy. Clin Dev Immunol 2013; 2013:1-14; http://dx.doi.org/10.1155/2013/281958
- Flechsig P, Dadrich M, Bickelhaupt S, Jenne J, Hauser K, Timke C, Peschke P, Hahn EW, Gröne HJ, Yingling J, et al. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-beta and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res: An Off J Am Assoc Cancer Res 2012; 18:3616-27; PMID:22547771; http://dx.doi.org/10.1158/1078-0432.CCR-11-2855
- Liu Y, Kudo K, Abe Y, Hu DL, Kijima H, Nakane A, Ono K. Inhibition of transforming growth factor-beta, hypoxia-inducible factor-1alpha and vascular endothelial growth factor reduced late rectal injury induced by irradiation. J Radiat Res 2009; 50:233-9; PMID:19346676; http://dx.doi.org/10.1269/jrr.08112
- Bouquet F, Pal A, Pilones KA, Demaria S, Hann B, Akhurst RJ, Babb JS, Lonning SM, DeWyngaert JK, Formenti SC, et al. TGFbeta1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin Cancer Res: An Off J Am Assoc Cancer Res 2011; 17:6754-65; PMID:22028490; http://dx.doi.org/10.1158/1078-0432.CCR-11-0544
