Abstract
T cell adoptive therapies for immune-mediated regression of cancers have attracted a great deal of recent attention. Clinical results are glamorous, yet much remains to be uncovered behind the basic science that allows us to engineer T cells and T cell receptors (TCRs) for clinical use. We discuss the development of TCRs for therapeutic use in the context of thymic selection toward central tolerance and we review therapies based on tumor infiltrating lymphocytes (TILs), endogenous antigen specific TCRs, and engineered TCRs. Further we discuss the development of low and high affinity TCRs and the extent to which each challenges central tolerance. Current results suggest that adaptation of TCR engineering of moderate affinity TCRs coupled with co-regulatory and stimulatory molecules may be the safest and most efficacious road for TCR development aimed at tumor abolition.
Abbreviations
| TCR | = | T cell receptor |
| CDR | = | complementarity determining region |
| MHC | = | major histocompatibility complex |
| SLEC | = | short-lived effector cell |
| mTEC | = | medullary thymic epithelial cell |
| TSA | = | tissue-specific self-antigen |
| AIRE | = | autoimmune regulator |
| TIL | = | tumor infiltrating lymphocyte |
| TAA | = | tumor-associated antigen |
| CTA | = | cancer testis antigen |
Introduction
T cells are a highly attractive immune cell subset for cancer immunotherapy. The intrinsic properties of T cells coupled to clinical developments in engineering them have made T cells potent anti-tumor agents. Optimal activation of T cells depends on 3 signals: TCR engagement (signal 1), co-stimulation (signal 2) and an inflammatory stimulus (signal 3) delivered via cytokine. Here we focus on the characteristics of signal 1 that lead to effective tumor clearance as well as the potential for amplification of signal 1 through modulation of signal 2. T cell receptors (TCRs) have the capacity for exquisite antigenic specificity and this can be employed toward tumor antigens.Citation1 Upon tumor-antigen recognition T cells impart potent cytotoxicity that can aid tumor cell death. Further, T cell memory function allows for cellular persistence to antigenic epitopes. Manipulation and amplification of the aforementioned T cell attributes poses a powerful anti-cancer agent.Citation2 A challenge to adoptive cellular therapy with T cells is that tumor antigens are often non-mutated self-antigens and development of high affinity TCRs to tumor antigens faces the paradigm of central tolerance. Moderate affinity TCRs aimed at tumor antigen harbor less potential for toxic responses and may be as effective at mediation of anti-tumor activity, when enhanced by co-receptor and co-stimulatory molecules, as high affinity TCRs.
Composition
The T cell receptor (TCR) on a majority of T cells is composed of 2 chains, α and β. In this review, T cells bearing γ and δ receptors known as γδ T cells will not be discussed. The chains converge at the cell surface to form a heterodimeric receptor that has exquisite antigen specificity. Variability in the α and β chain sequences allows for 1 × 1013 individual TCRs after thymic selection with the potential to recognize a similarly diverse set of antigenic epitopes.Citation3 TCR precision for antigen is endowed by 3 complementarity determining regions (CDRs) that comprise each TCR chain. Somatic recombination, or V(D)J recombination, is the process of random gene rearrangement within each hypervariable region of the CDR that allows for vast TCR diversity. CDR1 and CDR2 segments are positioned to make primary contact with the major histocompatibility complex (MHC) during antigen presentation.Citation4 CDR3 is situated to interact with the central region of presented antigen concomitant with the fact that CDR3 shows the greatest variability of gene recombination.Citation5
Kinetics
Though the structure of the TCR determines the peptide sequence that is recognized, the added parameter of TCR signal strength and subsequent outcome, on account of affinity for antigen, is under investigation. Several models exist that attempt to decipher the precise strength of a TCR signal generated upon peptide-MHC (pMHC) interaction.Citation6 It was first proposed that the dissociation rate (Kd) (Kd = [TCR][pepMHC]/[TCR:pepMHC]) of TCR complexed with pMHC was determinant of the magnitude of T cell activation.Citation7,8 Interaction of TCR:pMHC with sufficiently long dwell time leads to an intracellular signaling cascade that promotes Ca2+ release associated with T cell activation.Citation9,10 A second proposal marked the idea that the number of TCR-pMHC interactions, as a function of affinity for antigen and ligand density, was determinant of T cell activation levels.Citation11 Further work showed that TCR affinity for pep:MHC may regulate the type of signal generated and subsequent progeny produced.Citation12 High affinity TCRs interact long enough with pep-MHC to allow an asymmetric generation of daughter T cells and subsequent development of short-lived effector cells (SLECs). In contrast, low affinity TCRs were not in contact with APCs at the time of cell division and offspring lacked SLEC capacity, resultant in a feeble immune response.Citation13
Signals
The downstream signals that are generated by TCR engagement are complex and continue to be revealed. Recent data suggest that the early event of actin remodeling is critical for ZAP70 and LCK phosphorylation and induction of Ca2+ flux.Citation14 The very early TCR signal cascade leads to perturbations in Ca2+ at the level of the endoplasmic reticulum.Citation15 For the CD4 T cell lineage differential Ca2+ signals are a determinant of lineage commitment.Citation16 Ca2+-mediated de-phosphorylation of NFAT and its subsequent nuclear translocation are early hallmarks of T cell activation.Citation17 Taken together, very early signaling events have been shown to be graded in intensity and may provide a plausible mechanism to target for enhanced levels of T cell activation that circumvent the TCR altogether.
Downstream measurements that affect T cells engineered for the tumor microenvironment are cytokine and cytotoxic granule release resultant in tumor cell killing as well as ability to differentiate into an effector cell. Study of the immunological synapse has shown the link between early TCR driven molecular signaling through ZAP70 and initiation of cytotoxic granule polarization and release.Citation18 The ability to initiate a cytotoxic response as measured by granule release may be a property of high affinity TCR interactions.Citation19 Elucidation of these data is critical for the development of tenable T cells raised against tumor antigen.
II. Thymic selection: The natural process
Central tolerance
Selection of a high affinity TCR specific for tumor antigen is a dichotomous pursuit. On one hand high affinity TCRs produce strong signals that enable the aforementioned traits of T cells to flourish. On the other hand, a high affinity TCR to tumor antigen may, and has, caused adverse reaction to self-antigens (tumor-antigen). Central tolerance encompasses the mechanisms in the thymus that are in place to insure lack of T cell reaction to self-peptide upon entry into the periphery.Citation20 Clonal deletion, elimination of TCRs that present with high affinity to self peptide, and clonal diversion, conversion of moderate to high affinity TCRs to regulatory T cell (Tregs) lineage are the key processes that impact central tolerance.Citation21,22 Events that lead to central tolerance are key to the development of TCRs for adoptive cell therapy.
Clonal deletion
In the thymus cortical and medullary thymic epithelial cells present fragments of self-peptide loaded into the groove of MHC class I or II molecules directly after processing in the endoplasmic reticulum and endocytic vesicles, respectively.Citation23,24 Through this process the TCRα chain continually rearranges, with CDR3 region having the most promiscuity, and CDR1 and CDR2 poised to stabilize the interaction with MHC.Citation25 The affinity hypothesis posits that TCRs with low affinity for self-antigen are selected to survive and are primed for interaction with foreign antigen (positive selection), while TCRs with high affinities for self-peptide are sentenced to death (clonal deletion) or enter an anergic state.Citation26-28
Thymic Signals for TCR Development
Advances have begun to elucidate the signals that lead to survival or anergy/death to define the TCR repertoire available in the periphery, yet more research is needed. As methods for antigen-specific T cell selection and TCR engineering proceed, it is important to keep in mind that very specialized APCs present to naturally tune TCRs. Medullary thymic epithelial cells (mTECs) are specialized to present tissue specific self-antigens (TSAs).Citation29,30 mTECs express the transcription factor AIRE (autoimmune regulator) and AIRE promotes presentation of a large variety of TSAs to TCRs. AIRE induces chemokine expression in the thymus to direct cellular localization.Citation31 The importance of specialized cells for antigen presentation to mature affinity specific TCRs is highlighted by the fact that AIRE deficient mice show altered maturation of thymocytes and incidentally develop spontaneous multi-organ disease. Citation32
The signals that are orchestrated within a T cell upon TCR sampling in the thymus are under intense investigation. The zipper model proposes that dwell time of pMHC complexed to TCR-CD3 leads to positive or negative selection. A dwell time of greater than 4 seconds effectively “zippers” the TCR together with its co-receptor to allow complete phospohorylation of CD3 ITAMs and subsequent negative selection due to strong affinity TCR. A low affinity TCR will have a dwell time of less than 4 seconds and incomplete or transient activation of ITAMs will occur, ultimately inducing a survival signal, or positive selection.Citation33
Downstream of early TCR signal events, the kinetics of Ca2+ flux are essential to appropriate TCR selection. Ca2+ stores and ERK work in tandem at the level of the ER. Strong TCR signals result in Ca2+-mediated robust activation of pERK and subsequent negative selection. Positive selection is associated with less Ca2+ flux and low-level pERK activation that leads to a sustained response.Citation34 The protein themis is expressed at high levels in the thymus and was recently shown to regulate Ca2+ flux as an intricate player in TCR selection.Citation35 The precise mechanism of how high affinity and low affinity TCRs are discriminated between in the thymus remains unknown and further discovery of this process is important to the field of TCR engineering for immunotherapy.Citation20
Clonal Diversion: A Treg is Born
The process of clonal deletion is incomplete and TCRs with low affinities for self-antigen “escape” into the periphery with the potential to create autoimmune responses. Tregs are a specialized set of CD4/Foxp3+ T cells endowed with capacity to regulate auto-reactive T cells and comprise the heart of peripheral tolerance.Citation36 Tregs must undergo their own clonal selection in the thymus and this process is dubbed clonal diversion. TCR specificity for self-peptide plays a role in the formation of Foxp3+ cells. Early studies with transgenic TCRs specific to foreign antigen show that Tregs developed intra-thymically only when foreign antigen was present.Citation37,38 More recent work has used transgenic TCRs cloned from naturally occurring Tregs to show that self-reactive TCRs are required for direction of Foxp3+ cell formation in the thymus and that a selection niche is also at play.Citation21,22
Separate signals?
Downstream signals, pERK and Ca2+ flux, in regards to the zipper model that mediate Treg formation need to be addressed.Citation33 One study used a TCR signal strength reporter mouse to show that cells that underwent clonal deletion had greater expression of a fluorescent reporter protein than did Tregs.Citation39 These data agree with the prevailing idea in the field that Treg TCR affinity is intermediate to positively selected TCRs and those targeted for deletion. Interestingly, Tregs in the periphery do not flux Ca2+ efficiently, similar to a cell with anergic properties, or produce IL-2, a downstream product of Ca2+ driven NFAT activation.Citation40 As Treg specific tissue-restricted antigens are discovered, it will be important to elucidate the TCR:pMHC affinities and resultant signals that occur to drive the Treg lineage. This information will aid in development of high affinity TCRs for tumor eradication and elucidate requirements for self-antigen signals in order to develop novel therapeutics that enhance TCR-mediated signals.
Peripheral Tolerance
In the context of cancer Tregs mediate cancer-induced inflammation in a protective manner.Citation41 In contrast, Tregs are key players in tumorigenic tolerance and growth as suppressors of cytotoxic immunity.Citation42 It is critical to grasp Treg-mediated immune suppression of T cells that have escaped central tolerance in order to appropriately design T cells armed to overcome tumorigenic tolerance. Tregs have several modes of suppression and we will highlight those with potential to target effector cell TCRs in the tumor microenvironment.
Tregs inhibit TCR Induced Proliferation of Effector T Cells
Several groups have demonstrated that Tregs inhibit activation of effector T cells upon TCR activation with CD3/CD28 in the presence and absence of APCs. Further studies saw rapid inhibition of cytokine transcription (IL-2) in conventional T cells.Citation43,44 A recent report elegantly demonstrated a TCR driven mechanism for these aforementioned data in which pre-activated Tregs co-cultured with conventional T cells led to immediate suppression of effector T cell signaling events through inhibition of Ca2+-store release and subsequent de-phosphorylation of NFAT. Further, Ca2+-independent events in conventional T cells were not affected.Citation45
CTLA4
Cytotoxic T lymphocyte-associated protein 4 (CTLA4) is a co-inhibitory molecule expressed constitutively at the cell surface of activated Tregs. The mechanisms of effector T cell suppression that occur via CTLA4 upregulation on Tregs are indirect and mediated by CTLA4 interaction with APCs.Citation46 CTLA4 expression causes down modulation of co-stimulatory molecules CD80 and CD86 on dendritic cells (DCs) and this inhibition can be recused by blockade of CTLA4. Treg-CTLA4 conditioned APCs cause impaired proliferation of effector T cells.Citation47 Further, decreased glutathione synthesis in DCs mediated by Treg-CTLA4 leads to a higher redox potential at the immunological synapse and results in an unfavorable environment for effector T cell survival and proliferation.Citation48,49
Abrogation of CTLA4 expression in mice leads to fatal multi-organ tissue destruction due to hypo-proliferative lymphocytes and indicates the importance of CTLA4 as a negative regulator upon T lymphocyte activation.Citation50 Treg-specific deletion of CTLA4 leads to loss of Treg-mediated mechanisms of suppression.Citation51 Blockade of CTLA4 is an approved FDA therapy that aims to reverse tumor-mediated tolerance of tumor infiltrating lymphocytes (TILs) and has resulted in increased survival times in metastatic melanoma patients when compared to traditional treatments. Not surprisingly, CTLA4 blockade results in immune-related adverse events in metastatic melanoma patients, namely hepatitis, enterocolitis, hypophysitis and uveitis. These adverse events can be attributed to loss of peripheral regulatory immunity through impairment of endogenous CTLA4-Treg-mediated control of auto-reactive T cell response to self-antigen.Citation52
PD-1
Programmed cell death 1/ Programmed cell death ligand-1 (PD-1/PD-L1) pathway blockade similarly aims to abrogate immune checkpoint signals to enhance effector T cell cytotoxicity in the tumor microenvironment.Citation53 PD-1 is induced by TCR signals and is highly expressed on antigen-experienced T cells at sites of inflammation in tissues. Further, while PD-1 deletion in mice results in lupus-like autoimmune disease, mice do not develop a lethal lympho-proliferative phenotype as seen in CTLA4 deficient animals.Citation54 Along these lines, PD-1 expression has proven critical for the development and function of inducible Tregs more so than natural Tregs.Citation55 Blockade of PD-1 in mice with chronic viral infection was able to reverse PD-1-mediated CD8 T cell exhaustion and restore viral control associated with virus-specific T cell function.Citation56 These data present PD-1 as a favorable target to reverse TIL exhaustion, or tumor-tolerance, in the tumor microenvironment. Indeed, clinical trial data suggest that PD-1 blockade enhances patient survival through restoration of TIL function with limited immune-related toxicities in patients.Citation53 Further, recent repots also identify PD-1 as a novel biomarker for highly tumor-reactive CD8 T cells in metastatic melanoma patients.Citation57
III. TCRs present
Here we present an overview of endogenous and engineered TCRs that have been used clinically for treatment. We discuss selection of TCR affinities and highlight adverse trial outcomes associated with central tolerance. Lastly we discuss ways to augment T cell activation and killing capacity in tumor.
A. Endogenous T cells
Manipulation of the endogenous T cell pool for tumor-antigen specificity poses the question: Where T cells? Antigen-specific T cell therapies and TIL therapies are based on the concept that antigen-specific T cells are present within a patient's T cell pool and can be expanded to target and kill tumor.Citation58 Key studies led to the proof that T cells specific for tumor antigen could be isolated from the periphery. ∼2% of peripheral CD8 T cells were specific for metastatic melanoma tumor antigen MART-1.Citation59,60 A second rich source of T cells proved to be the tumor itself. A T cell line established from metastatic melanoma tumor was cultured and re-infused, along with high-dose IL-2, into the subject to result in cancer regression. The precise T cell line was found to be specific to melanoma tumor-antigen gp100.Citation61 These striking data are the result of methodologies developed to identify endogenous cytotoxic T cells with TCR specific for tumor antigen.
Peripheral Antigen-Specific T Cells
While ∼2% of T cells in peripheral circulation showed MART-1 tumor-antigen specificity, the true percentage for the majority of cancer types of tumor antigen-specific T cells is likely 10–20-fold lower.Citation62,63 Thus, the methodologies for isolation of antigen-specific T cells are intricate. Firstly, T cells from peripheral blood must be stimulated for selection with one of several antigen presentation methods. Next, clonal selection or cell sorting to target the antigen-specific T cell is undertaken. Finally, cytokine cocktails and CD3 stimulation are used for expansion of the antigen-specific T cell population and subsequent reinfusion to the subject.Citation58,64 Expansion of antigen-specific T cells from the periphery has drawbacks that will hinder entry into mainstream cancer care. Firstly, paucity of antigen-specific T cells in the peripheral pool makes this method analogous to “finding a needle in a haystack.” Secondly, methodology for the steps of clonal stimulation and T cell selection need to be centralized.
TILs
The method of TIL isolation, culture, and reinfusion is promising and has been refined toward centralized methodology. The nature of TILs is that they are found at the source of antigenic response, thus the potential is enhanced to culture antigen-specific T cells.Citation65 Along these lines, TILs have potent killing capacity when compared to lymphokine-activate killer cells and this is a likely property of natural activation to tumor-antigen at tumor site.Citation66 The young TIL method begins with dissociation of tumor tissue and culture of TILs with IL-2 for 4–5 weeks to expand the T cell population. Further, CD8 T cells are enriched via magnetic bead sort and re-infused to a patient after he is conditioned with lymphodepleting chemotherapy regimen.Citation67 Clinical drawbacks of TIL are largely associated with the lymphodepletion phase that must occur. First, patients must be hospitalized in acute care setting, which is costly. Second, engraftment of a highly active T cell clone to immune-depleted patients may lead to loss of normal immune repertoire and regeneration of skewed immune responses. Citation68
Low Affinity TCRs Challenge Central Tolerance
Whether tumor-antigen specific T cells are harvested from the periphery or tumor, each method proposes to expand TCRs specific for self-antigen, and thus challenges the paradigm of central tolerance. Early data from metastatic melanoma patients treated with immune-modulatory therapies led to the observation that patients experienced autoimmune vitiligo.Citation69 Specific data showed that MART1 tumor-antigen specific CD8 T cells, isolated and expanded from subject peripheral blood, mounted an autoimmune response and caused damage to melanocytes that expressed MART1.Citation70 Similarly, TILs expanded from metastatic melanoma patients produced uveitis or vitiligo in 5 of 13 patients treated.Citation68 The aforementioned adverse events were non-lethal and treatable. Application of endogenous-derived T cells in cancer types other than melanoma will be important to determine safety of these therapies.
Low-Affinity TCRs
It is unexpected that self-reactive T cells can be found in the periphery or migrated to the tumor at all, given that clonal deletion aims to rid the body of such T cells. Further, clonal diversion posits that TCRs with moderate affinity for self-antigen will be redirected toward Treg lineage. Indeed, the majority of tumor antigens discussed above that have led to severe autoimmune responses in adoptive T cell therapy settings are found in the thymus and presented by mTECs during T cell selection.Citation71 Thus, endogenous T cells that are selected and expanded for T cell therapies are truly low affinity TCRs for self-antigen, as high affinity TCRs have undergone clonal deletion ().
Figure 1. Graduated increase in TCR affinity also increases with predicted tumor regression and autoimmune risk (or challenge to central tolerance). In accordance, peripherally isolated antigen-specific T cells are predicted to be slightly less potent for tumor regression given that TILs develop in the tumor microenvironment under more significant selection pressures than peripheral T cells. Therapeutically manipulated TCRs have paramount antigenic specificity and predicted anti-tumor activity, and challenge central tolerance to the greatest degree.
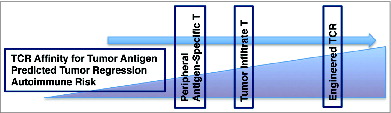
A recent study in TCR transgenic mice examined the affinity of a TCR specific for a tissue-restricted antigen. A sub-population of transgenic T cells escaped negative selection and was found in the periphery, although they possessed high affinity for self-restricted antigen. Further, these cells were able to activate and cause unchecked autoimmune responses in the face of infection.Citation72 These results challenge the belief that negative selection eliminates highly self-specific TCRs and show that endogenous tumor-antigen specific T cells may circulate in the periphery and possess reactive potential to self-antigen when provoked.
The posit that high affinity TCRs have profoundly enhanced functions as compared to low affinity TCRs comes from the in vitro setting where TCR:pMHC affinities may be unusually strong. Further, antigens that have been analyzed have not been true-self antigens and this may skew results.Citation73 A microbial infection model was used to delineate the kinetics of response for individual T cell clones of varying TCR affinities. Surprisingly, T cells with low affinity TCRs became activated, proliferated, and generated memory CD8 T cell responses at levels equal to high affinity clones. However, TCR affinity for ligand did impact the timing of T cell expansion, extravasation from lymphoid organs, and onset of contraction phase.Citation74 Further, it was shown that low affinity virus-specific T cells efficiently mediate viral response and clearance.Citation75 In vitro, NY-ESO-specific TCRs of various affinities were generated to assess the role of TCR affinity on CD8 T cell activation. Intermediate TCR-pMHC parameters conferred maximum CD8 T cell responsiveness as measured by calcium flux, chemokine/cytokine secretion, and cytotoxicity.Citation76 In line with these findings, various affinity TCRs to gp100 human melanoma antigen were assessed in vivo to determine the optimal TCR:pMHC strength for anti-tumor CD8 T cell activity. Strikingly, high affinity TCRs with greater pERK, Ca2+ flux, and cytokine release did not lead to greater anti-tumorigenicity. Instead, a plateau existed where maximum cytotoxicity and autoimmune reactivity were reached at the level of moderate TCR affinity to tumor antigen.Citation73 Further, TCR transgeneic mice with recognition for tissue-restricted TRP-1 melanoma antigen were generated. Two lines of mice differed in their affinity for TRP-1 antigen (high or low affinity TCRs) as evidenced by tetramer dissociation rate and peptide responsiveness. Surprisingly, the high and low affinity TCRs generated showed equivalent anti-tumor activity as assessed by CD8 cytotoxic parameters.Citation77 Taken together these data present a salient picture that calls into question the relevance of high affinity TCRs for adoptive immunotherapy.
B. Genetically Engineered T cells
Endogenous low affinity TCRs obtained from patient peripheral blood or TILs require higher levels of pMHC to be present for the number of peptide:ligand interactions to be sufficient for activation to occur.Citation78 Further, low-affinity TCRs require increased CD8 co-receptor stabilization, DC-derived co-stimulation, and blockade of co-inhibitory receptors to enhance activation and function.Citation73 In keeping with the zipper model, co-stimulatory modifications may allow increased dwell time of low affinity TCRs and result in full activation of T cells. Thus, the potential for high affinity TCRs engineered to combat tumor antigen is relevant. Many possibilities exist with the advantageous strategy of engineering T cells to express a pre-specified TCR with high affinity for tumor antigen.
High Affinity TCRs
High affinity TCRs are effective therapies that may improve anti-tumor cytotoxicity as the number of peptide:ligand interactions necessary for T cell activation is minimized and the dwell time for signal activation is optimal. TCR genes that encode α and β chains can be derived from highly effective TILs or transgenic mice that are engineered to express human MHC.Citation79 Retroviral or lentiviral transduction strategies are applied to introduce TCR genes into the T cell.Citation80 Early technical problems for TCR based therapies were low levels of expression of transferred TCR at the cell surface and mispairings of host α and transferred β chains (or vice versa).Citation81 Recent advances in TCR engineering resolved these difficulties. Knock down of endogenous TCRs has increased expression of transferred TCRs at the T cell surface. Introduction of cysteine residue technology allowed for transferred α and β chains to pair appropriately.Citation80
Which Antigen?
An obvious benefit of TCR engineering is the ready availability of a high affinity tumor-antigen specific TCR. However, this technology begs the question: Which antigen? A requisite for tumor-associated antigen (TAA) is that it is expressed exclusively by tumor cells and at levels sufficient to evoke a T cell response. Thus far, differentiation antigens and cancer testis antigens (CTAs) have been attractive targets. RT-PCR data demonstrated lack of expression of CTAs in normal tissues and upregulation of CTAs was found in tumor tissues of various origins.Citation82 Further, CTAs have been shown to evoke favorable effector T cell responses in cancer patients when CTA-specific peptides were loaded to DCs and vaccinated to metastatic melanoma patients.Citation83 Taken together, the aforementioned data set describes motives behind tumor antigen selection for development of high affinity TCRs.
High Affinity TCRs Challenge Central Tolerance
High affinity TCRs against differentiation antigens MART-1, gp100 and CTAs: MAGE-A1, MAGE-A3, and NY-ESO1 have been developed and placed in clinical trials. Induced autoimmune responses due to introduction of high affinity self-specific TCRs have been the hamartia of clinical trials thus far. Of 8 clinical trials assessed reviewed by Kuert et al, 5 report therapy-associated toxicities and 3 of 8 trials reported lethal toxicities likely owing to specificity of transferred TCRs for tissue-restricted antigen.Citation84 2 of 3 studies with lethal toxicities were targeted to MAGE-A3 antigen. Autopsy of patients showed dysregulated brain function and pointed to a previously unappreciated expression of MAGE-family proteins in brain tissue.Citation85 Histological analysis confirmed strong cross-reactivity of the MAGE-A3 specific TCR with brain-associated MAGE protein. Cardiac tissue from patients with lethal toxicities associated with heart failure showed no expression of MAGE protein in cardiac tissues; however, it was determined that the high affinity TCR cross-reacted with the cardiac protein of striated-muscle, titin.Citation86
Differential results in clinical trials regarding efficacy and toxicities between low affinity and high affinity TCRs have brought to light several critical thoughts. First, it is key to note that low affinity TCRs are able to cause tumor regression when harvested and expanded from the tumor microenvironment. Just as mTECs express AIRE, it is likely that APCs in the tumor microenvironment play an underappreciated role in maturation of low affinity TCRs to TAAs that endow cytotoxic T cells with not yet understood specificities. Second, demonstration that increasing TCR affinities plateau with regard to tumor-regression capacity and ability to induce autoimmunity lite the thoughts that we may not be adapted to, or stand to benefit from, introduction of genetically manipulated high affinity TCRs.
Patient-Specific Antigen Selection
Recent data suggest that patient-specific selection of mutated self-antigens from tumor DNA may be a successful strategy to overcome the treatment barrier of central tolerance. A novel approach allowed identification of mutated gene products from whole exome sequencing of patient-specific tumor. Peptides synthesized from mutated DNA sequences were presented by patient-specific tumor lines to patient derived TILs and response was measured by IFN-g production. Strikingly, in 3 metastatic melanoma patients tested, the top several isolated patient-specific mutated tumor antigens produced a strong cytotoxic response when incubated with patient-specific TILs.Citation87 This approach was further bolstered when regression of metastatic cholangiocarcinoma was seen upon treatment with patient-specific TILs specific for endogenous mutated tumor antigen.Citation88,Citation89
A key consideration in the aforementioned studies is that mutated antigens among patients and between cancer types differed greatly. Thus, patient-specific TIL therapies may hold more impact than high affinity TCRs that are aimed to treat large patient populations.
IV. Enhanced TCR Efficacy
Whether future trials prove low or high affinity TCRs to be effective in cancer regression, the role of TCR signal enhancement through co-receptor stabilization, engagement of co-stimulatory molecules, and diversion of inhibitory signals promise to augment the capacity of the TCR. Here we highlight the current understanding of how accessory molecules strengthen TCR signals.
CD8 Co-receptor
Through direct binding of invariant domains of MHC I, the CD8 co-receptor contributes to extracellular interactions with pMHC that enhances T cell signals during antigen presentation.Citation90,91 Blockade of CD8 activity through disruption of microdomain organization at the T cell surface led to decreased binding efficiency and association kinetics with pMHCI.Citation92 pMHC molecules with impaired CD8 binding capacity were used to assess the kinetics of TCR:pMHC interactions in live cytotoxic T cells. Low affinity TCR:pMHC binding and dissociation were substantially enhanced by the CD8 co-receptor. Less of a contribution was measured when high affinity TCRs were tested.Citation91 In vivo data assessed anti-tumor responses of CD8 T cells with varied TCR affinities to melanoma antigen gp100. The CD8 co-receptor enhanced anti-tumor activity measured by pERK activation, Ca2+ flux, and cytokine release of low affinity TCRs. Citation73 Together these data highlight role of CD8 for TCR stabilization and enhanced activation for low affinity TCRs.
Tumor Tolerance of TCRs
High affinity CD8 T cells in the tumor microenvironment become tolerized and lose function. Antigen-specific T cells that were infused into melanoma patients upregulated inhibitory receptors and lacked proliferative capacity resultant in trial failure. Citation93 Murine studies that employ high and low affinity transgenic TCRs to examine antigen-specific tumor regression demonstrate that high affinity TCRs become tolerized in the tumor microenvironment through loss of cytotoxic cytokine production and tumor killing capacity. Low affinity TCRs do not show significant loss of function as compared to high affinity TCRs, yet low affinity tumor-antigen specific TCRs were not capable of significant anti-tumor activity.Citation94,95 Further, in a T cell transgenic mouse model of prostate cancer, prostate-antigen specific CD8 T cells that infiltrated the tumor microenvironment became tolerized and converted to a suppressive phenotype as measured by TGF-β production.Citation96 It is interesting to note that a fate of high affinity TCRs to self-antigen in thymic selection is diversion to the suppressive Treg lineage. Thus, a mechanism of tumor tolerance that remains to be explored is diversion of high affinity TCRs to suppressor cells that aid tumor progression.
Methods are underway to overcome tumor tolerance and re-establish TCR tumor-antigen specific killing. CTLA-4 and PD-1 are negative regulators of CD8 T cell activity and are upregulated on the cell surface in association with functional exhaustion. Blockade of CTLA-4 is an approved therapy to promote reversion of tumor tolerance mechanisms. Unfortunately, CTLA-4 obstruction results in profound autoimmunity. Targeting of PD-1 to enhance blunted T cell function may be more feasible and clinical trials are underway. Citation97 Secondary methods to revert T cell exhaustion in the tumor microenvironment are amplification of co-stimulatory molecules CD28, CD134, CD137.Citation98 Insertion into third generation of chimeric antigen receptor constructs has been achieved and clinical trials that employ this technology have positive results and show enhanced T cell persistence and function.Citation80 Technologies that have been developed to deliver high affinity TCR genes to T cells will be effective to modulate enhanced co-stimulatory molecule expression as well.
Concluding Remarks
As adoptive T cell therapies progress, basic research that explores signals that mediate thymic selection is necessary. Along these lines, the cooperative signals between mTECs and T cells that determine low, moderate, and high affinity TCRs need to be elucidated. Further, signal requirements downstream of the TCR that pertain to modulation of pERK and Ca2+ harbor the potential to amplify T cell activity in TCR-independent manner. Low to moderate affinity TCRs are proving to be as efficacious and safer than high affinity TCRs. Recent reports suggest that patient-specific antigen selection may be key to T cell mediated tumor regression. Future research efforts that focus on amplification of T cell effector capacity of low to moderate affinity TCRs directs toward patient-specific tumor antigens will be critical to progress research and treatment.
Disclosure of Potential Conflicts of Interest
No conflicts of interest were disclosed.
Acknowledgments
We thank Brian Riesenberg, Caroline Wallace, and EJD for thoughtful insight and critical reading of the manuscript. We thank the Li laboratory and M&I Department for continued support.
Jessica Thaxton is funded by Department of Defense BCRP fellowship W81×1H.
References
- Linnemann C, Mezzadra R, Schumacher TN. TCR repertoires of intratumoral T-cell subsets. Immunol Rev 2014; 257:72-82;PMID:24329790;http://dx.doi.org/10.1111/imr.12140
- Muul LM, Spiess PJ, Director EP, Rosenberg SA. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol (Baltimore, Md. : 1950) 1987; 138:989-995; PMID:3100623
- Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nature reviews. Immunology 2004; 4:123-132; PMID:15040585; http://dx.doi.org/10.1038/nri1292
- Chlewicki LK, Holler PD, Monti BC, Clutter MR, Kranz DM. High-affinity, peptide-specific T cell receptors can be generated by mutations in CDR1, CDR2 or CDR3. J Mole Biol 2005; 346:223-239; PMID:15663940; http://dx.doi.org/10.1016/j.jmb.2004.11.057
- Reiser JB, Grégoire C, Darnault C, Mosser T, Guimezanes A, Schmitt-Verhulst AM, Fontecilla-Camps JC, Mazza G, Malissen B, Housset D. A T cell receptor CDR3beta loop undergoes conformational changes of unprecedented magnitude upon binding to a peptide/MHC class I complex. Immunity 2002; 16:345-354; PMID:11911820
- Hurwitz AA, Cuss SM, Stagliano KE, Zhu Z. T Cell Avidity and Tumor Immunity: Problems and Solutions. Cancer Microenviron 2013; PMID:24357332; http://dx.doi.org/10.1007/s12307-013-0143-1
- Kalergis AM, Boucheron N, Doucey MA, Palmieri E, Goyarts EC, Vegh Z, Luescher IF, Nathenson SG. Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex. Nat Immunol 2001; 2:229-234; PMID:11224522; http://dx.doi.org/10.1038/85286
- Stone JD, Chervin AS, Kranz DM. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology 2009; 126:165-176; PMID:19125887; http://dx.doi.org/10.1111/j.1365-2567.2008.03015.x
- Rabinowitz JD, Beeson C, Wülfing C, Tate K, Allen PM, Davis MM, McConnell HM. Altered T cell receptor ligands trigger a subset of early T cell signals. Immunity 1996; 5:125-135; PMID:8769476
- Kersh GJ, Kersh EN, Fremont DH, Allen PM. High- and low-potency ligands with similar affinities for the TCR: the importance of kinetics in TCR signaling. Immunity 1998; 9:817-826; PMID:9881972
- Tian S, Maile R, Collins EJ, Frelinger JA. CD8+ T cell activation is governed by TCR-peptide/MHC affinity, not dissociation rate. J Immunol (Baltimore, Md. : 1950) 2007; 179:2952-2960; PMID:17709510
- Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science (New York, N.Y.) 2007; 315:1687-1691; PMID:17332376; http://dx.doi.org/10.1126/science.1139393
- King CG, Koehli S, Hausmann B, Schmaler M, Zehn D, Palmer E. T cell affinity regulates asymmetric division, effector cell differentiation, and tissue pathology. Immunity 2012; 37:709-720; PMID:23084359; http://dx.doi.org/10.1016/j.immuni.2012.06.021
- Tan YX, Manz BN, Freedman TS, Zhang C, Shokat KM, Weiss A. Inhibition of the kinase Csk in thymocytes reveals a requirement for actin remodeling in the initiation of full TCR signaling. Nat Immunol 2014; 15:186-194; PMID:24317039; http://dx.doi.org/10.1038/ni.2772
- Oh-hora M. Calcium signaling in the development and function of T-lineage cells. Immunol Rev 2009; 231:210-224; PMID:19754899; http://dx.doi.org/10.1111/j.1600-065X.2009.00819.x
- Weber KS, Miller MJ, Allen PM. Th17 cells exhibit a distinct calcium profile from Th1 and Th2 cells and have Th1-like motility and NF-AT nuclear localization. J Immunol (Baltimore, Md. : 1950) 2008; 180:1442-1450; PMID:18209039
- Fracchia KM, Pai CY, Walsh CM. Modulation of T Cell Metabolism and Function through Calcium Signaling. Front Immunol 2013; 4:324; PMID:24133495; http://dx.doi.org/10.3389/fimmu.2013.00324
- Jenkins MR, Stinchcombe JC, Au-Yeung BB, Asano Y, Ritter AT, Weiss A, Griffiths GM. Distinct structural and catalytic roles for Zap70 in formation of the immunological synapse in CTL. eLife 2014; 3:e01310; PMID:24596147; http://dx.doi.org/10.7554/eLife.01310
- Jenkins MR, Tsun A, Stinchcombe JC, Griffiths GM. The strength of T cell receptor signal controls the polarization of cytotoxic machinery to the immunological synapse. Immunity 2009; 31:621-631; PMID:19833087; http://dx.doi.org/10.1016/j.immuni.2009.08.024
- Xing Y, Hogquist KA. T-cell tolerance: central and peripheral. Cold Spring Harb Perspect Biol 2012; 4; PMID:22661634; http://dx.doi.org/10.1101/cshperspect.a006957
- Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh CS. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol 2009; 10:610-617; PMID:19430476; http://dx.doi.org/10.1038/ni.1739
- Leung MW, Shen S, Lafaille JJ. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J Exp Med 2009; 206:2121-2130; PMID:19737865; http://dx.doi.org/10.1084/jem.20091033
- Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL, Wiley DC. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature 1993; 364:33-39; PMID:8316295; http://dx.doi.org/10.1038/364033a0
- Scott CA, Peterson PA, Teyton L, Wilson IA. Crystal structures of two I-Ad-peptide complexes reveal that high affinity can be achieved without large anchor residues. Immunity 1998; 8:319-329; PMID:9529149
- Sim BC, Zerva L, Greene MI, Gascoigne NR. Control of MHC restriction by TCR Valpha CDR1 and CDR2. Science (New York, N.Y.) 1996; 273:963-966; PMID:8688082
- Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NR. T-cell-receptor affinity and thymocyte positive selection. Nature 1996; 381:616-620; PMID:8637599; http://dx.doi.org/10.1038/381616a0
- Williams CB, Engle DL, Kersh GJ, Michael White J, Allen PM. A kinetic threshold between negative and positive selection based on the longevity of the T cell receptor-ligand complex. J Exp Med 1999; 189:1531-1544; PMID:10330432
- Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature 1999; 402:255-262; PMID:10580495; http://dx.doi.org/10.1038/46218
- Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol 2001; 2:1032-1039; PMID:11600886; http://dx.doi.org/10.1038/ni723
- Gotter J, Brors B, Hergenhahn M, Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J Exp Med 2004; 199:155-166; PMID:14734521; http://dx.doi.org/10.1084/jem.20031677
- Laan M, Peterson P. The Many Faces of Aire in Central Tolerance. Front Immunol 2013; 4:326; PMID:24130560; http://dx.doi.org/10.3389/fimmu.2013.00326
- Gavanescu I, Benoist C, Mathis D. B cells are required for Aire-deficient mice to develop multi-organ autoinflammation: A therapeutic approach for APECED patients. Proc Natl Acad Sci U S A 2008; 105:13009-13014; PMID:18755889; http://dx.doi.org/10.1073/pnas.0806874105
- Palmer E, Naeher D. Affinity threshold for thymic selection through a T-cell receptor-co-receptor zipper. Nature reviews. Immunology 2009; 9:207-213; PMID:19151748; http://dx.doi.org/10.1038/nri2469
- McNeil LK, Starr TK, Hogquist KA. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc Natl Acad Sci U S A 2005; 102:13574-13579; PMID:16174747; http://dx.doi.org/10.1073/pnas.0505110102
- Fu G, Casas J, Rigaud S, Rybakin V, Lambolez F, Brzostek J, Hoerter JA, Paster W, Acuto O, Cheroutre H, et al. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature 2013; 504:441-445; PMID:24226767; http://dx.doi.org/10.1038/nature12718
- Rudensky AY, Gavin M, Zheng Y. FOXP3 and NFAT: partners in tolerance. Cell 2006; 126:253-256; PMID:16873058; http://dx.doi.org/10.1016/j.cell.2006.07.005
- Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol (Baltimore, Md. : 1950) 1999; 162:5317-5326; PMID:10228007
- Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol 2001; 2:301-306; PMID:11276200; http://dx.doi.org/10.1038/86302
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 2011; 208:1279-1289; PMID:21606508; http://dx.doi.org/10.1084/jem.20110308
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 2005; 6:345-352; PMID:15785760; http://dx.doi.org/10.1038/ni1178
- Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, Beckhove P, Gounari F, Khazaie K. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res 2009; 69:5490-5497; PMID:19570783; http://dx.doi.org/10.1158/0008-5472.can-09-0304
- Kryczek I, Wei S, Zou L, Zhu G, Mottram P, Xu H, Chen L, Zou W. Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol (Baltimore, Md. : 1950) 2006; 177:40-44; PMID:16785496
- Ermann J, Szanya V, Ford GS, Paragas V, Fathman CG, Lejon K. CD4(+)CD25(+) T cells facilitate the induction of T cell anergy. J Immunol (Baltimore, Md. : 1950) 2001; 167:4271-4275; PMID:11591749
- Oberle N, Eberhardt N, Falk CS, Krammer PH, Suri-Payer E. Rapid suppression of cytokine transcription in human CD4+CD25 T cells by CD4+Foxp3+ regulatory T cells: independence of IL-2 consumption, TGF-beta, and various inhibitors of TCR signaling. J Immunol (Baltimore, Md. : 1950) 2007; 179:3578-3587; PMID:17785792
- Schmidt A, Oberle N, Weiss EM, Vobis D, Frischbutter S, Baumgrass R, Falk CS, Haag M, Brügger B, Lin H, et al. Human regulatory T cells rapidly suppress T cell receptor-induced Ca(2+), NF-kappaB, and NFAT signaling in conventional T cells. Sci Signal 2011; 4:ra90; PMID:22375050; http://dx.doi.org/10.1126/scisignal.2002179
- Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of treg-mediated T cell suppression. Front Immunol 2012; 3:51; PMID:22566933; http://dx.doi.org/10.3389/fimmu.2012.00051
- Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology 2006; 118:240-249; PMID:16771859; http://dx.doi.org/10.1111/j.1365-2567.2006.02362.x
- Yan Z, Garg SK, Kipnis J, Banerjee R. Extracellular redox modulation by regulatory T cells. Nat Chem Biol 2009; 5:721-723; PMID:19718041; http://dx.doi.org/10.1038/nchembio.212
- Yan Z, Garg SK, Banerjee R. Regulatory T cells interfere with glutathione metabolism in dendritic cells and T cells. J Biol Chem 2010; 285:41525-41532; PMID:21037289; http://dx.doi.org/10.1074/jbc.M110.189944
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995; 3:541-547; PMID:7584144
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science (New York, N.Y.) 2008; 322:271-275; PMID:18845758; http://dx.doi.org/10.1126/science.1160062
- Della Vittoria Scarpati G, Fusciello C, Perri F, Sabbatino F, Ferrone S, Carlomagno C, Pepe S. Ipilimumab in the treatment of metastatic melanoma: management of adverse events. OncoTargets Ther 2014; 7:203-209; PMID:24570590; http://dx.doi.org/10.2147/ott.s57335
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews. Cancer 2012; 12:252-264; PMID:22437870; http://dx.doi.org/10.1038/nrc3239
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999; 11:141-151; PMID:10485649
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009; 206:3015-3029; PMID:20008522; http://dx.doi.org/10.1084/jem.20090847
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439:682-687; PMID:16382236; http://dx.doi.org/10.1038/nature04444
- Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest 2014; 124:2246-2259; PMID:24667641; http://dx.doi.org/10.1172/jci73639
- Yee C. The use of endogenous T cells for adoptive transfer. Immunol Rev 2014; 257:250-263; PMID:24329802; http://dx.doi.org/10.1111/imr.12134
- Pittet MJ, Valmori D, Dunbar PR, Speiser DE, Liénard D, Lejeune F, Fleischhauer K, Cerundolo V, Cerottini JC, Romero P. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med 1999; 190:705-715; PMID:10477554
- Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med 1999; 5:677-685; PMID:10371507; http://dx.doi.org/10.1038/9525
- Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E, Yannelli JR, Adema GJ, Miki T, Rosenberg SA. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci U S A 1994; 91:6458-6462; PMID:8022805
- Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol (Baltimore, Md. : 1950) 2004; 172:5967-5972; PMID:15128778
- Bioley G, Jandus C, Tuyaerts S, Rimoldi D, Kwok WW, Speiser DE, Tiercy JM, Thielemans K, Cerottini JC, Romero P. Melan-A/MART-1-specific CD4 T cells in melanoma patients: identification of new epitopes and ex vivo visualization of specific T cells by MHC class II tetramers. J Immunol (Baltimore, Md. : 1950) 2006; 177:6769-6779; PMID:17082590
- Jotereau F, Gervois N, Labarriere N. Adoptive transfer with high-affinity TCR to treat human solid tumors: how to improve the feasibility? Targeted Oncol 2012; 7:3-14; PMID:22350487; http://dx.doi.org/10.1007/s11523-012-0207-z
- Goff SL, Smith FO, Klapper JA, Sherry R, Wunderlich JR, Steinberg SM, White D, Rosenberg SA, Dudley ME, Yang JC. Tumor infiltrating lymphocyte therapy for metastatic melanoma: analysis of tumors resected for TIL. J Immunother (Hagerstown, Md. : 1997) 2010; 33:840-847; PMID:20842052; http://dx.doi.org/10.1097/CJI.0b013e3181f05b91
- Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science (New York, N.Y.) 1986; 233:1318-1321; PMID:3489291
- Dudley ME, Gross CA, Langhan MM, Garcia MR, Sherry RM, Yang JC, Phan GQ, Kammula US, Hughes MS, Citrin DE, et al. CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res 2010; 16:6122-6131; PMID:20668005; http://dx.doi.org/10.1158/1078-0432.ccr-10-1297
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science (New York, N.Y.) 2002; 298:850-854; PMID:12242449; http://dx.doi.org/10.1126/science.1076514
- Uchi H, Stan R, Turk MJ, Engelhorn ME, Rizzuto GA, Goldberg SM, Wolchok JD, Houghton AN. Unraveling the complex relationship between cancer immunity and autoimmunity: lessons from melanoma and vitiligo. Adv Immunol 2006; 90:215-241; PMID:16730265; http://dx.doi.org/10.1016/s0065-2776(06)90006-6
- Yee C, Thompson JA, Roche P, Byrd DR, Lee PP, Piepkorn M, Kenyon K, Davis MM, Riddell SR, Greenberg PD. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of t cell-mediated vitiligo. J Exp Med 2000; 192:1637-1644; PMID:11104805
- Kyewski B, Klein L. A central role for central tolerance. Ann Rev Immunol 2006; 24:571-606; PMID:16551260; http://dx.doi.org/10.1146/annurev.immunol.23.021704.115601
- Enouz S, Carrie L, Merkler D, Bevan MJ, Zehn D. Autoreactive T cells bypass negative selection and respond to self-antigen stimulation during infection. J Exp Med 2012; 209:1769-1779; PMID:22987800; http://dx.doi.org/10.1084/jem.20120905
- Zhong S, Malecek K, Johnson LA, Yu Z, Vega-Saenz de Miera E, Darvishian F, McGary K, Huang K, Boyer J, Corse E, et al. T-cell receptor affinity and avidity defines antitumor response and autoimmunity in T-cell immunotherapy. Proc Natl Acad Sci U S A 2013; 110:6973-6978; PMID:23576742; http://dx.doi.org/10.1073/pnas.1221609110
- Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature 2009; 458:211-214; PMID:19182777; http://dx.doi.org/10.1038/nature07657
- Turner MJ, Jellison ER, Lingenheld EG, Puddington L, Lefrancois L. Avidity maturation of memory CD8 T cells is limited by self-antigen expression. J Exp Med 2008; 205:1859-1868; PMID:18625745; http://dx.doi.org/10.1084/jem.20072390
- Irving M, Zoete V, Hebeisen M, Schmid D, Baumgartner P, Guillaume P, Romero P, Speiser D, Luescher I, Rufer N, et al. Interplay between T cell receptor binding kinetics and the level of cognate peptide presented by major histocompatibility complexes governs CD8+ T cell responsiveness. J Biol Chem 2012; 287:23068-23078; PMID:22549784; http://dx.doi.org/10.1074/jbc.M112.357673
- Dougan SK, Dougan M, Kim J, Turner JA, Ogata S, Cho HI, Jaenisch R, Celis E, Ploegh HL. Transnuclear TRP1-specific CD8 T cells with high or low affinity TCRs show equivalent anti-tumor activity. Cancer Immunol Res 2013; 1:99-111; PMID:24459675; http://dx.doi.org/10.1158/2326-6066.cir-13-0047
- Hebeisen M, Oberle SG, Presotto D, Speiser DE, Zehn D, Rufer N. Molecular insights for optimizing T cell receptor specificity against cancer. Front Immunol 2013; 4:154; PMID:23801991; http://dx.doi.org/10.3389/fimmu.2013.00154
- Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 2011; 19:620-626; PMID:21157437; http://dx.doi.org/10.1038/mt.2010.272
- Gill S, Kalos M. T cell-based gene therapy of cancer. Transl Res 2013; 161:365-379; PMID:23246626; http://dx.doi.org/10.1016/j.trsl.2012.11.002
- Morgan RA, Dudley ME, Yu YY, Zheng Z, Robbins PF, Theoret MR, Wunderlich JR, Hughes MS, Restifo NP, Rosenberg SA. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol (Baltimore, Md. : 1950) 2003; 171:3287-3295; PMID:12960359
- Hofmann O, Caballero OL, Stevenson BJ, Chen YT, Cohen T, Chua R, Maher CA, Panji S, Schaefer U, Kruger A, et al. Genome-wide analysis of cancer/testis gene expression. Proc Natl Acad Sci U S A 2008; 105:20422-20427; PMID:19088187; http://dx.doi.org/10.1073/pnas.0810777105
- Schuler-Thurner B, Schultz ES, Berger TG, Weinlich G, Ebner S, Woerl P, Bender A, Feuerstein B, Fritsch PO, Romani N, et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med 2002; 195:1279-1288; PMID:12021308
- Kunert A, Straetemans T, Govers C, Lamers C, Mathijssen R, Sleijfer S, Debets R. TCR-Engineered T Cells Meet New Challenges to Treat Solid Tumors: Choice of Antigen, T Cell Fitness, and Sensitization of Tumor Milieu. Front Immunol 2013; 4:363; PMID:24265631; http://dx.doi.org/10.3389/fimmu.2013.00363
- Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother (Hagerstown, Md. : 1997) 2013; 36:133-151; PMID:23377668; http://dx.doi.org/10.1097/CJI.0b013e3182829903
- Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013; 122:863-871; PMID:23770775; http://dx.doi.org/10.1182/blood-2013-03-490565
- Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nature medicine 2013; 19:747-752; PMID:23644516; http://dx.doi.org/10.1038/nm.3161
- Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science (New York, N.Y.) 2014; 344:641-645; PMID:24812403; http://dx.doi.org/10.1126/science.1251102
- Rosenberg SA. Finding suitable targets is the major obstacle to cancer gene therapy. Cancer Gene Ther 2014; 21:45-47; PMID:24535159; http://dx.doi.org/10.1038/cgt.2014.3
- van den Berg HA, Wooldridge L, Laugel B, Sewell AK. Coreceptor CD8-driven modulation of T cell antigen receptor specificity. J Theor Biol 2007; 249:395-408; PMID:17869274; http://dx.doi.org/10.1016/j.jtbi.2007.08.002
- Laugel B, van den Berg HA, Gostick E, Cole DK, Wooldridge L, Boulter J, Milicic A, Price DA, Sewell AK. Different T cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties. J Biol Chem 2007; 282:23799-23810; PMID:17540778; http://dx.doi.org/10.1074/jbc.M700976200
- Gakamsky DM, Luescher IF, Pramanik A, Kopito RB, Lemonnier F, Vogel H, Rigler R, Pecht I. CD8 kinetically promotes ligand binding to the T-cell antigen receptor. Biophys J 2005; 89:2121-2133; PMID:15980174; http://dx.doi.org/10.1529/biophysj.105.061671
- Wang A, Chandran S, Shah SA, Chiu Y, Paria BC, Aghamolla T, Alvarez-Downing MM, Lee CC, Singh S, Li T, et al. The stoichiometric production of IL-2 and IFN-gamma mRNA defines memory T cells that can self-renew after adoptive transfer in humans. Sci Transl Med 2012; 4:149ra120; PMID:22932225; http://dx.doi.org/10.1126/scitranslmed.3004306
- Janicki CN, Jenkinson SR, Williams NA, Morgan DJ. Loss of CTL function among high-avidity tumor-specific CD8+ T cells following tumor infiltration. Cancer Res 2008; 68:2993-3000; PMID:18413769; http://dx.doi.org/10.1158/0008-5472.can-07-5008
- Zhu Z, Singh V, Watkins SK, Bronte V, Shoe JL, Feigenbaum L, Hurwitz AA. High-avidity T cells are preferentially tolerized in the tumor microenvironment. Cancer Res 2013; 73:595-604; PMID:23204239; http://dx.doi.org/10.1158/0008-5472.can-12-1123
- Shafer-Weaver KA, Anderson MJ, Stagliano K, Malyguine A, Greenberg NM, Hurwitz AA. Cutting Edge: Tumor-specific CD8+ T cells infiltrating prostatic tumors are induced to become suppressor cells. J Immunol (Baltimore, Md. : 1950) 2009; 183:4848-4852; PMID:19801511; http://dx.doi.org/10.4049/jimmunol.0900848
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res 2013; 19:5300-5309; PMID:24089443; http://dx.doi.org/10.1158/1078-0432.ccr-13-0143
- Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol (Baltimore, Md. : 1950) 2004; 172:104-113; PMID:14688315
