Abstract
Indoleamine 2,3-dioxygenase 1 (IDO1), IDO2 and tryptophan 2,3-dioxygenase (TDO) represent some of the key immune regulators. Their increased activity has been demonstrated in a number of human malignancies but not yet in chronic myeloid leukemia (CML). In the present study, the activity of these enzymes was tested in 29 CML patients and 28 healthy subjects by monitoring the kynurenine (KYN)/tryptophan ratio. Serum samples taken prior to the therapy displayed a highly significant difference in KYN levels between the patient and control groups. However, increased KYN levels were detected in only 13 (44.8%) of these CML patients. The KYN levels in pretreatment sera of the patients correlated with the tumor burden. There was also a strong correlation between KYN levels and uric acid levels (UA). This suggests but does not prove the possible involvement of UA in activating IDO family of enzymes. Whenever tested, the increased KYN levels normalized in the course of the therapy. Patients with normal KYN levels in their pretreatment sera and subsequently treated with interferon-α, showed a transitory increase in their KYN levels. The present data indicate that CML should be added to the malignancies with an increased activity of the IDO family of enzymes and suggest that IDO inhibitors may be used in the treatment of CML patients.
Abbreviations:
- CML, chronic myeloid leukemia
- IDO, indoleamine 2, 3-dioxygenase
- INFα, interferon- α
- INFγ, interferon-γ
- KYN, kynurenine
- KTI, kynurenine/tryptophan index
- NK, natural killer
- Ph+, Philadelphia chromosome positive
- PBMC, peripheral blood mononuclear cells
- TDO, tryptophan 2, 3-dioxygenase
- TKI, tyrosine-kinase inhibitors
- T regs, regulatory T cells
- TRY, tryptophan
- UA, uric acid.
Introduction
Tryptophan (TRY) is an essential amino acid that is required for cell proliferation and differentiation and that plays an important role in the functions of the immune system and the CNS. IDO is a monomeric cytosolic heme-containing enzyme that catalyzes the first and the rate-limiting step during TRY metabolism along the KYN pathway. It is now designated as IDO1, because two other enzymes, viz. IDO2Citation1 and TDOCitation2 exhibit a similar activity. In the past decade most attention has been paid to IDO1. It is produced by a wide variety of cells, including endothelial, renal, and liver epithelial cells and cells of the immune system. It is found at low levels in healthy people, but its production is markedly increased during the course of infection and inflammation and in a wide spectrum of malignancies (see below).
Interferon-γ (INFγ) is the most potent inducer of IDO1Citation3 (and most likely also IDO2); however, other inflammatory cytokines also support its production. TDO is primarily produced in the liver and is apparently induced by glucocorticoids, but not by inflammatory cytokines.Citation4 The first catabolic product of TRY induced by these enzymes is N-formyl-kynurenine, which is then rapidly converted by kynurenine-formimidase to kynurenine.Citation5 The ratio of KYN to TRY levels is an established index of the activity of the IDO family of enzymes. A number of other enzymes that are specifically expressed in different cell types are involved in additional KYN processing.Citation6 These products are sometimes collectively designated “kynurenines.” Quite recently it has also been reported that in mice, KYN levels were markedly increased following an induction of hyperuricemia by treatment with the uricase inhibitor oxonic acid.Citation7 The authors have shown that in their system hyperuricemia was associated with increased levels of TRY, KYN, and kynurenic acid and hypothesized that IDO activity might be induced by increased levels of UA.
IDO1 has been recognized as an important factor of innate immunity being involved in suppression of replication of a wide spectrum of infectious agents,Citation8 suppression of the immune reactions of a mother against a fetusCitation9,10 and controlling autoimmune reactions.Citation11 Although IDO1 was for some time also considered to be an inhibitor of tumor cell proliferation,Citation12 it is currently generally accepted that the activity of this family of enzymes represents one of the most efficient immune escape mechanisms for malignant tumors. The immunosuppression is mediated by proliferation of Treg cellsCitation13-17 but also by blocking CD8+ T cell functionCitation18-20 and suppression of NK cells.Citation21 Most investigators are convinced that the immunoregulatory activities of IDOs are due to the combined effects of both TRY depletion and the activities of KYNs.
Apparently, most human cancers constitutively express IDO1 and at least some of them also IDO2Citation22 and TDO.Citation23 Increased IDO1 activity as revealed by increased KYN levels has been found in patients with melanoma,Citation24,25 colorectal cancer,Citation26 acute myeloid leukemia,Citation27 hepatocellular carcinomaCitation28, prostate cancer,Citation29 osteosarcomaCitation30, lung carcinoma,Citation31 endometrial, ovarial and vulvar carinomas,Citation32 multiple myeloma,Citation33 chronic lyphocytic leukemia,Citation34 glioma,Citation35 Hodgkin lymphoma,Citation36 and laryngeal carcinoma.Citation37 Correlations between increased KYN levels and poor clinical outcomes were repeatedly reported in the aforementioned studies.
To the best of our knowledge, there have been no reports on KYN levels in chronic myeloid leukemia (CML) patients. Thus, the purpose of the present study was to determine whether KYN levels are increased in CML patients and, if so, how these increased levels are associated with the clinical status of these patients and, based on the above mentioned observation,Citation7 with the UA levels.
Results
KYN levels in pre-treatment sera from CML patients and in sera from healthy control subjects
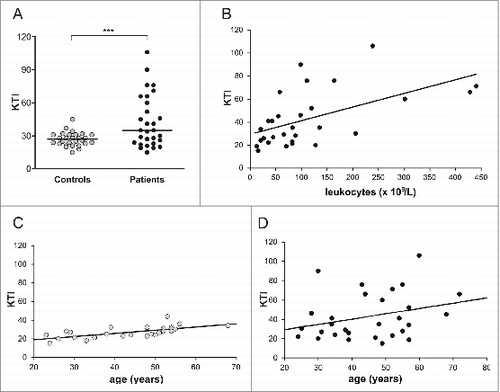
The serum KYN levels obtained in CML patients prior to the start of any therapy and in healthy control subjects are summarized in . The KYN levels as expressed by KTI values were significantly raised in the CML patients (p < 0.001). In general, KTI values tended to be higher for men than for women; however, these differences were not statistically significant (p = 0.285; results not shown). Based on these results, a cut-off value for increased KYN levels was arbitrarily chosen as KTI = 40 (mean KTI value of healthy control subjects + 2 SD; i.e., 26.5 + 13.3 = 39.8).
Table 1. KTI values in untreated CML patients and healthy control subjects.
A closer look at these data indicated that increased KYN levels (i.e., high KTI values) were found in less than half of the CML patients (). In the other CML patients, they were comparable to those of healthy subjects. To get more information on the reason for these differences, the associations between KYN levels and leukocyte counts and patient age were determined. The results, shown in , indicated that there was a strong, albeit not exceptionless correlation between leukocyte counts and KTI values (r = 0.549 p = 0.002). In the healthy subjects, KYN levels were age dependent as shown in (r = 0.675 p < 0.001). However, this was not found for the CML patients, as shown in (r = 0.291 p = 0.126), which suggested that the tumor burden was a more important factor than age in inducing IDO.
Figure 1. KTI values as determined for pre-treatment sera of CML patients and healthy control subjects (A). KTI in pre-treatment sera of CML patients and control subjects. Bars indicate median KTI values (B). Correlation between KTI and leukocyte count in sera of pre-treatment CML patients. Regression KTI = 29.409 + 0.118 x leukocytes; r = 0.549 (p = 0.002) (C). Correlation between KTI in sera of healthy control subjects and their age. Regression KTI = 11.748 + 0.345 x age; r = 0.675 (p < 0.001) (D). Correlation between KTI in sera of pre-treatment CML patients and their age. Regression KTI = 18.304 + 0.545 x age; r =0.291 (p = 0.126)
Changes in KYN levels during the course of therapy
Next, we determined whether KYN levels changed in the course of treatment and whether these changes could be correlated with the achievement of a remission. For 7 of the 13 CML patients with KYN levels ≥ 40 prior to the start of any therapy, additional (one or more) serum samples were available, which had been collected in the course of treatment 6 or more months after their first samples had been drawn. The KTI values of these patients are shown in . All seven of these patients displayed a marked decrease in KTI, which was associated with a significant reduction in their leukocyte counts. In contrast, as shown in , five patients whose first sample KTI values were < 40, showed an increase in KTI above the cut-off value in the course of the observation period. It may be of interest that four of these patients had been treated with INFα; in fact, all INFα-treated patients that did not have increased KTI values in their pre-treatment sera showed this trend. The same phenomenon (i.e., an increase in KTI) was seen in only 1 of 8 patients for whom additional serum samples were available and who had been treated exclusively with tyrosine kinase inhibitors (TKI).
Table 2. Decrease of KTI in patients with originally enhanced KTI values (≥ 40) in the course of therapy
Table 3. Increase of KTI in patients with normal values of KTI in their pre-treatment sera in the course of therapy
Relationship between KYN and UA levels
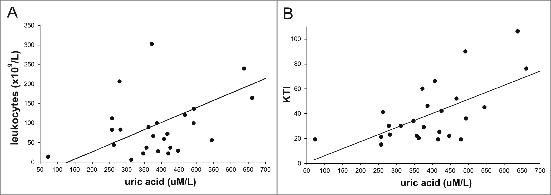
Results of UA determination in the pretreatment sera were available in 26 patients. Of these, 11 patients possessed increased KYN levels. UA levels in the respective group of patients are shown in . It can be seen that in the patients with increased KYN levels, the UA levels were significantly higher than in patients with normal KYN levels (p = 0.0038). As indicated in , there was a strong correlation between the leukocyte count and UA levels (r = 0.524, p = 0,006) () and between the KTI values and UA levels (r = 0.656, p < 0.001) (). In all but one patient, in whom two or more serum samples were available, achieving the hematological remission was associated with a marked drop in the UA level (results not shown). Hyperuricemia detected in the pretreatment sera of 14 patients was associated with somewhat increased TRY levels. However, the correlation lacked statistical significance (r = 0.4628, p = 0.0956, results not shown). When taking into consideration all patients there was no significant correlation between the UA and TRY levels (r = −0.3007, p = 0.1351, results not shown).
Table 4. Uric acid levels in pretreatment sera of CML patients with increased and normal KTI values in their pretreatment sera
Figure 2. Correlations between uric acid (UA) levels and leukocyte count and between UA levels and KTI values in sera of pre-treatement CML patients (A). Correlation between UA levels and leukocyte count. Regression leukocytes = −49.67 + 0.376 x UA, r = 0.524, (p = 0.006) (B). Correlation between UA levels and KTI values. Regression KTI = −5.52 + 0.144 x UA , r = 0.656 (p < 0.001).
Discussion
The major aim of our group is to contribute to the development of a therapeutic vaccine against CML. We are convinced that, for future vaccination studies, it is necessary to improve our understanding of CML immunology. We also believe that any therapeutic cancer vaccine will not be fully effective unless we can keep down tumor-induced immunosuppressive factors that militate against anti-tumor responses acting at both the local and systemic levels. Therefore, an important component of our current strategy is to identify such factors in CML.Citation38,39
The aim of the present study was to determine whether the activity of enzymes that catabolize TRY to KYN is enhanced in CML as has been shown to be in a number of human cancers (see above). Our results indicate that CML should be added to this group of malignancies, although we could only demonstrate increased KYN levels in less than a half of our patients. This is not quite surprising because in previous studies of various human cancers, raised KYN levels have not been detected in all patients either. Our data showed that KYN levels were most markedly raised in those patients who had high leukocyte counts, which indicated a strong correlation between the tumor burden and KYN level. However, this correlation was only incomplete, which suggested that individual CML patients differed in their rates of IDO production. This has previously been observed in patients with osteosarcoma,Citation30 glioma,Citation35 and laryngeal carcinoma.Citation37 Using anti-IDO and anti-TDO monoclonal antibodies and Western blotting and indirect immunofluorescenece test, we failed to convincingly demonstrate the presence of these enzymes in PBMC from patients with high levels of KYN. This suggests that other than tumor cells were involved. It should be recalled that in two other hematological malignancies, in which increased KYN levels have been demonstrated, the tumor cells have not been identified with IDO producers.Citation34,36
Several additional factors may account for the lack of complete correlation between the tumor cell counts and the KYN levels. It may be attributable to individual differences in production rates of INFγ, the most important IDO inducer. It is known that a single nucleotide polymorphism in the first intron of the human INFγ gene is associated with the amount of this cytokine that is produced.Citation40 Other factors might also be involved, however. For example, it has recently been reported that cellular immune responses against IDO1 may occur in both healthy subjects and, more frequently, in cancer patients.Citation41,42 The presence of cytotoxic T cell reactivity against IDO2 producing cells has also been reported.Citation43 These responses may represent “a striking back of the immune system.” This might have resulted in the selection of low IDO producers or even IDO antigen-lost cells. Another possibility is that the low KYN levels in some patients may be associated with a high activity of enzymes that are involved in further KYN processing. This phenomenon is possibly involved in cervical carcinoma patients in whom increased levels of KYN metabolites but not of KYN itself have been detected.Citation44 All of these alternatives, which are not mutually exclusive, remain open for further investigation.
It has been shown that activation of the KYN pathway increases with age, most probably owing to an age-dependent increase in INFγ production triggered by a shift in the balance between pro- and anti-inflammatory states.Citation45 Consequently, it has been suggested that increased KYN levels are involved in the pathogenesis of various psychiatric disorders associated with advanced age.Citation46 In accordance with this earlier observation, we demonstrated a significant association of IDO activity with age in healthy control subjects. Thus, it was rather unexpected that we did not find a significant association between KTI values and patient age. The lack of a strong age-dependence for KTI values in our CML patients may imply that the tumor burden eclipsed the influence of age on inducing IDO activity.
Similarly as in our previous study in which a number of immunity markers had been tested in CML patients,Citation47,48 hematological remission was also associated with normalization of KYN levels. In contrast, several other patients with normal KTI values in their pre-treatment sera displayed a transitory increase in KYN levels. It is likely that this enhancement was conditioned by intensive INFα treatment. Although INFγ is the major inducer of IDO, INFα can also induce this enzyme.Citation49
It may be of interest that in the present study, a rather strong correlation was revealed between the KYN and UA levels. It has recently been demonstrated in mice that experimentally induced hyperuricemia affects TRY metabolism.Citation7 The present data suggest that a similar phenomenon might also be operative in the present system. However, the observation that both the KYN levels and UA levels were dependent on the tumor burden raises the question what was the major inducer of IDO in CML patients. One can imagine two different scenarios. First, increased KYN levels were associated with IDO activity of the tumor and hyperuricemia is the consequence of a high death rate of cancer cells as it in other hematological malignancies.Citation50,51 Thus, KYN production and hyperuricemia can be two independent consequencies of the tumor burden. Second, it is the hyperuricemia resulting from the high cancer-related cell death rate, which activates the IDO enzymes in a variety of cells. At this writing, it seems reasonable to assume that both the tumor burden and the hyperuricemia are involved in KYN production, though their contribution may be markedly different. Further experiments are needed to clarify the point.
It has been demonstrated in various pathological settings that increased levels of KYN are associated with a poor prognosis. Because of the current highly effective therapy for CML, this aspect could not be examined in the present study. Thus, it is unclear whether KYN levels can serve as prognostic marker in CML. However, we continue the follow-up of the patients included in the present study.
To conclude, the present data indicate that CML should be added to the malignancies with an increased activity of the IDO family of enzymes and suggest that IDO inhibitors may be used in the treatment of CML patients. Such inhibitors have already entered clinical studies. They may be used to supplement current chemotherapy and future immunotherapy.Citation17,52
Materials and Methods
Patients
We enrolled 29 CML patients (13 males and 16 females), whose median age was 44 y (range: 24–72 y), and 28 healthy subjects (11 males and 17 females) whose median age was 46 y (range: 24–68 y). Prior to their sampling, informed consent was obtained from all these subjects and the study had been approved by the Ethics Committee of the Institute of Hematology and Blood Transfusion. All of the CML patients underwent routine haematological, biochemical, and molecular tests (including UA level determination). All were Ph+ positive. Blood samples were collected at the time of diagnosis (i. e., prior to starting any therapy). From 21 patients, additional blood samples were taken during the period of 4–58 mo after the start of therapy; they totalled 58 serum samples. These samples and the serum samples from healthy control subjects were stored at −20°C until being assayed.
Those patients who had been enrolled before 2004 were initially treated with INFα2a (Roferon, F.Hoffmann-La Roche Ltd, Basel, Switzerland), which was later replaced with TKI. Those patients who had been enrolled after 2004 were exclusively treated with TKI (imatinib mesylate, dasatitib or nilotinib). Patients with hyperuricemia (> 420 μM/L in men and > 360 μM/L in women) were treated with Apo-Allopurinol (Chanelle Medical Ltd, Laughrea, Ireland). For all patients who were followed, a haematological remission, and in most of them cytogenetic or molecular remission, was achieved during the course of the observation period.
Kynurenine and tryptophan detection
The methods used for KYN and TRY detection have been described in detail elsewhere.Citation53 In this paper, KYN levels are expressed by a KYN/TRY index (KTI) calculated as: (KYN levels in μmol/L / TRY levels in μmol/L) x 1000. KTI values of ≥ 40 were considered to indicate increased IDO activity. This cut-off value was derived from the results obtained for the healthy control subjects (see above) and represents the mean KTI value + 2 SDs.
Statistical analysis
The Student t-test was used to compare the results obtained in the CML patients and control group. Fisher's exact test was used to compare the results obtained with the different variables in the patients and the control group. Associations between different variables were assessed using the Pearson correlation analysis. Linear regression analysis was used to assess associations between the KYN or UA levels and leukocyte counts and subject age. A significance level of α = 0.05 was used for all statistical tests.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the grant No. NT-12363–4/2011 of the Internal Granting Agency of Ministry of Health of the Czech Republic and by the Project for conceptual development of research organization No 0023736 UHKT (Ministry of Health of the Czech Republic).
References
- Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007; 67:7082-7; PMID:17671174; http://dx.doi.org/10.1158/0008-5472.CAN-07-1872
- Capece L, Arrar M, Roitberg AE, Yeh SR, Marti MA, Estrin DA. Substrate stereo-specificity in tryptophan dioxygenase and indoleamine 2,3-dioxygenase. Proteins 2010; 78:2961-72; PMID:20715188; http://dx.doi.org/10.1002/prot.22819
- Brown RR, Ozaki Y, Datta SP, Borden EC, Sondel PM, Malone DG. Implications of interferon-induced tryptophan catabolism in cancer, auto-immune diseases and AIDS. Adv. Exp. Med. Biol. 1991; 294:425-35; PMID:1722946; http://dx.doi.org/10.1007/978-1-4684-5952-4_39
- Danesch U, Gloss B, Schmid W, Schutz G, Schule R, Renkawitz R. Glucocorticoid induction of the rat tryptophan oxygenase gene is mediated by two widely separated glucocorticoid-responsive elements. EMBO J. 1987; 6:625-30; PMID:3582368
- Takikawa O, Yoshida R, Kido R, Hayaishi O. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J. Biol. Chem. 1986; 261:3648-53; PMID:2419335
- Muller N, Schwarz MJ. Immune System and Schizophrenia. Curr. Immunol. Rev. 2010; 6:213-20; PMID:21057585; http://dx.doi.org/10.2174/157339510791823673
- Dankers AC, Mutsaers HA, Dijkman HB, van den Heuvel LP, Hoenderop JG, Sweep FC, Russel FG, Masereeuw R. Hyperuricemia influences tryptophan metabolism via inhibition of multidrug resistance protein 4 (MRP4) and breast cancer resistance protein (BCRP). Biochim. Biophys. Acta 2013; 1832:1715-22; PMID:23665398; http://dx.doi.org/10.1016/j.bbadis.2013.05.002
- Mackenzie CR, Gonzalez RG, Kniep E, Roch S, Daubener W. Cytokine mediated regulation of interferon-gamma-induced IDO activation. Adv. Exp. Med. Biol. 1999; 467:533-9; PMID:10721097; http://dx.doi.org/10.1007/978-1-4615-4709-9_66
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998; 281:1191-3; PMID:9712583; http://dx.doi.org/10.1126/science.281.5380.1191
- Blaschitz A, Gauster M, Fuchs D, Lang I, Maschke P, Ulrich D, Karpf E, Takikawa O, Schimek MG, Dohr G et al. Vascular endothelial expression of indoleamine 2,3-dioxygenase 1 forms a positive gradient towards the feto-maternal interface. PLoS. One. 2011; 6:e21774; PMID:21755000; http://dx.doi.org/10.1371/journal.pone.0021774
- Fallarino F, Volpi C, Zelante T, Vacca C, Calvitti M, Fioretti MC, Puccetti P, Romani L, Grohmann U. IDO mediates TLR9-driven protection from experimental autoimmune diabetes. J. Immunol. 2009; 183:6303-12; PMID:19841163; http://dx.doi.org/10.4049/jimmunol.0901577
- Ozaki Y, Edelstein MP, Duch DS. Induction of indoleamine 2,3-dioxygenase: a mechanism of the antitumor activity of interferon gamma. Proc. Natl. Acad. Sci. U. S. A 1988; 85:1242-6; PMID:3124115; http://dx.doi.org/10.1073/pnas.85.4.1242
- Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003; 4:1206-12; PMID:14578884; http://dx.doi.org/10.1038/ni1003
- Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene 2008; 27:3889-900; PMID:18317452; http://dx.doi.org/10.1038/onc.2008.35
- Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J. Immunol. 2009; 183:2475-83; PMID:19635913; http://dx.doi.org/10.4049/jimmunol.0900986
- Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010; 185:3190-8; PMID:20720200; http://dx.doi.org/10.4049/jimmunol.0903670
- Munn DH. Indoleamine 2,3-dioxygenase, Tregs and cancer. Curr. Med. Chem. 2011; 18:2240-6; PMID:21517755; http://dx.doi.org/10.2174/092986711795656045
- Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J. Immunol. 2006; 176:6752-61; PMID:16709834; http://dx.doi.org/10.4049/jimmunol.176.11.6752
- Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, Koni PA, Iwashima M, Munn DH. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J. Immunol. 2003; 171:1652-5; PMID:12902462; http://dx.doi.org/10.4049/jimmunol.171.4.1652
- Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death. Differ. 2002; 9:1069-77; PMID:12232795; http://dx.doi.org/10.1038/sj.cdd.4401073
- Song H, Park H, Kim J, Park G, Kim YS, Kim SM, Kim D, Seo SK, Lee HK, Cho D et al. IDO metabolite produced by EBV-transformed B cells inhibits surface expression of NKG2D in NK cells via the c-Jun N-terminal kinase (JNK) pathway. Immunol. Lett. 2011; 136:187-93; PMID:21277902; http://dx.doi.org/10.1016/j.imlet.2011.01.009
- Witkiewicz AK, Costantino CL, Metz R, Muller AJ, Prendergast GC, Yeo CJ, Brody JR. Genotyping and expression analysis of IDO2 in human pancreatic cancer: a novel, active target. J. Am. Coll. Surg. 2009; 208:781-7; PMID:19476837; http://dx.doi.org/10.1016/j.jamcollsurg.2008.12.018
- Pilotte L, Larrieu P, Stroobant V, Colau D, Dolusic E, Frederick R, De PE, Uyttenhove C, Wouters J, Masereel B et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc. Natl. Acad. Sci. U. S. A 2012; 109:2497-502; PMID:22308364; http://dx.doi.org/10.1073/pnas.1113873109
- Lee JR, Dalton RR, Messina JL, Sharma MD, Smith DM, Burgess RE, Mazzella F, Antonia SJ, Mellor AL, Munn DH. Pattern of recruitment of immunoregulatory antigen-presenting cells in malignant melanoma. Lab Invest 2003; 83:1457-66; PMID:14563947; http://dx.doi.org/10.1097/01.LAB.0000090158.68852.D1
- Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology 2007; 214:8-14; PMID:17191041; http://dx.doi.org/10.1159/000096906
- Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Gobel G et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin. Cancer Res. 2006; 12:1144-51; PMID:16489067; http://dx.doi.org/10.1158/1078-0432.CCR-05-1966
- Curti A, Aluigi M, Pandolfi S, Ferri E, Isidori A, Salvestrini V, Durelli I, Horenstein AL, Fiore F, Massaia M et al. Acute myeloid leukemia cells constitutively express the immunoregulatory enzyme indoleamine 2,3-dioxygenase. Leukemia 2007; 21:353-5; PMID:17170728; http://dx.doi.org/10.1038/sj.leu.2404485
- Pan K, Wang H, Chen MS, Zhang HK, Weng DS, Zhou J, Huang W, Li JJ, Song HF, Xia JC. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2008; 134:1247-53; PMID:18438685; http://dx.doi.org/10.1007/s00432-008-0395-1
- Feder-Mengus C, Wyler S, Hudolin T, Ruszat R, Bubendorf L, Chiarugi A, Pittelli M, Weber WP, Bachmann A, Gasser TC et al. High expression of indoleamine 2,3-dioxygenase gene in prostate cancer. Eur. J. Cancer 2008; 44:2266-75; PMID:18619832; http://dx.doi.org/10.1016/j.ejca.2008.05.023
- Urakawa H, Nishida Y, Nakashima H, Shimoyama Y, Nakamura S, Ishiguro N. Prognostic value of indoleamine 2,3-dioxygenase expression in high grade osteosarcoma. Clin. Exp. Metastasis 2009; 26:1005-12; PMID:19802733; http://dx.doi.org/10.1007/s10585-009-9290-7
- Suzuki Y, Suda T, Furuhashi K, Suzuki M, Fujie M, Hahimoto D, Nakamura Y, Inui N, Nakamura H, Chida K. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer 2010; 67:361-5; PMID:19487045; http://dx.doi.org/10.1016/j.lungcan.2009.05.001
- de Jong RA, Nijman HW, Boezen HM, Volmer M, Ten Hoor KA, Krijnen J, van der Zee AG, Hollema H, Kema IP. Serum tryptophan and kynurenine concentrations as parameters for indoleamine 2,3-dioxygenase activity in patients with endometrial, ovarian, and vulvar cancer. Int. J. Gynecol. Cancer 2011; 21:1320-7; PMID:21720257
- Bonanno G, Mariotti A, Procoli A, Folgiero V, Natale D, De RL, Majolino I, Novarese L, Rocci A, Gambella M et al. Indoleamine 2,3-dioxygenase 1 (IDO1) activity correlates with immune system abnormalities in multiple myeloma. J. Transl. Med. 2012; 10:247; PMID:23232072; http://dx.doi.org/10.1186/1479-5876-10-247
- Lindstrom V, Aittoniemi J, Jylhava J, Eklund C, Hurme M, Paavonen T, Oja SS, Itala-Remes M, Sinisalo M. Indoleamine 2,3-dioxygenase activity and expression in patients with chronic lymphocytic leukemia. Clin. Lymphoma Myeloma. Leuk. 2012; 12:363-5; PMID:22981691; http://dx.doi.org/10.1016/j.clml.2012.06.001
- Mitsuka K, Kawataki T, Satoh E, Asahara T, Horikoshi T, Kinouchi H. Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery 2013; 72:1031-8; PMID:23426156; http://dx.doi.org/10.1227/NEU.0b013e31828cf945
- Choe JY, Yun JY, Jeon YK, Kim SH, Park G, Huh JR, Oh S, Kim JE. Indoleamine 2,3-dioxygenase (IDO) is frequently expressed in stromal cells of Hodgkin lymphoma and is associated with adverse clinical features: a retrospective cohort study. BMC. Cancer 2014; 14:335; PMID:24886161; http://dx.doi.org/10.1186/1471-2407-14-335
- Ye J, Liu H, Hu Y, Li P, Zhang G, Li Y. Tumoral indoleamine 2,3-dioxygenase expression predicts poor outcome in laryngeal squamous cell carcinoma. Virchows Arch. 2013; 462:73-81; PMID:23179760; http://dx.doi.org/10.1007/s00428-012-1340-x
- Vonka V. Immunotherapy of chronic myeloid leukemia: present state and future prospects. Immunotherapy. 2010; 2:227-41; PMID:20635930; http://dx.doi.org/10.2217/imt.10.2
- Vonka V. Comments on therapeutic cancer vaccines. Immunotherapy. 2012; 4:133-5; PMID:22339454; http://dx.doi.org/10.2217/imt.11.173
- Awad M, Pravica V, Perrey C, El GA, Yonan N, Sinnott PJ, Hutchinson IV. CA repeat allele polymorphism in the first intron of the human interferon-gamma gene is associated with lung allograft fibrosis. Hum. Immunol. 1999; 60:343-6; PMID:10363726; http://dx.doi.org/10.1016/S0198-8859(98)00133-5
- Sorensen RB, Hadrup SR, Svane IM, Hjortso MC, Thor SP, Andersen MH. Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood 2011; 117:2200-10; PMID:21079151; http://dx.doi.org/10.1182/blood-2010-06-288498
- Munir S, Larsen SK, Iversen TZ, Donia M, Klausen TW, Svane IM, Straten PT, Andersen MH. Natural CD4+ T-cell responses against indoleamine 2,3-dioxygenase. PLoS. One. 2012; 7:e34568; PMID:22539948; http://dx.doi.org/10.1371/journal.pone.0034568
- Sorensen RB, Kollgaard T, Andersen RS, van den Berg JH, Svane IM, Straten P, Andersen MH. Spontaneous cytotoxic T-Cell reactivity against indoleamine 2,3-dioxygenase-2. Cancer Res. 2011; 71:2038-44; PMID:21406395; http://dx.doi.org/10.1158/0008-5472.CAN-10-3403
- Fotopoulou C, Sehouli J, Pschowski R, VON HS, Domanska G, Braicu EI, Fusch G, Reinke P, Schefold JC. Systemic changes of tryptophan catabolites via the indoleamine-2,3-dioxygenase pathway in primary cervical cancer. Anticancer Res. 2011; 31:2629-35; PMID:21778315
- Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr. Opin. Hematol. 2001; 8:131-6; PMID:11303144; http://dx.doi.org/10.1097/00062752-200105000-00001
- Capuron L, Schroecksnadel S, Feart C, Aubert A, Higueret D, Barberger-Gateau P, Laye S, Fuchs D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol. Psychiatry 2011; 70:175-82; PMID:21277567; http://dx.doi.org/10.1016/j.biopsych.2010.12.006
- Humlova Z, Klamova H, Janatkova I, Sandova P, Sterzl I, Sobotkova E, Hamsikova E, Haskovec C, Pisacka M, Cetkovsky P et al. Immunological profiles of patients with chronic myeloid leukaemia. I. State before the start of treatment. Folia Biol. (Praha) 2006; 52:47-58; PMID:17089915
- Humlova Z, Klamova H, Janatkova I, Malickova K, Kralikova P, Sterzl I, Roth Z, Hamsikova E, Vonka V. Changes of immunological profiles in patients with chronic myeloid leukemia in the course of treatment. Clin. Dev. Immunol. 2010; 2010:137320; PMID:21197073; http://dx.doi.org/10.1155/2010/137320
- Curreli S, Romerio F, Mirandola P, Barion P, Bemis K, Zella D. Human primary CD4 + T cells activated in the presence of IFN-alpha 2b express functional indoleamine 2,3-dioxygenase. J. Interferon Cytokine Res. 2001; 21:431-7; PMID:11440641; http://dx.doi.org/10.1089/107999001750277916
- Nagai A, Kubota M, Tang L, Adachi S, Usami I, Matsubara K. Hyperuricemia in pediatric malignancies before treatment. Nucleosides Nucleotides Nucleic Acids 2011; 30:1060-5; PMID:22132957; http://dx.doi.org/10.1080/15257770.2011.591745
- Yamauchi T, Negoro E, Lee S, Takai M, Matsuda Y, Takagi K, Kishi S, Tai K, Hosono N, Tasaki T et al. A high serum uric acid level is associated with poor prognosis in patients with acute myeloid leukemia. Anticancer Res. 2013; 33:3947-51; PMID:24023333
- Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, Link CJ, Prendergast GC. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012; 1:1460-8; PMID:23264892; http://dx.doi.org/10.4161/onci.21716
- Krcmova L, Solichova D, Melichar B, Kasparova M, Plisek J, Sobotka L, Solich P. Determination of neopterin, kynurenine, tryptophan and creatinine in human serum by high throuput HPLC. Talanta 2011; 85:1466-71; PMID:21807211; http://dx.doi.org/10.1016/j.talanta.2011.06.027
