Abstract
Tumor-associated myeloid cells undermine the therapeutic efficacy of cancer immunotherapy by their inhibitory properties on immune effector cells. Development of therapeutic agents to deplete suppressive myeloid cells in tumor microenvironment requires identification of cell-specific targets. A competitive phage display technique on live cells paves the way to discovery of such a target.
Myeloid cells composed of tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) are a distinctive feature of many human cancers. For example, in non-Hodgkin lymphoma, variation between patients in the density of TAMs in their tumors is usually negatively correlated with survival. In follicular lymphoma, a landmark gene expression profiling study found that in patients treated with chemotherapy, prognostic gene signatures came not from neoplastic B cells but from T cells and MΦ, with respectively good and bad effects on outcome.Citation1 In our recent publication, we demonstrated that MDSC are also present in the tumor microenvironment of mouse lymphomas and exert immunosuppressive and protumoral effects.Citation2 Taken together, these reports suggest that tumor-associated myeloid cells in B-cell lymphomas exist in different functional states and may collectively promote tumor growth and confer poor prognosis.
Studies using syngeneic, transplantable mouse tumor models showed that MDSC are a major component of tumor-induced immune suppression, that allows malignant cells to escape from immune attack.Citation3 In mouse MDSC are a heterogeneous cell population that co-expresses Gr-1 and CD11b myeloid-cell lineage differentiation markers.Citation4 Due to lack of human homology of the Gr-1 gene, identification of MDSC in cancer patients relies on combination of a range of lineage markers; however, none of these markers is unique for MDSC. Studies confirmed that HLA-DR−/CD33+/CD11b+/CD14+ and/or CD15+ myeloid cells found in various cancer patients were immune suppressive and correlated with poor prognosisCitation5,6. It is speculated that novel agents targeting MDSC may ameliorate tumor-induced immune suppression and be combined with existing therapies, including chemotherapy, immunotherapies, and targeted therapies, potentially improving their efficacy without increasing toxicity. However, what hampers the current development of MDSC depletion therapy is the lack of a human MDSC-specific marker. Accordingly, discovery of such a therapeutic target has become a top priority in the field.
Using competitive biopanning with a peptide phage library on splenocytes freshly prepared from tumor-bearing mice, we overcame current limitations and identified two novel, mouse MDSC-specific peptides.Citation2 The peptides were further developed into therapeutic agents (peptibodies) that efficiently depleted splenic and intratumoral MDSC in tumor-bearing mice, but did not affect other proinflammatory cell types including dendritic cells, T, B, and NK lymphocytes, and immature myeloid cells in bone marrow, suggesting limited off-target activity. The peptibody treatment was associated with retardation of tumor growth in vivo highlighting the role of MDSC in tumorogenesis.Citation2 Proteomic analysis of cell surface membrane proteins precipitated by the peptibodies suggests that the lead candidate target on the surface of MDSC is S100 family proteins (S100A9/A8).Citation2 Through this proof-of-principle study, we established a technical platform of cell-specific marker discovery and a promising method to develop therapeutic reagents.
Specific immunotherapy against cancer has been a long-sought, yet-to-be-achieved goal. Optimization of the therapeutic efficacy of specific cancer immunotherapy, to a large extent, may depend on correction of immune suppressive mechanisms in the tumor microenvironment, which renders tumor-specific cytotoxic T cells dysfunctional. Recently, a controlled vaccine clinical trial in patients with follicular lymphoma validated the concept of specific immunotherapy. The variable regions of the clonal Ig receptor on the surface of malignant B-cells contain determinants that can themselves be recognized as antigens, termed idiotypes (Id). Early pilot studies had demonstrated the immunogenicity of human lymphoma Id proteins, including CD8+ T-cell responses against processed Id peptides and molecular remissions.Citation7 In the randomized controlled multi-center clinical trial,Citation8 patients with previously untreated advanced stage follicular lymphoma were treated with a standard chemotherapy. Those achieving complete remission were randomized at a ratio of 2:1 to receive Id-KLH plus GM-CSF or KLH plus GM-CSF (control). Of 234 enrolled patients, 177 achieved complete response and were subsequently randomized to receive either active or control vaccine. Of these, 117 maintained a complete remission for the 6 m rest period and received vaccine. 76 patients received Id-KLH plus GM-CSF and 41 patients received KLH plus GM-CSF. Study arms were balanced for International Prognostic Index and other relevant clinical factors. After a median follow-up of 56.6 mo (range 12.6–89.3 mo), median time to relapse after randomization for the Id-KLH/GM-CSF arm was 44.2 mo, vs. 30.6 mo for the control arm, suggesting benefit for this vaccine (p-value = 0.045; HR = 1.6). Even though this trial was not suitable for regulatory approval (because the control arm did not include rituximab, which became standard treatment for follicular lymphoma after this trial started), these results validated Id as a tumor rejection antigen in humans.
The major value of MDSC-depleting reagents, then, is that they may be combined with cancer vaccines or adoptive T-cell therapy to improve and optimize the clinical benefits of specific immunotherapy (Fig. 1). Agents capable of depleting multiple immunosuppressive cell types, such as Treg and TAMs, will also be required. At least in one recent study, suppression of MDSC was sufficient to eradicate metastatic tumors which were resistant to Treg and immune checkpoint blockade.Citation9 Such clinical reagents will allow us to design clinical studies using such combinatorial immunotherapy strategies to treat cancer patients.
Thus, future directions should be focused on developing clinical-grade reagents capable of depleting human MDSC. Obviously, S100 family proteins are one such candidate for developing monoclonal antibody therapy. This is also supported by the recent report of S100A9 on human MDSC isolated from patients with colon cancer.Citation10 Therefore, screening tumor-infiltrating human MDSC from cancer patients for cell surface expression of S100A9/A8 should be prioritized. Concurrently, the live cell-based peptide phage display library technology used to successfully identify S100 family proteins as cell surface targets on murine MDSC could be applied to screen human MDSCs for specific binding peptide ligands to identify novel markers that may be distinct from S100A9/A8 and that can be targeted. Finding such a marker will enable development of diagnostic and depleting therapeutic reagents specific for MDSC which are positioned to enter first-in-human clinical trials in patients.
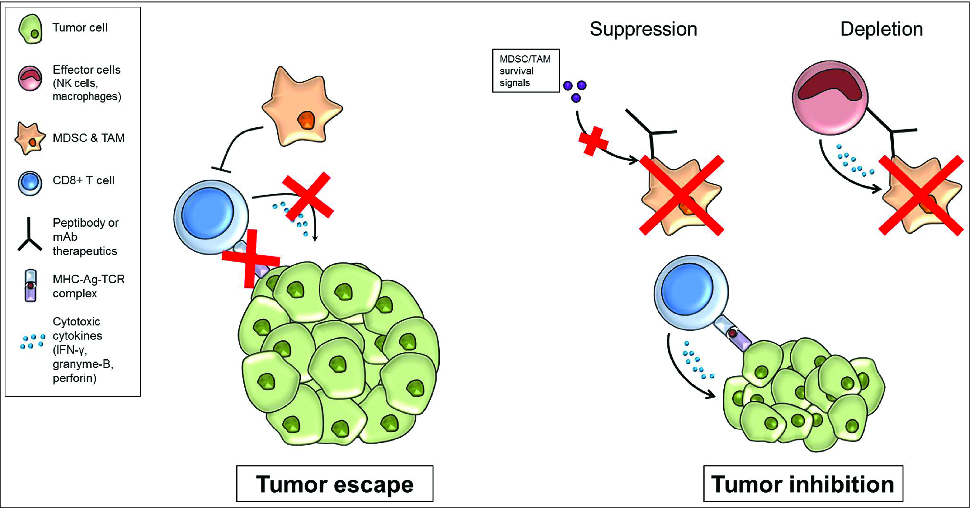
Figure 1. Targeting tumor associated myeloid cells for release of immunosuppression. In the tumor microenvironment, tumor associated myeloid cells can impair antitumor immunity though inhibitory effects on immune effector cells, including CD8+ T cells. Therapeutic peptibodies or monoclonal antibodies that specifically target tumor-associated myeloid cells can suppress the function of these cells and/or deplete these cells from the tumor microenvironment. This strategy has the potential to correct tumor-induced immunosuppression, and may be combined with other cancer treatments including immunotherapy or chemotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by NCI SPORE grant P50CA136411 (LWK).
References
- Dave S.S, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, Fisher RI, Braziel RM, Rimsza LM, Grogan TM et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med 2004 351, 2159-69; PMID:15548776; http://dx.doi.org/10.1056/NEJMoa041869
- Qin, H, Lerman B, Sakamaki I, Wei G, Cha SC, Rao SS, Qian J, Hailemichael Y, Nurieva R, Dwyer KC et al. Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med 2014 20, 676-81; PMID:24859530; http://dx.doi.org/10.1038/nm.3560
- Gabrilovich DI , Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009 9, 162-74; PMID:19197294; http://dx.doi.org/10.1038/nri2506
- Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol 2010 22, 238-44; PMID:20171075; http://dx.doi.org/10.1016/j.coi.2010.01.021
- Gorgun GT, Whitehill G, Anderson JL, Hideshima T, Maguire C, Laubach J, Raje N, Munshi NC, Richardson PG, Anderson KC. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood 2013 121, 2975-87; PMID:23321256; http://dx.doi.org/10.1182/blood-2012-08-448548
- Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 2007 25, 2546-53; PMID:17577033; http://dx.doi.org/10.1200/JCO.2006.08.5829
- Bendandi M, Gocke CD, Kobrin CB, Benko FA, Sternas LA, Pennington R, Watson TM, Reynolds CW, Gause BL, Duffey PL et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med 1999 5, 1171-7; PMID:10502821; http://dx.doi.org/10.1038/13928
- Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, Winter JN, Flowers CR, Nikcevich DA, Sotomayor EM et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol 2011 29, 2787-94; PMID:21632504; http://dx.doi.org/10.1200/JCO.2010.33.3005
- Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, Diaz LA Jr, Papadopoulos N, Kinzler KW, Vogelstein B et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A 2014 111, 11774-9; PMID:25071169; http://dx.doi.org/10.1073/pnas.1410626111
- Zhao F, Hoechst B, Duffy A, Gamrekelashvili J, Fioravanti S, Manns MP, Greten TF, Korangy F. S100A9 a new marker for monocytic human myeloid-derived suppressor cells. Immunology 2012 136, 176-83; PMID:22304731; http://dx.doi.org/10.1111/j.1365-2567.2012.03566.x
