Abstract
Although inflammation and metastasis are two well-known hallmarks of malignant disease, the relationship between inflammation and lymphatic metastasis is an unexplored research area. We recently elucidated a sophisticated mechanism by which TNFα-induced tumor inflammation conscripts macrophage-mediated VEGF-C-VEGFR3 signaling in lymphangiogenesis and metastasis.
Solid tumors are considered to be inflamed pathological tissues that, as such, contain exceptionally high numbers of inflammatory cells including macrophages, leukocytes and various immune cells. While the primary mission of these inflammatory cells is to eliminate malignant cells, these immune cells are often manipulated by malignant cells to support tumor growth, invasion, and metastasis. Malignant and host cells in the tumor microenvironment produce various cytokines and growth factors to recruit inflammatory cells from the circulation.Citation1 Alternatively, these cytokines and growth factors could also stimulate migration and proliferation of resident inflammatory cells, such as macrophages, in a given tissue to augment infiltration in tumors. Inflammation and lymphangiogenesis are cohesively coupled processes simply because of the lymph node-mediated immune surveillance to eliminate pathogens. The same interaction between inflammation and lymphangiogenesis likely also exists in tumor tissues.
In one of our recent studies, we show that tumor necrosis factor α (TNFα), a potent inflammatory cytokine, robustly affects lymphangiogenesis in an in vivo mouse model of corneal lymphangiogenesis.Citation2,3 Notably, deletion of tumor necrosis factor receptor 1 (Tnfr1) in mice completely ablates lymphangiogenesis indicating the TNFR1-triggered signaling is essential for TNFα-induced lymphangiogenesis. Consistent with its in vivo effect of lymphangiogenesis, TNFα also stimulates lymphatic endothelial cell (LEC) proliferation and migration through a TNFR1-, but not TNFR2-, dependent mechanism. These in vitro studies demonstrate that TNFα is a direct lymphangiogenic cytokine. Despite these in vitro and in vivo findings, treatment of mice with an anti-vascular endothelial growth factor receptor 3 VEGFR3 neutralizing antibody (VEGFR3 blockade) completely blocked TNFα-induced lymphangiogenesis in the mouse cornea.Citation2 These findings are surprising because VEGFR3 blockade does not inhibit TNFα-induced LEC activity in vitro. In-depth examination of lymphatic vessel structures show that the VEGFR3 blockade-treated TNFα-induced lymphatic vessels lack LEC tips that are extensions of filopodia of migrating LECs at the leading edge of lymphatics. These findings suggest to us that the VEGFR3-mediated signals are essential for LEC tip cell formation of growing lymphatics. If so, what are ligands for VEGFR3 and what are the cellular sources for these ligands? VEGF-C and VEGF-D are the 2 known VEGFR3-binding ligands and therefore are likely to activate VEGFR3.Citation4 Indeed, the expression level of VEGF-C in TNFα-stimulated macrophages is markedly elevated. Similarly, tumor-associated macrophages (TAMs) isolated from TNFα tumors also express high levels of VEGF-C. Moreover, deletion of Tnfr1 in mice completely ablates upregulation of VEGF-C expression in TAMs, indicating that the TNFR1-mediated signaling is essential for VEGF-C expression in macrophages.
One of the surprising findings in this study is that inhibition of VEGFR3 signaling pathways by a neutralizing antibody blocked tumor lymphatic vessel development without completely abrogating TNFα-induced LEC proliferation. Consequently, continuous LEC proliferation without guidance by the VEGF-C-VEGFR3 signaling leads to accumulation of LECs that form mushroom-like shaped primitive lymphatics that most likely lack drainage functions. These data show a dual function of TNFα-TNFR1 signaling in modulating VEGF-C-VEGFR3-dependent and -independent lymphangiogenesis.
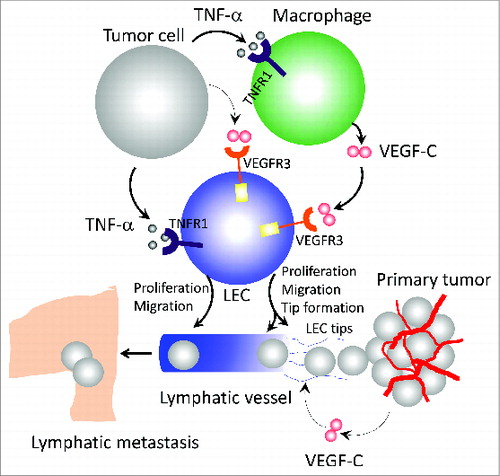
Figure 1. Mechanisms of TNFα-induced lymphangiogenesis and metastasis. Tumor cell-derived tumor necrosis factor α (TNFα) directly acts on lymphatic endothelial cells (LECs) to induce their proliferation and migration through interaction with TNF receptor 1 (TNFR1) receptor. In the tumor microenvironment, TNFα also recruits and activates macrophages that produce a high level of vascular endothelial growth factor C (VEGF-C). Through activation of VEGFR3, VEGF-C induces LEC tip cell formation, which is an essential process guiding lymphatic development. Additionally, the VEGF-C-VEGFR3 signaling pathway significantly contributes to LEC proliferation and migration. In primary tumors, the intimate interaction between TNFα-TNFR1 and VEGF-C-VEGFR3 signaling pathways in macrophages and LECs leads to ingrowth of tumor lymphatics and lymph node metastasis.
TNFα is known to stimulate tumor angiogenesis,Citation5 which is essential for tumor growth and blood stream metastasis. However, TNFα in facilitating lymphatic metastasis remains completely unknown. Our recent work shows that TNFα-induced lymphatics in tumor tissues are functional and drain tumor cells to sentinel lymph nodes. In several mouse tumor models, we demonstrate TNFα is a potent causal factor underlying lymphatic metastatic spread. Consistent with the notion of TAM-derived VEGF-C, depletion of macrophages in mice completely inhibits TNFα-induced lymphangiogensis and lymphatic metastasis (Fig. 1). Similarly, deletion of the Tnfr1 gene or treatment with VEGFR3 blockade in mice also abrogates TNFα-induced lymphatic metastasis. These findings suggest that targeting TNFα-TNFR1 signaling is a potential therapeutic approach for the treatment of cancer invasion and metastasis. Since the VEGF-C-VEGFR3 signaling pathway is essential for LEC tip cell formation and for guidance of lymphatic vessel development, inhibition of this signaling cascade would also block TNFα-promoted lymphatic metastasis. This surprising discovery led to an important question: Would the VEGF-C-VEGFR3-LEC-tip axis also be required for lymphatic vessel development in response to other lymphangiogenic factors? Another recent study of ours focused on fibroblast growth factor 2 (FGF-2)-induced lymphangiogenesis also supported this conclusion.Citation6 In the FGF-2 study, we provide another example of the VEGF-C-VEGFR3-dependent lymphangiogenesis in collaboration with FGF-2 in promoting cancer metastasis. If these findings could be further generalized, it would be reasonable to speculate that targeting of the VEGF-C-VEGFR3 signaling could, conceptually, universally block tumor lymphangiogenesis and metastasis. Unfortunately, several VEGF-C-VEGFR3-independent lymphangiogenic factors have also been described and these signals may thus be anticipated to induce cancer metastasis through alternative mechanisms.Citation7-10 Taking into consideration the complexities of the tumor microenvironment, it is perhaps understandable that blocking a single signaling pathway is unlikely to represent an effective therapeutic approach for all cancers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interests were disclosed.
Funding
This work is supported through research grants from the Swedish Research Council, the Swedish Cancer Foundation, the Karolinska Institute Foundation, the Karolinska Institute distinguished professor award, the Torsten Soderbergs foundation, and the European Research Council (ERC) advanced grant ANGIOFAT (Project no 250021), the NOVO Nordisk Foundation, the Advanced grant from the NOVO Nordisk foundation, and the Royal Alice Wallenberg foundation.
References
- Cao Y. Tumor angiogenesis and molecular targets for therapy. Front Biosci 2009; 14:3962-73; PMID:19273326; http://dx.doi.org/10.2741/3504
- Ji H, Cao R, Yang Y, Zhang Y, Iwamoto H, Lim S, Nakamura M, Andersson P, Wang J, Sun Y, et al. TNFR1 mediates TNF-α-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. Nat Commun 2014; 5:4944; PMID:25229256; http://dx.doi.org/10.1038/ncomms5944
- Cao R, Lim S, Ji H, Zhang Y, Yang Y, Honek J, Hedlund EM, Cao Y. Mouse corneal lymphangiogenesis model. Nat Protoc 2011; 6:817-26; PMID:21637201; http://dx.doi.org/10.1038/nprot.2011.359
- Cao Y, Zhong W. Tumor-derived lymphangiogenic factors and lymphatic metastasis. Biomed Pharmacothe 2007; 61:534-9; PMID:17904785; http://dx.doi.org/10.1016/j.biopha.2007.08.009
- Montrucchio G, Lupia E, Battaglia E, Passerini G, Bussolino F, Emanuelli G, Camussi G. Tumor necrosis factor α-induced angiogenesis depends on in situ platelet-activating factor biosynthesis. J Exp Med 1994; 180:377-82; PMID:7516414; http://dx.doi.org/10.1084/jem.180.1.377
- Cao R, Ji H, Feng N, Zhang Y, Yang X, Andersson P, Sun Y, Tritsaris K, Hansen AJ, Dissing S, et al. Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc Natl Acad Sci U S A 2012; 109:15894-9; PMID:22967508
- Cao R, Bjorndahl MA, Gallego MI, Chen S, Religa P, Hansen AJ, Cao Y. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood 2006; 107:3531-6; PMID:16424394; http://dx.doi.org/10.1182/blood-2005-06-2538.
- Cao Y. Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer 2005; 5:735-43; PMID:16079909; http://dx.doi.org/10.1038/nrc1693
- Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell 2004; 6:333-45; PMID:15488757; http://dx.doi.org/10.1016/j.ccr.2004.08.034
- Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ Res 2004; 94:664-70; PMID:14739162; http://dx.doi.org/10.1161/01.RES.0000118600.91698.BB
