Abstract
Toll-like receptor 7 (TLR7) agonists are under investigation for their ability to enhance antitumor immune responses. However, these agonists can also stimulate TLR7-expressing tumor cells. High TLR7 expression in the primary tumor confers poor clinical outcome and resistance to chemotherapy in lung cancer patients. This protumorigenic effect of TLR7 has been validated in murine models of lung carcinoma.
Keywords:
Introduction
TLR7, a receptor for single stranded RNA, is expressed on endosomes in immune cells including plasmacytoid and conventional dendritic cells (pDC and cDC), macrophages, B lymphocytes and NK cells. Stimulation of these cells with TLR7 ligands induces their maturation and activation, and the secretion of pro-inflammatory cytokines. These properties are currently being exploited in pre-clinical studies to improve antitumor therapies. Contrarily, stimulation of TLR7 on tumor cells can lead to tumor progression and resistance to treatment.
Antitumor effects of TLR7
The use of TLR7 agonists such as imiquimod, loxoribine, CL264, ssRNA40, R848, and SM-276 001, either alone or as vaccine adjuvants, induces potent immunity leading to antitumor therapeutic efficacy in several murine models. In line with these observations, it has been demonstrated that systemic TLR7 agonist injection reduces tumor progression and modulates the systemic and intratumoral immune contexture in colon, renal and mammary carcinomas. This has been illustrated by a decrease in intratumoral regulatory T cells, an increase of antigen-specific interferon γ (IFNγ) producing effector cells in the spleen,Citation1 an increased number of natural killer (NK), NKT cells and T lymphocytes,Citation2 and by activation and maturation of pDC able to efficiently stimulate antitumoral responses.Citation3 These results observed in animal models demonstrate the use of TLR7 agonists as an attractive strategy to treat several tumor types.
The antitumor effects arising from TLR7 stimulation have also been demonstrated in human skin cancers and cervical intraepithelial neoplasia. It has been shown that TLR7 stimulation by imiquimod is a successful treatment for actinic keratosis (intradermal neoplasia), with 27% of patients exhibiting a complete clinical response.Citation4 It has also been shown that the application of imiquimod on invasive primary melanoma results in local regression of tumor size, which is associated with increased levels of CD4+T and CD8+T lymphocytes both in the skin and in lymph nodes.Citation5
Protumorigenic effects of TLR7
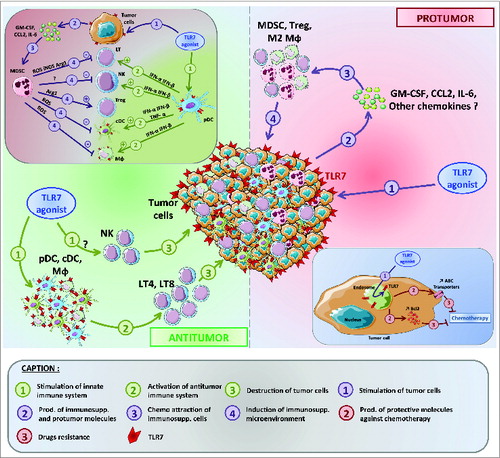
Contrary to the therapeutic benefits of TLR7 agonists on the immune cells, several studies have shown that TLR7 stimulation augments tumor progression. Previously we have shown that TLR7 is highly expressed on primary tumor cells from non-small-cell lung carcinoma (NSCLC) patients.Citation6 Furthermore, stimulation with TLR7 agonists induced a strong in vitro pro-tumorigenic effect and resistance to chemotherapeutic drugs currently used to treat NSCLC patients.Citation6 We recently verified these effects in both immunodeficient (NOD/SCID) and immunocompetent (C57BL/6) murine models, using subcutaneously grafted lung carcinoma cells.Citation7 In both models, we showed that repeated administration of CL264, loxoribine or imiquimod leads to increased tumor volume, which was similar to the results we obtained in TLR7-deficient mice. This protumorigenic effect could be mediated either by direct stimulation of TLR7-expressing tumor cells as we observed in experiments in vitro or by increased recruitment and differentiation of immunosuppressive cells in the tumor microenvironment. In support of the second scenario, we observed an increased frequency of myeloid-derived suppressor cells (MDSCs) and a reduction of CD8+T cells in the tumors of mice treated with TLR7 agonists compared to no treatment.Citation7 Additional experiments are needed to further characterize the role of MDSCs in the protumorigenic effects of TLR7 stimulation. We hypothesize that the recruitment of MDSCs could be induced by cytokines and/or chemokines produced by tumor cells upon TLR7 stimulation ().
Figure 1. Dichotomous effects of TLR7 stimulation on tumor progression and chemotherapy. Toll-like receptor 7 (TLR7) stimulation can modify the tumor microenvironment. This can induce either an antitumor or a protumorigenic effect via both direct or indirect stimulation of cancer cells or immune cells. TLR7 stimulation can also decrease the efficacy of chemotherapy.
Other groups have shown similar results in additional tumor models. For example, in a pancreatic cancer model composed of TLR7-expressing tumor cells, the stimulation of this receptor was shown to induce an acceleration of tumor growth and reduce the expression of several antitumor molecules such as PTEN, p16 and cyclin D1, concomitantly with an increase of pro-tumoral molecules, including p21, p27, p53, c-Myc and cyclin-B1.Citation8 Similarly, in a study of hepatocellular carcinoma tumor cells were shown to express TLR7, the stimulation of which induced increased proliferation of malignant cells.Citation9
In addition to effects on tumor progression, TLR stimulation also impacts the efficacy of cancer treatments, particularly in the case of chemotherapy. We first demonstrated in vitro that the addition of TLR7 agonist significantly reduced the effectiveness of different chemotherapeutic drugs (cisplatin, carboplatin, doxorubicin and navelbine), used alone or in combination, to kill human lung adenocarcinoma cells. We later reproduced these effects in murine models of lung carcinoma with the elimination of antitumor efficacy of cisplatin when co-injected with a TLR7 agonist. Finally, we found that among NSCLC patients those who highly express TLR7 on primary tumor cells have a significantly reduced response to chemotherapy. Drug resistance induced by TLR7 stimulation could, conceptually, be mediated by several distinct mechanisms, such as an increase in expression of anti-apoptotic molecules, an upregulation of members of the ABC drug-transporter family, or a decrease in the levels of apoptotic promoting factors. Such mechanisms have been demonstrated in an ovarian carcinoma model in which stimulation of another TLR, namely TLR4 induced resistance to paclitaxel treatment through the induction of anti-apoptotic proteins.Citation10
Conclusions
We have demonstrated that in opposition to the antitumor enhancement of immune cells, TLR7 stimulation of tumor cells induces tumor progression (summarized in ). Multiple parameters could underlie this apparent discrepancy, such as the type of tumor, the level of TLR7 expression, the downstream function of TLR7 signaling in particular tumor cells, or chemotaxis of suppressive cells into the tumor. These major pathways of TLR7 stimulation could act either directly or indirectly on both immune and tumor cells, converging on cancer patient outcome.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Wang D, Precopio M, Lan T, Yu D, Tang JX, Kandimalla ER, Agrawal S. Antitumor activity and immune response induction of a dual agonist of Toll-like receptors 7 and 8. Mol Cancer Ther 2010 Jun; 9(6):1788-97; PMID:20515950; http://dx.doi.org/10.1158/1535-7163.MCT-09-1198
- Koga-Yamakawa E, Dovedi SJ, Murata M, Matsui H, Leishman AJ, Bell J, Ferguson D, Heaton SP, Oki T, Tomizawa H, et al. Intratracheal and oral administration of SM-276001: a selective TLR7 agonist, leads to antitumor efficacy in primary and metastatic models of cancer. Int J Cancer J Int Cancer 2013 Feb 1; 132(3):580-90; PMID:22733292; http://dx.doi.org/10.1002/ijc.27691
- Le Mercier I, Poujol D, Sanlaville A, Sisirak V, Gobert M, Durand I, Dubois B, Treilleux I, Marvel J, Vlach J, et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res 2013 Aug 1; 73(15):4629-40; PMID:23722543; http://dx.doi.org/10.1158/0008-5472.CAN-12-3058
- Serra-Guillén C, Nagore E, Hueso L, Traves V, Messeguer F, Sanmartín O, Llombart B, Requena C, Botella-Estrada R, Guillén C. A randomized pilot comparative study of topical methyl aminolevulinate photodynamic therapy versus imiquimod 5% versus sequential application of both therapies in immunocompetent patients with actinic keratosis: clinical and histologic outcomes. J Am Acad Dermatol 2012 Apr; 66(4):e131-7; PMID:22226430; http://dx.doi.org/10.1016/j.jaad.2011.11.933
- Narayan R, Nguyen H, Bentow JJ, Moy L, Lee DK, Greger S, Haskell J, Vanchinathan V, Chang PL, Tsui S, et al. Immunomodulation by imiquimod in patients with high-risk primary melanoma. J Invest Dermatol 2012 Jan; 132(1):163-9; PMID:21850019; http://dx.doi.org/10.1038/jid.2011.247
- Cherfils-Vicini J, Platonova S, Gillard M, Laurans L, Validire P, Caliandro R, Magdeleinat P, Mami-Chouaib F, Dieu-Nosjean MC, Fridman WH, et al. Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. J Clin Invest. 2010 Apr; 120(4):1285-97; PMID:20237413; http://dx.doi.org/10.1172/JCI36551
- Chatterjee S, Crozet L, Damotte D, Iribarren K, Schramm C, Alifano M, Lupo A, Cherfils-Vicini J, Goc J, Katsahian S, et al. TLR7 promotes tumor progression, chemotherapy resistance, and poor clinical outcomes in non-small cell lung cancer. Cancer Res 2014 Sep 15; 74(18):5008-18; PMID:25074614 ; http://dx.doi.org/10.1158/0008-5472.CAN-13-2698
- Ochi A, Graffeo CS, Zambirinis CP, Rehman A, Hackman M, Fallon N, Barilla RM, Henning JR, Jamal M, Rao R, et al. Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. J Clin Invest. 2012 Nov 1; 122(11):4118-29; PMID:23023703; http://dx.doi.org/10.1172/JCI63606
- Mohamed FE, Al-Jehani RM, Minogue SS, Andreola F, Winstanley A, Olde Damink SWM, Habtesion A, Malagó M, Davies N, Luong TV, et al. Effect of toll-like receptor 7 and 9 targeted therapy to prevent the development of hepatocellular carcinoma. Liver Int Off J Int Assoc Study Liver 2014 Jul 2; PMID:24990399; 10.1111/liv.12626
- Kelly MG, Alvero AB, Chen R, Silasi D-A, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006 Apr 1; 66(7):3859-68; PMID:16585214; http://dx.doi.org/10.1158/0008-5472.CAN-05-3948
