Abstract
Natural Killer (NK) cells are a key component of tumor immunosurveillance and thus play an important role in rituximab-dependent killing of lymphoma cells via an antibody-dependent cellular cytotoxicity (ADCC) mechanism. We evaluated the phenotypic and functional assets of peripheral blood NK cell subsets in 32 newly-diagnosed diffuse large B-cell lymphoma (DLBCL) patients and in 27 healthy controls. We further monitored long-term modifications of patient NK cells for up to 12 months after rituximab-based immunochemotherapy. At diagnosis, patients showed a higher percentage of CD56dim and CD16+ NK cells, and a higher frequency of GrzB+ cells in CD56dim, CD56bright, and CD16+ NK cell subsets than healthy controls. Conversely, DLBCL NK cell killing and interferon γ (IFNγ) production capability were comparable to those derived from healthy subjects. Notably, NK cells from refractory/relapsed patients exhibited a lower “natural” cytotoxicity. A marked and prolonged therapy-induced reduction of both “natural” and CD16-dependent NK cytotoxic activities was accompanied by the down-modulation of CD16 and NKG2D activating receptors, particularly in the CD56dim subset. However, reduced NK cell killing was not associated with defective lytic granule content or IFNγ production capability. This study firstly describes tumor-associated and therapy-induced alterations of the systemic NK cell compartment in DLBCL patients. As these alterations may negatively impact rituximab-based therapy efficacy, our work may provide useful information for improving immunochemotherapeutic strategies.
Introduction
Natural killer (NK) cells are an essential component of innate immunity and surveillance against malignancies. Their anti-leukemic potential and ability to regulate normal and neoplastic haematopoietic precursors has recently raised considerable interest. Their effector functions also represent a crucial factor in determining the response to anticancer therapy.Citation1-3 Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive B-cell lymphoma, with heterogeneous genetic, phenotypic, and clinical features.Citation4-6 The immunochemotherapeutic association of anti-CD20 monoclonal antibody (mAb) rituximab, with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has significantly improved the survival of DLBCL patients, and represents the current standard-of-care with an effective outcome in 60–80% of cases.Citation6,7 NK cell-mediated, CD16-dependent antibody-dependent cytotoxicity (ADCC) likely plays a major role in determining the efficacy of rituximab-based therapy.Citation8-10 Indeed, structural and/or functional characteristics of CD16 receptor that affect anti-CD20 mAb-mediated ADCC in vitro,Citation11-13 also impact on NK cell activation statusCitation14 and patient outcome in vivo.Citation10,15-18
NK cells are resident in peripheral blood and in some lymphoid and non-lymphoid organs, and are promptly recruited to tumor sites.Citation19 The 2 main NK cell subsets in the periphery are thought to represent sequentially ordered differentiation steps. CD56dim NK cells with high cytotoxic potential account for the vast majority of peripheral blood NK cells, are primarily CD16+ (FcγRIIIA low-affinity Fc receptor+), and most likely represent the final maturation step. CD56bright NK cells also home to secondary lymphoid organs, bear low or absent CD16, display higher capability to produce cytokines and lower cytotoxic activity, and are thought to represent the immediately preceding differentiation stage.Citation20-22
Antitumor NK cell activation and effector functions are finely regulated by the balance between opposing signaling pathways initiated by activating and inhibitory, MHC Class I-specific, receptors.Citation23-26 While CD16 is responsible for ADCC against IgG-coated cells,Citation27,28 several activating receptors cooperatively trigger natural cytotoxicity against tumor cells.Citation23-26 Among these, killer cell lectin-like receptor K1 (KLRK1, better known as NKG2D) plays an important role in the immunosurveillance against human and mouse model lymphoma, by recognizing stress-induced ligands belonging to the MIC (MICA and MICB) and ULBP (ULBP1–6) families.Citation29-35 Expression and/or functional capability of activating receptors is orderly acquired during NK cell differentiationCitation20-22 and can be modulated upon activation and/or ligand engagement.Citation29-37 Activated NK cells also rapidly secrete a variety of cytokines and chemokines, such as interferon γ (IFNγ), that amplify the recruitment and activation of other participants to the antitumor response.Citation19,38
Although peripheral blood lymphopenia at diagnosis and during treatment has been widely reported as a negative prognostic factor in DLBCL,Citation39-41 this phenomenon, a reflection of host systemic immunity, remains poorly characterized. Few studies have addressed the characterization of the NK cell compartment in DLBCL patients. A notable study suggested the relevance of a low NK cell count as an important prognostic factor in the pre-rituximab era,Citation42 while a recent report described the concomitant defect of CD16 expression and ADCC in NK cells of a small group of DLBCL patients at diagnosis.Citation43 While it has been shown that rituximab infusion rapidly (within hours) induces changes in the NK cell phenotype, activation status, and ADCC activity,Citation14,44 information on the long-term effects of rituximab-based therapy on NK cells are still mostly lacking.
In this prospective study, we have longitudinally assessed the phenotypic and functional long-term dynamics of the peripheral blood NK cell compartment, in a cohort of newly diagnosed DLBCL patients undergoing rituximab-based immunochemotherapy. As the phenotypic and functional profile of the host's NK cells might significantly contribute to the success of immunochemotherapy strategies, the novel information provided by this study may prove useful for the development of focused and innovative therapeutic approaches.
Results
Phenotypic and functional assets of the peripheral NK cell compartment in DLBCL patients at diagnosis
We initially assessed the profile of the peripheral blood (PB) NK cell compartment in a cohort of newly diagnosed DLBCL patients and age- and sex-matched healthy controls. Although patients showed significantly lower total lymphocytes counts (), the absolute numbers of total CD3-CD56+ NK cells and of CD56dim, CD56bright, and CD16+ subsets were comparable between patients and healthy subjects (). However, the relative frequency of total CD3–CD56+ NK cells, and of CD56dim and CD16+, but not of CD56bright, NK cell subsets, were significantly higher in patients than in controls ().
Figure 1. Phenotypic and functional assessment of the peripheral NK cell compartment in DLBCL patients at diagnosis. Peripheral blood mononuclear cells PBMCs of newly diagnosed diffuse large B cell lymphoma (DLBCL) patients (gray boxes) and healthy age- and sex-matched controls (empty boxes) were analyzed for: (A) total lymphocyte counts, (B) natural killer (NK) cell subset absolute counts, and (C) NK cell subsets as a percentage of lymphocytes. (D) The percentage of Granzyme B-positive (GrzB+) cells was calculated within each NK cell subset. (E) “Natural” (anti-K562) and CD16-dependent (anti-P815 + anti-CD16 monoclonal antibody [mAb]) cytotoxic activities are expressed in lytic units at 10% cytotoxicity/106 PBMC. (F) The percentage of interferon γ (IFNγ)-producing cells was calculated on gated CD3-CD56+ NK cells. Bars represent median gated CD3-CD56+ NK cells. Bars represent median and 10–90 percentile; dots represent outliers. *P <0.05, **P ≤ 0.01, ***P < 0.0005, ****P = 0.000001.
![Figure 1. Phenotypic and functional assessment of the peripheral NK cell compartment in DLBCL patients at diagnosis. Peripheral blood mononuclear cells PBMCs of newly diagnosed diffuse large B cell lymphoma (DLBCL) patients (gray boxes) and healthy age- and sex-matched controls (empty boxes) were analyzed for: (A) total lymphocyte counts, (B) natural killer (NK) cell subset absolute counts, and (C) NK cell subsets as a percentage of lymphocytes. (D) The percentage of Granzyme B-positive (GrzB+) cells was calculated within each NK cell subset. (E) “Natural” (anti-K562) and CD16-dependent (anti-P815 + anti-CD16 monoclonal antibody [mAb]) cytotoxic activities are expressed in lytic units at 10% cytotoxicity/106 PBMC. (F) The percentage of interferon γ (IFNγ)-producing cells was calculated on gated CD3-CD56+ NK cells. Bars represent median gated CD3-CD56+ NK cells. Bars represent median and 10–90 percentile; dots represent outliers. *P <0.05, **P ≤ 0.01, ***P < 0.0005, ****P = 0.000001.](/cms/asset/57f3388c-477c-4369-885f-05b7bd5b73de/koni_a_990773_f0001_b.gif)
NK cells are endowed with cytotoxic activity and with the capability to promptly produce cytokines and chemokines.Citation19,38 A considerably higher frequency of cells expressing the cytotoxic granule marker Granzyme B (GrzB) characterized CD56dim, CD56bright and CD16+ NK cell populations in patients’ PBMC (); nevertherless, either “natural” (anti-K562 erythroleukemia cell line) and CD16-dependent (anti-P815+anti-CD16 mAb) cytotoxic activities were comparable between patient and control-derived NK cells (). NK cell capability to produce IFNγ, as evaluated by the frequency of cytokine-producing cells upon short-term stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin, was also comparable between patients and controls ().
Taken altogether, these data indicate that the peripheral blood NK cell compartment of newly diagnosed DLBCL patients (time point 1 [T1]), although being quantitatively and functionally normal, shows a higher representativity over lymphocytes, and displays a higher cytotoxic potential.
Long-term dynamics of peripheral blood NK cell subsets in DLBCL patients undergoing rituximab-based immunochemotherapy
The absolute counts of CD3−CD56+ NK cells, as well as their CD56dim and CD56bright subsets, were transiently decreased at mid-therapy time point (T2), and had recovered by the end of therapy (T3, within one month after the last treatment course); the diminution was significant, as compared to either healthy controls () or pre-therapy samples (T1, Table S1A-Clink>). Interestingly, the absolute count of CD16-expressing CD3-CD56+ NK cells showed a marked and prolonged reduction, as it persisted till the end of therapy time point (T3), and had recovered by 3 months later (T4) (; Table S1D).
Figure 2. CD56dim and CD16+ NK cell absolute counts transiently decrease in DLBCL patients during immunochemotherapy. Peripheral blood mononuclear cells (PBMCs) of diffuse large B cell lymphoma (DLBCL) patients at different time points (T1-T6, gray boxes) and of healthy controls (HC, empty boxes) were analyzed for: (A-D) the absolute counts of total CD3-CD56+ natural killer (NK) cells and their subsets, obtained by combining complete blood counts and immunocytofluorimetric analysis; (E-H) the percentage of total CD3-CD56+ NK cells and their subsets within lymphocytes. Bars represent median and 10–90 percentile; dots represent outliers. *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P < 0.0005 vs. controls.
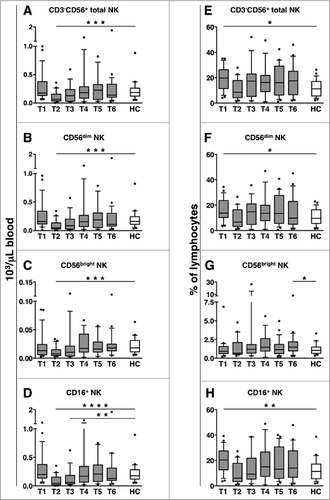
The percentage of total, CD56dim, and CD16-expressing NK cells (over lymphocytes), that were higher at diagnosis (T1), became comparable to controls from T2 till the end of the following observation period (12 months) (). CD56bright NK cells were slightly elevated only at 12 months after therapy (T6, ).
Altogether, these results show that while circulating CD56dim and CD56bright NK cell counts transiently decrease during therapy, the diminution of CD16-expressing NK cells is more prolonged.
Long-term dynamics of CD16 receptor expression on PB NK cells of DLBCL patients
Our findings suggest the occurrence of therapy-induced downregulation of CD16 receptor on NK cells in DLBCL patients. We next analyzed in depth the dynamics of CD16 expression on circulating NK cell subsets. Interestingly, the fraction of NK cells expressing CD16 receptor was markedly and significantly reduced at T3 (within one month upon therapy completion), with respect to healthy controls () or to pre-therapy levels (Table S2A-C). A significant diminution of CD16+ cells selectively occurred on CD56dim, and not on CD56bright NK cells (, respectively); however, CD16 receptor intensity (expressed as specific mean fluorescence intensity, MFI) was markedly reduced on CD56bright NK cells, at T3 (, respectively).
Figure 3. Long-term dynamics of CD16 expression on NK cell subsets in DLBCL patients upon immunochemotherapy. Peripheral blood mononuclear cells (PBMCs) of diffuse large B cell lymphoma (DLBCL) patients at different time points (T1-T6, gray boxes) and of healthy controls (HC, empty boxes) were analyzed for: (A) the percentage of CD16+ cells over CD3-CD56+ natural killer (NK) cells; (B-C) the percentage of CD16+ cells and (D-E) the specific mean fluorescence index (MFI) of CD16 receptor calculated within CD56dim (B, D) and CD56bright (C, E) NK cell subsets. Bars represent median and 10–90 percentile; dots represent outliers. *P < 0.05, ****P < 0.0005 vs. controls.
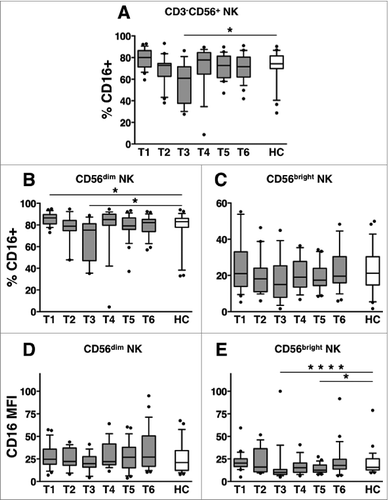
Taken altogether, these data suggest that immunochemotherapy induces the diminution of circulating CD16+ NK cells. This decrease: a) persists over one month after therapy completion; b) more deeply affects the CD56dim subset; and and c) is the combined result of diminished NK cell absolute counts, observed during therapy, and CD16 downregulation on NK cells, recorded after the completion of therapy.
Transient down-modulation of NKG2D activating receptor on NK cells in DLBCL patients undergoing immunochemotherapy
NKG2D is an important activating receptor for “natural” cytotoxicityCitation29-35 and also participates in CD16-dependent killing.Citation45,46 The frequency of NKG2D+ NK cells at diagnosis (T1) was comparable between DLBCL and healthy controls. However, it transiently decreased during therapy (T2), with the diminution more persistently observed in the CD16+ fraction (sustained until 3 months after the end of therapy, T4, ). NKG2D downregulation selectively occurred on CD56dim NK cells (i), notably, affecting only the CD16+ fraction (ii-iii). Conversely, NKG2D expression on either CD16+ or CD16- CD56bright NK cells did not vary significantly over the entire follow-up period (i-iii).
Figure 4. Long-term dynamics of NKG2D activating receptor on NK cell subsets in DLBCL patients upon immunochemotherapy. Peripheral blood mononuclear cells (PBMCs) of diffuse large B cell lymphoma (DLBCL) patients at different time points (T1-T6, gray boxes) and of healthy controls (HC, empty boxes) were analyzed for the percentage of NKG2D+ cells in: total CD3−CD56+ NK cells (A), CD16+ NK cells (B), or within CD56dim (C) and CD56bright (D) NK cell subsets; the frequency of NKG2D+ cells was evaluated in each subset, as a whole (i), and in the CD16+ (ii) and CD16- (iii) fractions. Bars represent median and 10–90 percentile; dots represent outliers. *P < 0.05, **P < 0.01, ***P ≤ 0.001 vs. controls.
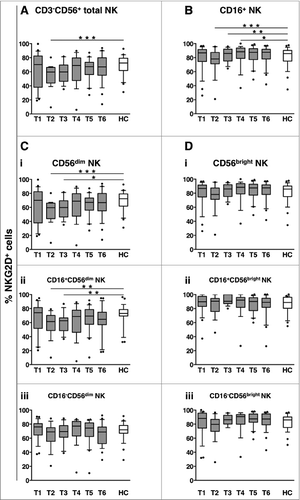
Soluble NKG2D ligands can induce NKG2D receptor downregulation in cancer patients.Citation29-33 However, plasma levels of the NKG2D ligands MICA, MICB, ULBP1 and ULBP2 did not differ significantly between pre-therapy (T1) DLBCL patients and healthy controls. MICB diminution was the only variation observed during therapy (T2) ( Fig. S1).
These results show that immunochemotherapy is associated with the down-modulation of NKG2D activating receptor on CD16+ CD56dim NK cells, and suggest that circulating NKG2D ligands are not involved in this phenomenon.
Long-term dynamics of “natural” and CD16-dependent cytotoxic activities of DLBCL PBMCs
The aforementioned data show that immunochemotherapy is associated with a prolonged downregulation of CD16 and NKG2D activating receptors on NK cells, particularly the highly lytic CD56dim subset.Citation20-22 Interestingly, both “natural” and CD16-dependent cytotoxic activities were strongly reduced during and up to one month after the end of therapy (T2 and T3, respectively), relative to either pre-therapy levels (Table S3A and B) or healthy controls (). NK cytotoxic activities returned to levels comparable to those of control NK cells by 3 months after therapy completion (T4).
Figure 5. Long-term dynamics of “natural” and CD16-dependent cytotoxic activities in peripheral blood of DLBCL patients. (A) “natural” (anti-K562) and (B) CD16-dependent (anti-P815 + anti-CD16 monoclonal antibody [mAb]) cytotoxic activities of peripheral blood mononuclear cells (PBMCs) of diffuse large B cell lymphoma (DLBCL) patients at different time points (T1-T6, gray boxes) and of healthy controls (HC, empty boxes); data are expressed in lytic units at 10% cytotoxicity/106 PBMC. Bars represent median and 10–90 percentile; dots represent outliers. ***P < 0.0001, ****P < 0.00005 vs. controls.
![Figure 5. Long-term dynamics of “natural” and CD16-dependent cytotoxic activities in peripheral blood of DLBCL patients. (A) “natural” (anti-K562) and (B) CD16-dependent (anti-P815 + anti-CD16 monoclonal antibody [mAb]) cytotoxic activities of peripheral blood mononuclear cells (PBMCs) of diffuse large B cell lymphoma (DLBCL) patients at different time points (T1-T6, gray boxes) and of healthy controls (HC, empty boxes); data are expressed in lytic units at 10% cytotoxicity/106 PBMC. Bars represent median and 10–90 percentile; dots represent outliers. ***P < 0.0001, ****P < 0.00005 vs. controls.](/cms/asset/0cd7f88c-1224-47af-84ce-6d1cb7e50d77/koni_a_990773_f0005_b.gif)
Taken together, our results show that immunochemotherapy-induced downregulation of CD16 and NKG2D activating receptors is temporally related to the impairment of both "natural" and CD16-dependent in vitro cytotoxic capability. In accordance, CD16-dependent cytotoxic activity of NK cells derived from individual patients at T3 significantly correlated with NKG2D expression on CD16+, but not on CD16- CD56dim NK cells (Fig. S2A-C).
Long-term dynamics of NK functional competence in DLBCL patients
The frequency of GrzB+ NK cells in patients remained significantly higher than in healthy controls till T4 and T5 (3 and 6 months after the end of therapy, respectively), in CD56dim and CD56bright NK cell subsets (). At variance, the percentage of IFNγ-producing NK cells was transiently elevated at T3, although comparable to NK cells derived from healthy controls at all the remaining time points ().
Figure 6. Long-term dynamics of NK cell functional competence in DLBCL patients. Peripheral blood mononuclear cells (PBMCs) of diffuse large B cell lymphoma (DLBCL) patients at different time points (T1-T6, gray boxes) and of healthy controls (HC, empty boxes) were analyzed for: the percentage of GrzB+ cells in (A) CD56dim and (B) CD56bright natural killer (NK) cell subsets. (C) The percentage of interferon γ (IFNγ)-producing cells was determined by intracellular staining and cytofluorimetric analysis, and calculated on gated CD3−CD56+ NK cells. Bars represent the median and 10–90 percentile; dots represent outliers. *P < 0.05, **P < 0.01, ***P < 0.00005, ****P < 0.000005 vs. controls.
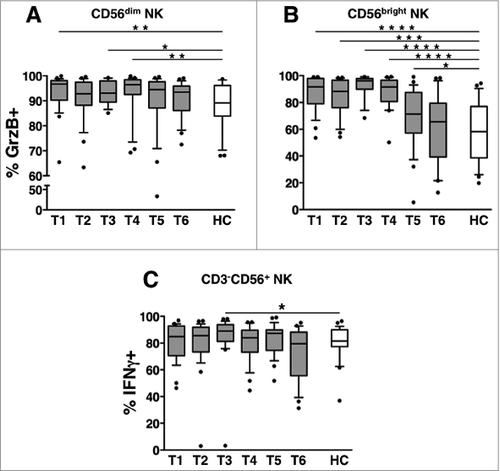
These results indicate that the expanded pool of cytotoxic granule-containing cells that characterized the DLBCL NK compartment at diagnosis recalcitrantly returned to normal levels, albeit well after the end of the treatment.
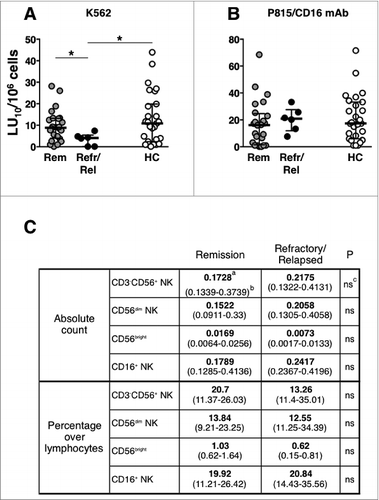
Refractory/early-relapsed patients show defective “natural” cytotoxicity at diagnosis
The analysis of PBMC cytotoxic activity, after patients stratification, shows that patients that were resistant to therapy, or that underwent early relapse (within one year after diagnosis), displayed a significantly lower “natural," but not CD16-dependent, cytotoxic activity at diagnosis, as compared either with controls, or with patients attaining remission lasting more than 2 y (). NK cell subset absolute counts and percentages were comparable between the 2 groups of patients (). Clinical parameters of remitting and resistant patients are reported in Table S4. This observation, although based on a limited number of subjects, suggests that defective NK cell functional activity at diagnosis may be associated with immunochemotherapy failure.
Figure 7. Defective “natural” cytotoxicity at diagnosis in refractory/early-relapse patients. (A) “natural” (anti-K562) and (B) CD16-dependent (anti-P815 + anti-CD16 mAb) cytotoxic activities of peripheral blood mononuclear cells (PBMCs) in diffuse large B cell lymphoma (DLBCL) patients that attained long-lasting remission (Rem, gray symbols, n = 25 ), or that were refractory/early-relapsed (Refr/Rel, black symbols, n = 6 ), and of healthy controls (HC, empty boxes, n = 27); data are expressed in lytic units at 10% cytotoxicity/106 PBMC. Bars represent median and 10–90 percentile. *P < 0.05. (C) Natural killer (NK) cell subset absolute counts and percentages in remitting and refractory/relapsed patients; amedian value; b(25–75 percentile range); cP value; ns, not significant.
Discussion
NK cells represent an important component of tumor immunosurveillance, and their effector functions may contribute significantly to the success of rituximab-based anti-lymphoma therapies.Citation8-10,15-18 Characterization of NK cell phenotypic and functional assets in newly diagnosed DLBCL patients has been scarce. Here, we first to report baseline and long-term analysis of the phenotypic and functional dynamics of peripheral blood NK cell subsets in DLBCL patients. Our data reveal tumor-associated as well as therapy-dependent alterations of the systemic NK cell compartment.
At diagnosis, the major NK cell subsets (CD56dim, CD56bright, CD16+), although in the normal range of peripheral blood absolute counts, had a higher frequency compared to age- and sex-matched healthy individuals. This can be explained by the decreased absolute counts of B and CD4+ T lymphocytes (MC Cox, G Palmieri et al., manuscript in preparation), which underlies the low absolute lymphocyte count observed in DLBCL patients. “Natural” and CD16-dependent cytotoxic activities were comparable between DLBCL and controls; however, refractory/early-relapsed patients showed a lower “natural” cytotoxic activity at diagnosis, as compared to either patients achieving durable remission or to healthy subjects.
Information on NK cell phenotypic and functional assets in newly diagnosed DLBCL patients has, so far, been scarce. In accordance with our data, an elevated NK cell frequency has been previously reported in patients.Citation47 Furthermore, higher absolute NK cell counts and “natural” cytotoxicity at diagnosis have both shown to positively correlate with response to therapy, either in the pre-rituximab era,Citation42,48-49 and more recently in immunochemotherapy-treated patients.Citation50 Our results, although obtained in a limited number of patients, confirm that defective basal “natural” killing activity associates with treatment failure, thus stressing the role of NK cell cytotoxic capability in the success of rituximab-based therapy. A recent report described defective NK cell degranulation as a proxy for "natural" and ADCC activities in a group of newly diagnosed DLBCL patients.Citation43 As these authors did not provide information on demographic and clinical parameters of patients and controls, and on therapy outcome, the discrepancy with our results may depend on unknown patient characteristics and/or differences in the experimental assays employed (NK cell degranulation vs. killing and ADCC vs. CD16-dependent cytotoxicity).
The frequency of cytotoxic granule-containing cells was markedly elevated in all major NK cell populations of DLBCL patients, whereas the percentage of IFNγ-producing cells was comparable to that of in healthy subjects. GrzB-containing cytotoxic granules are acquired during NK cell differentiation, and their biosynthesis is modulated by proinflammatory cytokines or members of the γc chain cytokine family.Citation20-22 The higher frequency of GrzB+ NK cells, particularly in the CD56bright subset, may thus underlie a state of chronic activation and/or a perturbed differentiation process, which may stem from a tumor-dependent altered microenvironment.
Rituximab-based immunochemotherapy induced a complex phenotypic and functional perturbation of peripheral blood NK compartment. A profound and protracted impairment of both “natural” and CD16-dependent in vitro cytotoxic activities was observed during therapy, it persisted well over one month after the end of treatment, and had recovered 3 months later; this was paralleled by a significant reduction of CD16 and NKG2D activating receptors, that more markedly affected the CD56dim NK cell subset. Interestingly, neither the lytic potential nor the capability to produce IFNγ were defective in DLBCL patients during the same time period. Moreover, the percentage of CD56dim and CD56bright subsets relative to total lymphocytes remained comparable to healthy subjects. However, NK absolute counts transiently diminished during therapy cycles, and returned to normal levels by one month after the end of therapy; this may be accounted by immunochemotherapy-induced NK cell margination and/or re-localization, or alternatively, drug-induced toxicity.
Taken together, these results suggest that the defective in vitro cytotoxic activity neither depends on a gross functional impairment, nor on the exhaustion of the lytic potential, nor on a reduced frequency of peripheral blood NK cells. Moreover, the short in vivo half-life of the chemotherapeutic drugs that were administered, together with the fast and reversible kinetics with which they partially affect in vitro and in vivo NK cell killing ability,Citation51-53 make it unlikely that the impaired NK cytotoxicity of DLBCL patients is attributable to the direct effect of cytotoxic drugs, especially for the end of treatment time point. In contrast, rituximab persists at detectable levels long after the end of therapy cycles.Citation10,53,54
The downregulation of CD16 and NKG2D activating NK cell receptors on the more lytic CD56dim subsetCitation19-22 suggests that defective recognition and/or activation mechanisms may rather play a role in the impairment of "natural" and CD16-dependent cytotoxic activities. The mechanisms underlying receptor down-modulation are presently unknown, although it has been widely reported that cell activation and/or ligand engagement can induce the modulation of NK activating receptors through several mechanisms.Citation29-37
CD16 down-modulation, the upregulation of activation markers, and enhanced ADCC capability occur in a matter of hours after rituximab infusion, with some of these effects depending on the presence of CD16 high affinity polymorphism;Citation14,44 however, the long-term effects of rituximab-based immunochemotherapy on CD16 expression levels have not been previously studied in DLBCL patients.
Immunosuppressive cytokines, as well as chronic contact with cell-associated or soluble ligands, may induce NKG2D receptor downregulation, as shown in vitro and in tumor patients in vivo.Citation29-34 However, plasma levels of several NKG2D ligands were not increased in DLBCL patients. Moreover, NKG2D dowregulation selectively affected the CD16+ CD56dim NK subset. This finding argues against a role for soluble, systemic mediators in NKG2D dowregulation. Our results suggest that a more effective, CD16-dependent, interaction with rituximab-coated, NKG2D ligand-expressing lymphoma cells may contribute to down-modulation of the NKG2D receptor. Indeed, DLBCL cells can constitutively express NKG2D ligands, that may be further enhanced by chemotherapy-triggered DNA damage response.Citation29-34,56,57
Overall, our results suggest that the therapy-driven, CD16-dependent, continuous stimulation leads to a prolonged NK cell phenotypic modulation and in vitro functional impairment. These alterations may be highly significant also for in vivo NK cell cytotoxic activity against DLBCL cells, and as such, may impact rituximab-based therapy efficacy. Although CD16 engagement by IgG-coated target cells is the sole trigger for ADCC,Citation26,27 its activity can be enhanced by NKG2D engagement;Citation45,46 moreover, it has been shown that NKG2D+ NK cells preferentially perform ADCC.Citation44 Indeed, the interplay between CD16 and NKG2D activating receptors is undergoing active investigation for therapeutic purposes.Citation58
Our study also revealed that the expanded pool of cytotoxic granule-containing NK cells in DLBCL patients at disease onset does normalize, albeit only after a prolonged interval following the completion of therapy. This delayed kinetics may reflect a slow NK cell turnover, possibly due to lymphopenia- and/or age-associated expansion of long-lived memory NK cells.Citation20-22,59 Additionally, a long-lasting perturbation in the NK differentiation/maturation process, either in the bone marrow and/or in the periphery, may also be involved in this process. This perturbation would be initially dependent on the presence of the tumor and afterward be sustained by chemotherapy-triggered alterations of the bone marrow microenvironment.Citation60,61
In sum, our pioneering and comprehensive analysis has shown that the peripheral NK cell compartment in DLBCL patients is affected by both tumor-associated alterations that normalize late after the achievement of clinical remission as well as immunochemotherapy-dependent quantitative, phenotypic and functional alterations that recover with distinct kinetics. Immunochemotherapy-induced alterations of NK cell functionality might partly explain why a dose-dense rituximab regime may not lead to improved outcome.Citation62,63
Innovative combination strategies aiming to potentiate NK cell effector functions and newly devised anti-CD20 antibodies to target malignant B cells are promising new tools in lymphoma therapy.Citation64-68 The novel information provided by our study may be relevant for the improvement of anticancer immunotherapy, as well as in other settings in which therapeutic efficacy relies on NK-dependent effector functions.
Materials and Methods
DLBCL patients and controls
All patients included in the prospective study were referred to the Hematology Unit of Sant'Andrea Hospital in Rome. Newly-diagnosed DLBCL patients with Stage II-IV or I bulky were included in the study; exclusion criteria included positivity for human immunodeficiency virus (HIV) or hepatitis (HBV/HCV), presence of opportunistic infections, central nervous system (CNS) involvement, leukemic dissemination, or previous chemotherapy-treated neoplasia. Demographic and clinical characteristics of patients and healthy controls are in . Patients with conventional DLBCL were treated with 6 cycles of R-CHOP-21 or R-CHOP-14 (high risk <60 years); primary mediastinal large B-cell lymphoma (PMLBCL) patients were treated with 12 cycles of RMACOPBCitation69 and consolidation radiotherapy. In R-CHOP-treated, rituximab was administered at days 0 and +7 of cycle 1, and with chemotherapy cycles thereafter. The 8th and last rituximab infusion was administered 25-30 d after the last course of immunochemotherapy.
Table 1. Demographic and clinical characteristics of DLBCL patients and controls
Patients were analyzed at diagnosis (T1), at mid-therapy (between 2nd and 3rd cycle of R-CHOP, and between 6th and 7th week of RMACOPB, T2), within one month after immunochemotherapy completion and immediately before last rituximab infusion (T3), at 3 (T4), 6 (T5), and 12 (T6) months after the end of immunochemotherapy. Patients that were considered to be non-responders after mid-treatment restaging,Citation70 or that relapsed during the study period, were not analyzed further.
The study was approved by our Institutional Review Board and was conducted in accordance with the regulations of health information protection policies, and with the Declaration of Helsinki. All patients and controls gave informed consent to the study.
PBMC isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood samples by Lymphoprep (Cedarlane, Ontario, Canada) density gradient centrifugation. PBMCs were immediately used for cytotoxicity assay, and stored at −80°C in 10% dimethyl sulfoxide (DMSO; Sigma Aldrich, St. Louis, USA) and 90% fetal calf serum (FCS; Euroclone, Milan, Italy), for phenotypic and functional analyses. Cells were rapidly thawed at 37°C, washed, and rested overnight in RPMI 1640 medium supplemented with 10% FCS and 1% glutamine (all from Euroclone; complete medium), at 37°C in 5% CO2 atmosphere, prior to stimulation, immunostaining and cytofluorimetric analysis.
Evaluation of intracellular IFNγ
PBMCs were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) and 0.5 μg/mL ionomycin at 37 °C for 6h in complete medium, in the presence of the intracellular trafficking inhibitors Monensin (50 μM, added at the beginning of stimulation) and Brefeldin A (10 μg/mL, added after 1h) (all from Sigma-Aldrich Srl, Milan, Italy). Cell samples were then fixed, permeabilized, and stained with phycoerythin (PE)-conjugated anti-IFNγ antibody or isotype control mAb (both from BD Biosciences, Milan, Italy).
Immunostaining procedures and cytofluorimetric analysis
NK cell subsets were identified by a combination of physical parameters and surface staining with optimal dilutions of fluorochrome-conjugated mAbs (anti-CD3 Alexafluor488, anti-CD16 PE-Cy7, anti-CD56 APC, all from BD Biosciences), for 30 min at 4°C. The values of complete blood counts and cytofluorimetric analysis percentages were used to calculate the absolute number of lymphocyte subsets.
For intracellular staining, cell samples were stained for surface antigens, washed with phosphate-buffered saline, fixed with 2% paraformaldehyde (Sigma-Aldrich) for 20 min, permeabilized with 0.5% saponin (Sigma-Aldrich) in 1% FCS for 30 min, and stained with PE-conjugated anti-IFNγ or anti-Granzyme B (Enzo Life Science, Inc., USA) antibodies, or isotype control mAb, in the presence of 0.5% saponin/1% FCS.
All samples were analyzed with a FACScalibur (BD Biosciences) flow cytometer, using CellQuest Pro (BD Biosciences) and FlowJo v9.3.2 (Treestar, Ashland, OR) data analysis software. Lymphocytes were defined by gating on forward and side scatter physical parameters. The threshold for antigen positivity was set on the basis of a matched isotype control mAb-stained sample, whose positivity never exceeded 0.5% of gated events.
Cytotoxicity assay
“Natural” (anti-K562 erythroleukemia cell line) and CD16-dependent (anti-P815 murine mastocytoma cell line + anti-CD16 mAb) cytotoxic activities were measured with a 4 h 51Cr-release assay, as previously described.Citation71 Briefly, K562 and P815 target cells were labeled with 51Cr (PerkinElmer, MA, USA) (100 μCi/1 × 106 cells) for 75 min at 37°C. Serial dilutions of PBMC, as effector cells, were plated in U-bottom 96-well plates in complete medium supplemented with 10 mM Hepes (Sigma-Aldrich), together with 51Cr-labeled target cells (5 × 103 cells/well), in the absence (K562) or in the presence (P815) of optimal concentration of anti-CD16 mAb (B73.1 clone, kindly provided by Dr G. Trinchieri, Cancer and Inflammation Program, NCI, Frederick, USA). After 4 h incubation at 37°C, released radioactivity in 100 μL of supernatant was counted with a Cobra Gamma Counter (Packard-PerkinElmer). Percent specific lysis was calculated according to the formula: % specific lysis = (experimental release cpm - spontaneous release cpm)/(maximum release cpm - spontaneous release cpm) × 100. Spontaneous release never exceeded 5% of total release. Basal cytotoxicity against P815 cell line (in the presence of anti-CD56 mAb, as isotype control) never exceeded spontaneous release (data not shown).
Cytotoxicity is expressed in lytic units at 10% cytotoxicity/million PBMC (LU10/106), calculated using D. Coggin's software. One lytic unit is defined as the number of effector cells necessary to lyse 10% of target cells.
Evaluation of soluble NKG2D ligands
Enzyme-linked immunosorbent assays (ELISA) to detect soluble MICA, MICB and ULBP1 were obtained from R&D Systems (Minneapolis, MN, USA), and performed using 2 μg/mL anti-MICA capture mAb (AMO1; BAMOMAB, Germany) essentially as previously describedCitation72. Soluble ULBP2 was detected as previously described.Citation73 Absorbance values of triplicate samples were obtained by subtracting readings at 540 nm from readings at 450 nm. Net absorbance was obtained by subtracting the reagent blank absorbance. Before the assay, plasma samples were diluted in PBS containing 0.1% Triton X-100 (vol/vol), and incubated for 30 min at 37°C.
Statistical analysis
Healthy controls and patients were compared with the 2-tailed Mann-Whitney U test. Patients at different time points were compared with the Wilcoxon signed rank test. Pearson correlation test was employed for correlation analysis. Statistical analysis was performed with PRISM v.6 (GraphPad Software, San Diego, CA, USA) and SPSS v.20 (IBM Italia SpA, Segrate, MI, Italy) softwares. P values <0.05 (2-sided) were considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
990773_Supplementary_Materials.zip
Download Zip (685.5 KB)Funding
This work was partially supported by grants from AIRC (A.S.), MIUR (G.P.), Onlus Sant’Andrea (M.C.C.).
References
- Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood 1990; 76:2421-38; PMID:2265240
- Knorr DA, Bachanova V, Verneris MR, Miller JS. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin Immunol 2014; 26:161-72; PMID:24618042; http://dx.doi.org/10.1016/j.smim.2014.02.002
- Chretien AS, Le Roy A, Vey N, Prebet T, Blaise D, Fauriat C, Olive D. Cancer-induced alterations of NK-mediated target recognition: current and investigational pharmacological strategies aiming at restoring NK-mediated anti-tumor activity. Front Immunol 2014; 5:122; PMID:24715892; http://dx.doi.org/10.3389/fimmu.2014.00122
- The Non-Hodgkin's Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. Blood 1997; 89:3909-18; PMID:9166827
- Pasqualucci L. The genetic basis of diffuse large B-cell lymphoma. Curr Opin Hematol 2013; 20:336-44; PMID:23673341; http://dx.doi.org/10.1097/MOH.0b013e3283623d7f
- Martelli M, Ferreri AJ, Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol 2013; 87:146-71; PMID:23375551; http://dx.doi.org/10.1016/j.critrevonc.2012.12.009
- Molina A. A decade of rituximab: improving survival outcomes in non-Hodgkin's lymphoma. Annu Rev Med 2008; 59:237-50; PMID:18186705; http://dx.doi.org/10.1146/annurev.med.59.060906.220345
- Boross P, Leusen JH. Mechanisms of action of CD20 antibodies. Am J Cancer Res 2012; 2:676-90; PMID:23226614
- Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol 2007; 44:3823-37; PMID:17768100; http://dx.doi.org/10.1016/j.molimm.2007.06.151
- Weiner GJ. Rituximab: mechanism of action. Semin Hematol 2010; 47:115-23; PMID:20350658; http://dx.doi.org/10.1053/j.seminhematol.2010.01.011
- Dall'Ozzo S, Tartas S, Paintaud G, Cartron G, Colombat P, Bardos P, Watier H, Thibault G. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res 2004; 64:4664-69; PMID:15231679; http://dx.doi.org/10.1158/0008-5472.CAN-03-2862
- Bowles JA, Wang SY, Link BK, Allan B, Beuerlein G, Campbell MA, Marquis D, Ondek B, Wooldridge JE, Smith BJ, et al. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood 2006; 108:2648-54; PMID:16825493; http://dx.doi.org/10.1182/blood-2006-04-020057
- Hatjiharissi E, Xu L, Santos DD, Hunter ZR, Ciccarelli BT, Verselis S, Modica M, Cao Y, Manning RJ, Leleu X, et al. Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the Fc{gamma}RIIIa-158 V/V and V/F polymorphism. Blood 2007; 110:2561-64; PMID:17475906; http://dx.doi.org/10.1182/blood-2007-01-070656
- Veeramani S, Wang SY, Dahle C, Blackwell S, Jacobus L, Knutson T, Button A, Link BK, Weiner GJ. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood 2011; 118:3347-49; PMID:21768303; http://dx.doi.org/10.1182/blood-2011-05-351411
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002; 99:754-58; PMID:11806974; http://dx.doi.org/10.1182/blood.V99.3.754
- Kim DH, Jung HD, Kim JG, Lee JJ, Yang DH, Park YH, Do YR, Shin HJ, Kim MK, Hyun MS, et al. FCGR3A gene polymorphisms may correlate with response to frontline R-CHOP therapy for diffuse large B-cell lymphoma. Blood 2006; 108:2720-25; PMID:16609067; http://dx.doi.org/10.1182/blood-2006-01-009480
- de Haij S, Jansen JH, Boross P, Beurskens FJ, Bakema JE, Bos DL, Martens A, Verbeek JS, Parren PW, van de Winkel JG, et al. In vivo cytotoxicity of type I CD20 antibodies critically depends on Fc receptor ITAM signaling. Cancer Res 2010; 70:3209-17; PMID:20354182; http://dx.doi.org/10.1158/0008-5472.CAN-09-4109
- Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol 2013; 4:76; PMID:23543707; http://dx.doi.org/10.3389/fimmu.2013.00076
- Trinchieri G. Biology of natural killer cells. Adv Immunol 1989; 47:187-376; PMID:2683611; http://dx.doi.org/10.1016/S0065-2776(08)60664-1
- Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol 2007; 7:703-14; PMID:17717540; http://dx.doi.org/10.1038/nri2154
- Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8⁺ T cells. Nat Rev Immunol 2011; 11:645-57; PMID:21869816; http://dx.doi.org/10.1038/nri3044
- Yu J, Freud AG, Caligiuri MA. Location and cellular stages of natural killer cell development. Trends Immunol 2013; 34:573-82; PMID:24055329; http://dx.doi.org/10.1016/j.it.2013.07.005
- Bottino C, Moretta L, Moretta A. NK cell activating receptors and tumor recognition in humans. Curr Top Microbiol Immunol 2006; 298:175-82; PMID:16323416
- Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008; 9:495-502; PMID:18425106; http://dx.doi.org/10.1038/ni1581
- Long EO, Sik Kim H, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol 2013; 31:227-58; PMID:23516982; http://dx.doi.org/10.1146/annurev-immunol-020711-075005
- Kruse PH, Matta J, Ugolini S, Vivier E. Natural cytotoxicity receptors and their ligands. Immunol Cell Biol 2014; 92:221-9; PMID:24366519; http://dx.doi.org/10.1038/icb.2013.98
- Trinchieri G, Valiante N. Receptors for the Fc fragment of IgG on natural killer cells. Nat Immun 1993; 12:218-34.
- Perussia B. Fc receptors on natural killer cells. Curr Top Microbiol Immunol 1998; 230:63-88; PMID:9586351
- Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene 2008; 27:5944-58; PMID:18836475; http://dx.doi.org/10.1038/onc.2008.272
- Burgess SJ, Maasho K, Masilamani M, Narayanan S, Borrego F, Coligan JE. The NKG2D receptor: immunobiology and clinical implications. Immunol Res 2008; 40:18-34; PMID:18193361; http://dx.doi.org/10.1007/s12026-007-0060-9
- El-Gazzar A, Groh V, Spies T. Immunobiology and conflicting roles of the human NKG2D lymphocyte receptor and its ligands in cancer. J Immunol 2013; 191:1509-15; PMID:23913973; http://dx.doi.org/10.4049/jimmunol.1301071
- Le Bert N, Gasser S. Advances in NKG2D ligand recognition and responses by NK cells. Immunol Cell Biol 2014; 92:230-6; PMID:24445601; http://dx.doi.org/10.1038/icb.2013.111
- Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol 2013; 31:413-41; PMID:23298206; http://dx.doi.org/10.1146/annurev-immunol-032712-095951
- Wiemann K, Mittrücker HW, Feger U, Welte SA, Yokoyama WM, Spies T, Rammensee HG, Steinle A. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J Immunol 2005; 175:720-9; PMID:16002667; http://dx.doi.org/10.4049/jimmunol.175.2.720
- Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 2008; 28:571-80; PMID:18394936; http://dx.doi.org/10.1016/j.immuni.2008.02.016
- Grzywacz B, Kataria N, Verneris MR. CD56(dim)CD16(+) NK cells downregulate CD16 following target cell induced activation of matrix metalloproteinases. Leukemia 2007; 21:356-9; PMID:17251901; http://dx.doi.org/10.1038/sj.leu.2404499
- Romee R, Foley B, Lenvik T, Wang Y, Zhang B, Ankarlo D, Luo X, Cooley S, Verneris M, Walcheck B, et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood 2013; 121:3599-608; PMID:23487023; http://dx.doi.org/10.1182/blood-2012-04-425397
- Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol 2006; 6:836-48; PMID:17063185; http://dx.doi.org/10.1038/nri1961
- Cox MC, Nofroni I, Ruco L, Amodeo R, Ferrari A, La Verde G, Cardelli P, Montefusco E, Conte E, Monarca B, et al. Low absolute lymphocyte count is a poor prognostic factor in diffuse-large-B-cell-lymphoma. Leuk Lymphoma 2008; 49; 1745-51; PMID:18798109; http://dx.doi.org/10.1080/10428190802226425
- Watanabe R, Tomita N, Itabashi M, Ishibashi D, Yamamoto E, Koyama S, Miyashita K, Takahashi H, Nakajima Y, Hattori Y, et al. Peripheral blood absolute lymphocyte/monocyte ratio as a useful prognostic factor in diffuse large B-cell lymphoma in the rituximab era. Eur J Haematol 2014; 92:204-10; PMID:24283206; http://dx.doi.org/10.1111/ejh.12221
- Porrata LF, Ristow KM, Habermann TM, Witzig TE, Colgan JP, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Nowakowski G, et al. Peripheral blood absolute lymphocyte/monocyte ratio during rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone treatment cycles predicts clinical outcomes in diffuse large B-cell lymphoma. Leuk Lymphoma 2014; 55:2728-2738; PMID:24547705; http://dx.doi.org/10.1038/sj.bmt.1705565
- Plonquet A, Haioun C, Jais JP, Debard AL, Salles G, Bene MC, Feugier P, Rabian C, Casasnovas O, Labalette M, et al. Peripheral blood natural killer cell count is associated with clinical outcome in patients with aaIPI 2–3 diffuse large B-cell lymphoma. Ann Oncol 2007; 18:1209-15; PMID:17496307; http://dx.doi.org/10.1093/annonc/mdm110
- Danielou-Lazareth A, Henry G, Geromin D, Khaznadar Z, Briere J, Tamouza R, Cayuela JM, Thieblemont C, Toubert A, Dulphy N. At diagnosis, diffuse large B-cell lymphoma patients show impaired rituximab-mediated NK-cell cytotoxicity. Eur J Immunol 2013; 43:1383-88; PMID:23400905; http://dx.doi.org/10.1002/eji.201242733
- Fischer L, Penack O, Gentilini C, Nogai A, Muessig A, Thiel E, Uharek L. The anti-lymphoma effect of antibody-mediated immunotherapy is based on an increased degranulation of peripheral blood natural killer (NK) cells. Exp Hematol 2006; 34:753-9; PMID:16728280; http://dx.doi.org/10.1016/j.exphem.2006.02.015
- Inagaki A, Ishida T, Yano H, Ishii T, Kusumoto S, Ito A, Ri M, Mori F, Ding J, Komatsu H, et al. Expression of the ULBP ligands for NKG2D by B-NHL cells plays an important role in determining their susceptibility to rituximab-induced ADCC. Int J Cancer 2009; 125:212-21; PMID:19358282; http://dx.doi.org/10.1002/ijc.24351
- Deguine J, Breart B, Lemaître F, Bousso P. Cutting Edge: Tumor-Targeting Antibodies Enhance NKG2D-Mediated NK Cell Cytotoxicity by Stabilizing NK Cell–Tumor Cell Interactions. J Immunol 2012; 189:5493-97; PMID:23183896; http://dx.doi.org/10.4049/jimmunol.1202065
- Kuriyama Y, Nakano M, Kawanishi Y, Iwase O, Aizawa S, Toyama K. Cytotoxic lymphocytes in the peripheral blood of patients with B cell lymphomas. Leukemia 1995; 9:2123-6; PMID:8609727
- Tursz T, Dokhelar MC, Lipinski M, Amiel JL. Low natural killer cell activity in patients with malignant lymphoma. Cancer 1982; 50:2333-5; PMID:6958348; http://dx.doi.org/10.1002/1097-0142(19821201)50:11%3c2333::AID-CNCR2820501119%3e3.0.CO;2-W
- Ono K. Clinical significance of natural killing activity in patients with advanced lymphoma. J Clin Immunol 1998; 18:132-41; PMID:9533657; http://dx.doi.org/10.1023/A:1023298917191
- Kim JK, Chung JS, Shin HJ, Song MK, Yi JW, Shin DH, Lee DS, Baek SM. Influence of NK cell count on the survival of patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood Res 2014; 49:162-9; PMID:25325035; http://dx.doi.org/10.5045/br.2014.49.3.162
- Markasz L, Stuber G, Vanherberghen B, Flaberg E, Olah E, Carbone E, Eksborg S, Klein E, Skribek H, Szekely L. Effect of frequently used chemotherapeutic drugs on the cytotoxic activity of human natural killer cells. Mol Cancer Ther 2007; 6:644-54; PMID:17308061; http://dx.doi.org/10.1158/1535-7163.MCT-06-0358
- Katz P, Zaytoun AM, Lee JH Jr. Mechanisms of human cell-mediated cytotoxicity. III. dependence of natural killing on microtubule and microfilament integrity. J Immunol 1982; 129:2816-25; PMID:6890568
- Riccardi C, Barlozzari T, Santoni A, Herberman RB, Cesarini C. Transfer to cyclophosphamide-treated mice of natural killer (NK) cells and in vivo natural reactivity against tumors. J Immunol 1981; 126:1284-89; PMID:7204966
- Cartron G, Blasco H, Paintaud G, Watier H, Le Guellec C. Pharmacokinetics of rituximab and its clinical use: thought for the best use? Crit Rev Oncol Hematol 2007; 62:43-52; PMID:17287129; http://dx.doi.org/10.1016/j.critrevonc.2006.09.004
- Arpon DR, Gandhi MK, Martin JH. A new frontier in haematology - combining pharmacokinetic with pharmacodynamic factors to improve choice and dose of drug. Br J Clin Pharmacol 2014; 78:274-81; PMID:24433338; http://dx.doi.org/10.1111/bcp.12318
- Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res 2002; 62:6178-86; PMID:12414645
- G Mentlik, James A, Cohen AD, Campbell KS. Combination immune therapies to enhance anti-tumor responses by NK cells. Front Immunol 2013; 4:1-12; PMID:23355837
- Kellner C, Hallack D, Glorius P, Staudinger M, Mohseni Nodehi S, de Weers M, van de Winkel JG, Parren PW, Stauch M, et al. Fusion proteins between ligands for NKG2D and CD20-directed single-chain variable fragments sensitize lymphoma cells for natural killer cell-mediated lysis and enhance antibody-dependent cellular cytotoxicity. Leukemia 2012; 26:830-4; PMID:22005785; http://dx.doi.org/10.1038/leu.2011.288
- Zhang Y, Wallace DL, de Lara CM, Ghattas H, Asquith B, Worth A, Griffin GE, Taylor GP, Tough DF, Beverley PC, et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology 2007; 121:258-65; PMID:17346281; http://dx.doi.org/10.1111/j.1365-2567.2007.02573.x
- Charbonneau B, Maurer MJ, Ansell SM, Slager SL, Fredericksen ZS, Ziesmer SC, Macon WR, Habermann TM, Witzig TE, Link BK, et al. Pretreatment circulating serum cytokines associated with follicular and diffuse large B-cell lymphoma: a clinic-based case-control study. Cytokine 2012; 60:882-9; PMID:23010502; http://dx.doi.org/10.1016/j.cyto.2012.08.028
- McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 2014; 16:717-27; PMID:25082194; http://dx.doi.org/10.1038/ncb3015
- Murawski N, Pfreundschuh M, Zeynalova S, Poeschel V, Hänel M, Held G, Schmitz N, Viardot A, Schmidt C, Hallek M, et al. Optimization of rituximab for the treatment of DLBCL (I): dose-dense rituximab in the DENSE-R-CHOP-14 trial of the DSHNHL. Ann Oncol 2014; 25:1800-06; PMID:24928834; http://dx.doi.org/10.1093/annonc/mdu208
- Lindorfer MA, Wiestner A, Zent CS, Taylor RP. Monoclonal antibody (mAb)-based cancer therapy: Is it time to reevaluate dosing strategies?. Oncoimmunology 2012; 1:959-61; PMID:23162771; http://dx.doi.org/10.4161/onci.20368
- Gluck WL, Hurst D, Yuen A, Levine AM, Dayton MA, Gockerman JP, Lucas J, Denis-Mize K, Tong B, Navis D, et al. Phase I studies of interleukin (IL)-2 and rituximab in B-cell non-hodgkin's lymphoma: IL-2 mediated natural killer cell expansion correlations with clinical response. Clin Cancer Res 2004; 10:2253-64; PMID:15073100; http://dx.doi.org/10.1158/1078-0432.CCR-1087-3
- Eisenbeis CF, Grainger A, Fischer B, Baiocchi RA, Carrodeguas L, Roychowdhury S, Chen L, Banks AL, Davis T, Young D, et al. Combination immunotherapy of B-cell non-Hodgkin's lymphoma with rituximab and interleukin-2: a preclinical and phase I study. Clin Cancer Res 2004; 10:6101-10; PMID:15447996; http://dx.doi.org/10.1158/1078-0432.CCR-04-0525
- Ysebaert L, Gross E, Kühlein E, Blanc A, Corre J, Fournié JJ, Laurent G, Quillet-Mary A. Immune recovery after fludarabine-cyclophosphamide-rituximab treatment in B-chronic lymphocytic leukemia: implication for maintenance immunotherapy. Leukemia 2010; 24:1310-16; PMID:20463751; http://dx.doi.org/10.1038/leu.2010.89
- Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, Fuseri N, Bonnafous C, Czerwinski D, Rajapaksa A, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood 2014; 123:678-86; PMID:24326534; http://dx.doi.org/10.1182/blood-2013-08-519199
- Du J, Lopez-Verges S, Pitcher BN, Johnson J, Jung SH, Zhou L, Hsu K, Czuczman MS, Cheson B, Kaplan L, et al. CALGB 150905 (Alliance): rituximab broadens the antilymphoma response by activating unlicensed NK cells. Cancer Immunol Res 2014; 2:878-89; PMID:24958280; http://dx.doi.org/10.1158/2326-6066.CIR-13-0158
- Klimo P, Connors JM. MACOP-B chemotherapy for the treatment of diffuse large-cell lymphoma. Ann Intern Med 1985; 102:596-602; PMID:2580468; http://dx.doi.org/10.7326/0003-4819-102-5-596
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25:579-86; PMID:17242396; http://dx.doi.org/10.1200/JCO.2006.09.2403
- Palmieri G, Serra A, De Maria R, Gismondi A, Milella M, Piccoli M, Frati L, Santoni A. Cross-linking of alpha 4 beta 1 and alpha 5 beta 1 fibronectin receptors enhances natural killer cell cytotoxic activity. J Immunol 1995; 155:5314-22; PMID:7594545
- Cerboni C, Ardolino M, Santoni A, Zingoni A. Detuning CD8+ T lymphocytes by down-regulation of the activating receptor NKG2D: role of NKG2D ligands released by activated T cells. Blood 2009; 113:2955-64; PMID:19124832; http://dx.doi.org/10.1182/blood-2008-06-165944
- Waldhauer I, Steinle A. Proteolytic release of soluble UL16-binding protein 2 from tumor cells. Cancer Res 2006; 66:2520-6; PMID:16510567; http://dx.doi.org/10.1158/0008-5472.CAN-05-2520
