Abstract
Understanding molecular mechanisms involved in creating and sustaining the tumor suppressive microenvironment is critical for the development of novel antitumor therapeutic strategies. We have identified the induction of T cell senescence as a novel mechanism utilized by human tumor cells to induce immune suppression, and provided a new strategy using TLR8 ligands to reverse tumor immunosuppressive effects for tumor immunotherapy.
It is now well recognized that the tumor suppressive microenvironment created by malignant tumors is a major obstacle for effective antitumor immunity and successful tumor immunotherapy. Tumor cells can utilize different strategies, including recruitment of regulatory T (Treg) cells and tumor-derived macrophages and myeloid suppressor cells (MSCs), production of suppressive factors (IL-10, TGF-β, and IDO), and expression of immune inhibitory molecules (FasL and PD-L1), to inhibit tumor-specific T cell proliferation and functions in the tumor microenvironment.Citation1 A better understanding of these molecular processes within the immune suppressive microenvironment is critical for the development of novel tumor vaccines and therapeutic strategies active against human cancers.
Increasing evidence shows that significant accumulation of senescent CD8+CD28null T cells has been found in certain types of cancer patients, strongly suggesting that it might be a strategy utilized by malignant tumors to evade immune surveillance.Citation2,3 Senescent T cells were initially characterized in age-associated dysregulation of the immune system during the normal aging process.Citation4 We recently found that human naturally occurring CD4+ Treg and tumor-derived γδ Treg cells can strongly suppress naïve/effector T cells through the induction of responder T cell senescence.Citation5,6 In the current study, we further showed that multiple types of tumor cells, including breast cancer, melanoma, colon cancer, prostate cancer, ovarian cancer, and head and neck cancer, can also utilize the same mechanism as Treg cells and directly induce T cell senescence.Citation7 Senescent T cells develop significant phenotypic alterations, such as permanent loss of CD28 expression, cell cycle arrest, and secret proinflammatory and suppressive cytokines.Citation4-7 More importantly, senescent T cells have exhibited functional changes, including defective killing abilities and the development of potent suppressive activity (). In addition, our in vivo adoptive transfer studies further confirmed that tumor-bearing microenvironments induced both adoptively transferred human naïve T cells and tumor-specific effector T cells to become senescent T cells possessing suppressive function.Citation7 These studies clearly indicate that senescent tumor-infiltrating T cells are dysfunctional and could indirectly amplify and maintain the immunosuppressive effects mediated by tumor cells and Treg cells in the tumor microenvironment.Citation5-7 These results suggest a potential mechanism for the failures seen in multiple clinical trials of tumor vaccines and adoptive T cell therapies. In addition, the possibility of blocking the induction of T cell senescence and restoring the effector function of senescent T cells are critical goals for enhancing antitumor immunity.
Figure 1. Reversal of T cell senescence induced by tumor and Treg cells in the tumor microenvironment for tumor immunotherapy. Both tumor cells and Treg cells can induce naïve/effector T cell senescence in the tumor microenvironment. Tumor-derived cAMP can be transferred from tumor cells to responder T cells via gap junction and is responsible for tumor-induced T cell senescence. Senescent T cells develop significant phenotypic alterations and exhibit suppressive function, which further amplify immune suppression mediated by Treg and tumor cells. Activation of TLR8 signaling in Treg and tumor cells, as well as the combinations with MAPK and cAMP signaling inhibition can prevent the induction of responder T cell senescence.
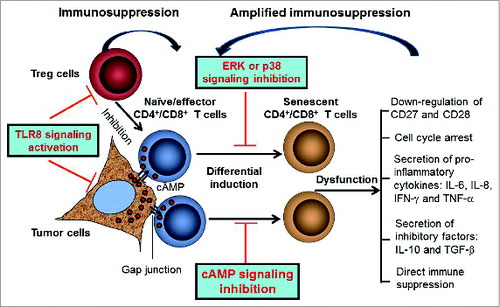
Dissecting molecular mechanisms used by tumor and Treg cells in the generation of senescent T cells will open new avenues to restoring T-cell function and will help in the design of novel vaccines for cancers. Metabolic dysregulation of tumor cells is one of the key factors involved in tumor-induced immune suppression. Tumor cells create a hypoxic microenvironment, resulting in accumulation of adenosine and cAMP within the tumor sites.Citation8 These hypoxia-derived metabolites are potent immunosuppressors that can protect tumor cells from antitumor immune responses mediated by tumor-specific T cells. In our efforts to identify the molecules responsible for the induction of T cell senescence mediated by different types of tumor cells, we found that tumor-derived endogenous cAMP is responsible for the induction of senescence in T cells.Citation7 We further demonstrated that tumor cells can transfer cAMP to targeted T cells via gap junctions, significantly increasing cAMP levels in senescent T cells. Notably, human Treg cells can also induce responder T cell senescence, and cAMP is a key component of Treg cell suppression.Citation5 These novel studies identifying cAMP-mediated T cell senescence not only identify mechanistic links between tumor immunosuppression, hypoxia, and metabolic dysregulation, but also should lead to novel strategies capable of augmenting immune responses directly against cancer.
Our studies strongly indicate that the induction of T cell senescence possessing potent suppressive function is a general phenomenon utilized by malignant tumors to escape immune responses. Therefore, development of strategies to prevent the generation of senescence and control the fate and function of tumor-specific T cells is critical for antitumor immunity. Recent strategies, including depletion of CD4+ Treg cells or targeting immune checkpoint molecules CTLA-4 or PD1, have been utilized in clinical trials for cancer immunotherapy, and have yielded promising results. However, these strategies may concurrently eliminate activated effector T cells, prevent effector T cell activities and induce Treg cell replenishment.Citation9 Thus, alternative novel strategies targeting more specific checkpoint molecules or interrupting tolerogenic pathways are needed. Identification of this novel suppressive mechanism utilized by tumor and Treg cells in our recent studies further suggests that blockage of senescence in tumor-specific T cells is also a critical checkpoint to control tumor suppression, which will provide a novel alternative target for cancer immunotherapy.Citation5–7 We have previously demonstrated that human Toll-like receptor 8 (TLR8) signaling completely reversed the suppressive functions of naturally occurring CD4+CD25+ Treg and tumor-derived Treg cells.Citation10 In addition, our more recent studies have shown that TLR8 ligand treatment in Treg cells could prevent the senescence induction and reverse the suppressor function of senescent responder T cells.Citation5,6 Our current studies further showed that human TLR8 signaling can also directly target multiple types of tumor cells and prevent their ability to induce T cell senescence.Citation7 The mechanism regulated by TLR8 signaling appears to involve modulation of the levels of the endogenous secondary messenger cAMP in tumor cells, strongly implicating the involvement of metabolic regulation mediated by TLR8 signaling. Importantly, we further demonstrated that TLR8 ligand enhanced antitumor immunity by preventing tumor-induced senescence in tumor-specific effector T cells in vivo in an adoptive transfer therapy model. Collectively, our studies indicate that human TLR8 signaling can reverse the suppressive effects mediated by tumor microenvironments and switch it into an effector microenvironment, by targeting different types of cells at different levels. In addition to TLR8 signaling, we have demonstrated that the induction and differentiation of Treg-induced senescent T cells could be controlled by ERK1/2 and P38 signaling in vitro and in vivo, which could lead to other useful strategies for senescence prevention.Citation5,6
In summary, our studies strongly indicate that differential induction of naïve/effector T cells into senescent cells possessing potent suppressive activity is a novel mechanism mediated by human tumor cells and Treg cells to induce immune suppression. Importantly, TLR8 signaling in tumor cells and Treg cells can block the induction of senescence in naïve and tumor-specific effector T cells and reverse their suppressive effects in vitro and in vivo, resulting in enhanced antitumor immunity (). These studies provide new insights relevant for the development of strategies to prevent and/or overcome tumor-induced immune suppression for tumor immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008 27:5904-12; PMID:18836471; http://dx.doi.org/10.1038/onc.2008.271
- Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(-) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother 2003 52:599-607; PMID:12827303; http://dx.doi.org/10.1007/s00262-003-0395-6
- Mondal AM, Horikawa I, Pine SR, Fujita K, Morgan KM, Vera E, Mazur SJ, Appella E, Vojtesek B, Blasco MA, Lane DP, Harris CC. p53 isoforms regulate aging- and tumor-associated replicative senescence in T lymphocytes. J Clin Invest 2013 123:5247-57; PMID:24231352; http://dx.doi.org/10.1172/JCI70355
- Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev 2005 205:147-57; PMID:15882351; http://dx.doi.org/10.1111/j.0105-2896.2005.00259.x
- Ye J, Huang X, Hsueh EC, Zhang Q, Ma C, Zhang Y, Varvares MA, Hoft DF, Peng G. Human regulatory T cells induce T-lymphocyte senescence. 2012 Blood 120:2021-31; PMID:22723548; http://dx.doi.org/10.1182/blood-2012-03-416040
- Ye J, Ma C, Hsueh EC, Eickhoff CS, Zhang Y, Varvares MA, Hoft DF, Peng G. Tumor-derived gammadelta regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J Immunol 2013 190:2403-14; PMID:23355732; http://dx.doi.org/10.4049/jimmunol.1202369
- Ye J, Ma C, Hsueh EC, Dou J, Mo W, Liu S, Han B, Huang Y, Zhang Y, Varvares MA, Hoft DF, Peng G. TLR8 signaling enhances tumor immunity by preventing tumor-induced T-cell senescence. EMBO Mol Med 2014 6:1294-311; PMID:25231413; http://dx.doi.org/10.15252/emmm.201403918
- Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res 2008 14:5947-52; PMID:18829471; http://dx.doi.org/10.1158/1078-0432.CCR-08-0229
- Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer 2007 7:880-7; PMID:17957190; http://dx.doi.org/10.1038/nrc2250
- Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science 2005 309:1380-4; PMID:16123302; http://dx.doi.org/10.1126/science.1113401
