Abstract
The latent membrane protein 1 (LMP1), which is encoded by the Epstein–Barr virus (EBV), has been suggested to be one of the major oncogenic factors in nasopharyngeal carcinoma (NPC). In previous studies, we experimentally demonstrated that downregulation of LMP1 expression by targeting EBV-LMP1 DNAzyme (Dz1) could increase the radiosensitivity of NPC. However, how Dz1 contributes to the radiosensitivity in NPC is still not clear. In the present study, we confirmed that Dz1 decreases LMP1 expression and downregulates the expression of the catalytic subunit of telomerase (hTERT), both at the protein and mRNA levels (P < 0.01), and therefore, consequently inhibits telomerase activity (P < 0.05) in LMP1-positive NPC cells. We also observed that LMP1 mediated Akt phosphorylation is involved in the regulation of hTERT expression and phosphorylation. After LMP1 and hTERT expression was silenced by Dz1 and hTERT-targeted siRNA, respectively, the radiosensitivity increased in CNE1-LMP1 cells (P < 0.05). The inhibition was more significant after Dz1 treatment was combined with siRNA (P < 0.01). We concluded that hTERT expression and phosphorylation could be regulated by LMP1 through the Akt pathway, and Dz1 enhances radiosensitivity of LMP1-positive NPC cells by inhibiting telomerase activity.
Keywords: :
Introduction
Nasopharyngeal carcinoma (NPC) is an endemic disease that occurs with high incidence in south China. Because NPC has high radiosensitivity, currently the primary treatment for NPC is radiotherapy. However, radioresistance has emerged as a significant impediment to effective NPC therapy.Citation1 EBV is a lymphotropic human gamma herpes virus that has been implicated in the pathogenesis of several human malignancies including Burkitt and Hodgkin lymphomas, NPC, and gastric carcinoma.Citation2 Among the EBV proteins expressed in NPC cells, LMP1 exhibits properties of a classical oncoprotein. It activates different signaling pathways, such as nuclear factor kappa B (NFκB), c-jun N-terminal kinases (JNK), mitogen-activated protein kinases (MAPK), Janus kinase (JAK), serine–threonine protein kinase Akt, activators of transcription protein (STAT), and others involved in the proliferation, apoptosis, and metastasis of tumor cells.Citation3-Citation7 Therefore, LMP1 is considered the primary oncogene of the virus and thus a potential target for NPC biotherapy.Citation8 Downregulation of LMP1 using RNA interference has been shown to be potentially effective in the prevention of NPC metastasis and reduction of NPC radioresistance.Citation9,Citation10
DNAzymes have emerged as a promising new class of therapeutic agents. These single-stranded, catalytic DNA molecules bind their target RNA via Watson-Crick base-pairing. DNAzymes of the “10–23” subtype cleave RNA at a predetermined junction through a de-esterification reaction.Citation11 The targeting EBV-LMP1 DNAzyme (Dz1) is a “10–23” DNAzyme with a phosphorothioate-modified for stability that cleaves the mRNA of LMP1.Citation12 Our previous study showed that Dz1 downregulates the expression of LMP1 and inhibits signaling pathways that are abnormally initiated by LMP1, which consequently increases cell apoptosis, and inhibits cell proliferation, invasion, and metastasis in LMP1-positive NPC cells.Citation12,Citation13 The radiosensitivity of NPC cells was significantly increased after combining Dz1 treatment with irradiation.Citation14 In a recent study, we found that Dz1 decreased ATM production by attenuating the binding of NFκB to the ATM promoter, which suggests that Dz1 may be involved in radioresistance.Citation15 Nevertheless, how Dz1 contributes to radiosensitivity in NPC is not yet fully understood.
Radiation-induced cell death is usually attributed to DNA damage in tumor cells, which induces cell apoptosis and/or necrosis. Telomerase can play a role in the healing of chromosomes or chromatid breaks produced by this damage and, therefore, might enhance the radioresistance of cells.Citation16,Citation17 Telomerase activity is controlled by the regulation of the catalytic subunit of telomerase (hTERT), through modulating the expression and posttranslational modifications of hTERT.Citation18,Citation19 Expression of hTERT is tightly regulated at the transcriptional level and the hTERT promoter contains a variety of binding sites for both activating and inhibiting transcription factors. Studies have reported that levels of hTERT mRNA are directly upregulated by c-Myc overexpression.Citation20 The most common type of posttranslational modifications is phosphorylation and it was reported that the expression and activation of hTERT could be regulated by several intracellular kinases including Akt kinase, which enhances human telomerase activity through phosphorylation of hTERT.Citation21-Citation23 Therefore, these findings might provide a new insight into the molecular mechanism by which LMP1 promotes the expression and phosphorylation of hTERT and further improve the telomerase activity.
Although LMP1 is known to interact with the telomerase complex,Citation24,Citation25 it is not known whether inactivation of LMP1 can influence cell killing in the presence and absence of telomerase by irradiation, especially when irradiation is combined with Dz1 treatment. In the present study, we tested whether Dz1 results in suppression of hTERT expression and phosphorylation with subsequent loss of telomerase activity and enhances the radiosensitivity in LMP1-positive NPC cells. Furthermore, we explored the key signaling pathway through which LMP1 regulates the expression and phosphorylation of hTERT.
Results
Dz1 inhibits the hTERT expression by downregulation of LMP1
We performed western blot and quantitative RT-PCR analysis to examine the changes in hTERT expression of NPC cells after treatment with Dz1. The results showed that constitutive LMP1 expression in CNE1-LMP1 cells resulted in approximately 10-fold increase in the levels of hTERT mRNA (P < 0.01, ) and a 3-fold increase in the levels of hTERT protein (P < 0.01, ). Silencing of LMP1 in the CNE1-LMP1 cells treated with Dz1 efficiently induced downregulation of LMP1 mRNA and protein. The levels of LMP1 mRNA were reduced approximately 80% compared with the control cells and concordantly, the levels of LMP1 protein were reduced approximately 60% (P < 0.05, ). The CON used at the same concentrations served as a negative control. Knocking down of LMP1 resulted in a decrease in the hTERT expression at both the protein and mRNA levels. After treatment with Dz1, the levels of hTERT mRNA were reduced approximately 90% () and concordantly, the levels of hTERT protein were reduced approximately 70% compared with the control cells in the whole cell extracted fraction (P < 0.01, ). These results suggest that LMP1 upregulates hTERT expression in the NPC cells and treatment with Dz1 induces the downregulation of hTERT both at the protein and mRNA levels through suppression of LMP1 gene expression.
Figure 1. Dz1 inhibits the hTERT mRNA level by downregulation of LMP1. Cells were seeded at 4 × 104 cells/ml and treated with 2 µM Dz1 or 2 µM control oligo (CON) for 24 h. (A) Total RNA was isolated using the TRIzol reagent and quantitative RT-PCR was performed for LMP1 mRNA expression. (B) Quantitative RT-PCR was performed for hTERT mRNA expression. Values are the means ± SD of 3 replicates, *P < 0.05, **P < 0.01 compared with the control cells.
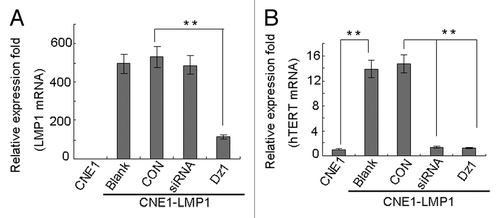
Figure 2. Dz1 inhibits the hTERT protein expression by downregulation of LMP1. Cells were treated with 2 µM Dz1 or 2 µM control oligo (CON) for 24 h and western blot analysis was performed using antibodies against LMP1, hTERT. β-actin was used as a loading control. Values are the means ± SD of 3 replicates, *P < 0.05, **P < 0.01 compared with the control cells.
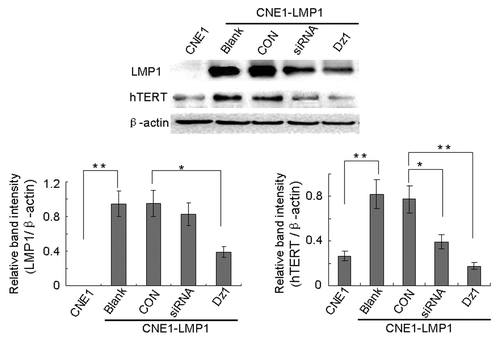
In addition, the effects of hTERT- targeted siRNA on hTERT expression were investigated in CNE1-LMP1 cells. The data showed that treatment of CNE1-LMP1 with siRNA induced downregulation of hTERT expression at both the protein and mRNA levels ( and ).
Dz1 inhibits telomerase activity by downregulation of LMP1
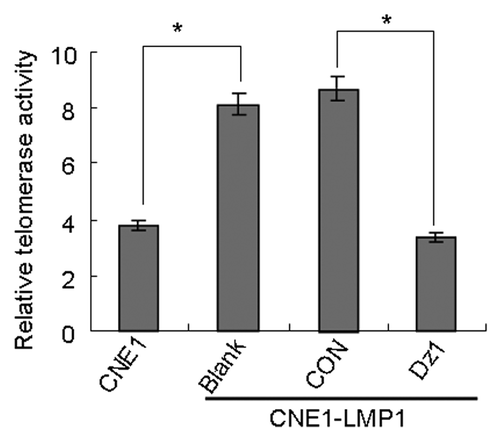
As shown in , the telomerase activity in CNE1-LMP1 cells is increased 2-fold compared with the CNE1 cells. After treatment with Dz1, the levels of telomerase activity were reduced by 60% compared with the control cells (P < 0.05). These results suggested that Dz1 suppressed LMP1 expression and sequentially induced downregulation of telomerase activity through suppression of hTERT.
Figure 3. Dz1 inhibits telomerase activity by downregulation of LMP1. Cells were treated with 2 µM Dz1 or 2 µM control oligo (CON), respectively for 24 h and telomerase activity was analyzed by the Telomerase PCR ELISA kit. Sample absorbance was measured with Model 550 Microplate Reader at a wavelength of 450 or 690 nm. Values are the means ± SD of 3 replicates, *P < 0.05, **P < 0.01 compared with the control cells.
LMP1 promotes expression and phosphorylation of hTERT through the Akt pathway
There is accumulating evidence that telomerase activity is regulated by phosphorylation of hTERT besides the expression of hTERT which is tightly coupled to telomerase activity. To determine which protein kinase is responsible for the enhancement of hTERT activity, we analyzed the expression levels of hTERT and phosphorylated hTERT from CNE1-LMP1 cells treated with different protein kinase inhibitors that included most of the protein kinases induced by LMP1 such as the Akt inhibitor LY294002, JAK3 inhibitor WHIP131, p38 inhibitor SB203580, MEK inhibitor PD98059, and JNK inhibitor SP600125. The results showed that pharmacologic inhibition of Akt by LY294002 resulted in a significant decrease of the phosphorylated hTERT expression and also found it had a slight inhibitory effect of hTERT protein. Moreover, WHIP131 and PD98059 also had a weak inhibitory effect, whereas the other inhibitors almost had no effect (). Thus, we assumed that the increased expression of phosphorylated hTERT in the CNE1-LMP1 cells may be mediated through the Akt signaling pathway.
Figure 4. LMP1 promotes expression and phosphorylation of hTERT through the Akt pathway. (A) Cells were seeded at 4 × 104 cells/ml and treated with the indicated concentrations of LY294002, WHIP131, SB203580, PD98059, and SP600125 for 24 h. Phosphorylated hTERT expression in CNE1-LMP1 cells was measured by western blot and β-actin was used as a loading control. (B) Cells were treated with the indicated concentrations of Dz1 and LY294002 for 24 h and western blot was performed to measure the levels of total hTERT, phosphorylated hTERT, total Akt and phosphorylated Akt. β-actin was used as a loading control.
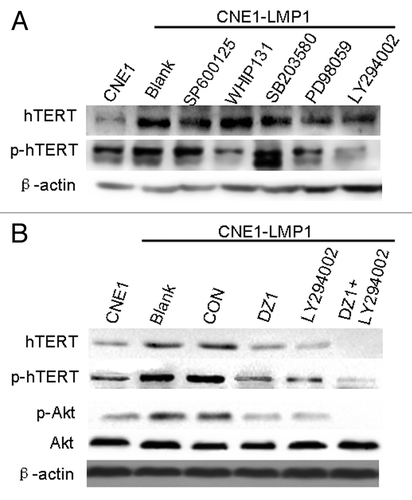
To further verify the direct involvement of Akt in the upregulation of telomerase mediated by LMP1, we used Dz1 and LY294002 for our study. As shown in , Akt kinase activity was upregulated in CNE1-LMP1 cells compared with the CNE1 cells in spite of no change in the total Akt levels. Moreover, the expression and phosphorylated hTERT was also upregulated. However, after treatment with Dz1, the expression and phosphorylated hTERT was reduced. Similarly, treatment with LY294002 significantly decreased the levels of phosphorylated Akt. Furthermore, hTERT expression and phosphorylation were suppressed by the combined treatment with Dz1 and LY294002. Thus, these findings indicated that Akt is involved in the LMP1-dependent induction of expression and phosphorylation of hTERT and telomerase activity in the LMP1-positive NPC cells.
LMP1 is associated with the expression of phosphorylated Akt and hTERT in vivo
To gain additional evidence of the Dz1-mediated suppression of phosphorylated Akt and hTERT levels, a xenograft model in nude mice was used. CNE1-LMP1 cells were used in the xenograft model and treated with Dz1 as described in a previous study.Citation14 Immunohistochemical staining was conducted on the cross-sections of paraffin-embedded formalin-fixed tissues from different treatment groups. The data showed that LMP1 expression was suppressed by Dz1 in these tumors. The expression of phosphorylated Akt and hTERT was also examined and found to be markedly reduced in the Dz1 group in comparison with the control groups. The quantification analysis revealed a significant correlation between LMP1 and p-Akt expression (correlation coefficient = 0.745, P < 0.05) and between LMP1 and hTERT expression (correlation coefficient = 0.887, P = 0.001) (; ). This inhibitory effect was in agreement with the data at the cellular level.
Figure 5. LMP1 is associated with the expression of phosphorylated Akt and hTERT in vivo. Immunohistochemical staining was performed for LMP1, phosphorylated Akt and hTERT in Dz1- or CON-treated NPC tumors of Athymic Balb/c mice. Data were derived from 10 xenograft tumors in 2 groups (200×).
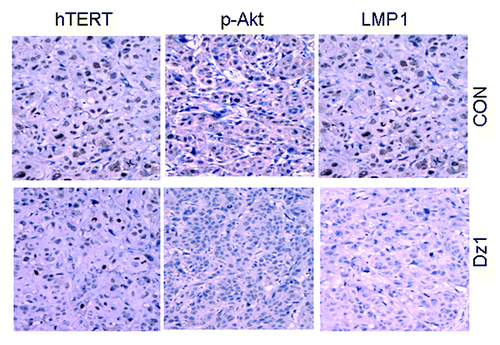
Table 1. Correlation between LMP1 and p-Akt or hTERT expression in Dz1-treated NPC tumors
Inhibition of LMP1 and hTERT decreases the survival of LMP1-positive NPC cells
We investigated the levels of expression and phosphorylation of hTERT following irradiation. The results indicated that the expression of hTERT at the mRNA level in both irradiated CNE1 and CNE1-LMP1 cells was increased compared with the non-irradiated cells, and LMP1 could increase the expression of hTERT mRNA in NPC cells both under irradiation and non-irradiation (). Moreover, the levels of phosphorylated hTERT were decreased approximately 60% after treatment with Dz1 under irradiation ().
Figure 6. Inhibition of LMP1 and hTERT decreases the survival of LMP1-positive NPC cells. (A) Cells were irradiated at 2 Gy and after 4 h, the mRNA expression of hTERT was analyzed using quantitative real time RT-PCR. (B) Cells were seeded at 4 × 104 cells/ml and treated with the 2 µM Dz1, control oligo (CON), or hTERT-targeted siRNA for 20 h, and irradiated at 2 Gy. After 4 h, the protein expression levels of LMP1 and hTERT were analyzed by western blots. (C) CNE1-LMP1 cells were transfected with hTERT-targeted siRNA or Dz1. After 20 h, the cells were irradiated at 2 Gy and then incubated for 24, 48, and 72 h before cell survival was quantified by MTS. Values are the means ± SD of 3 replicates, *P < 0.05, **P < 0.01 compared with the control.
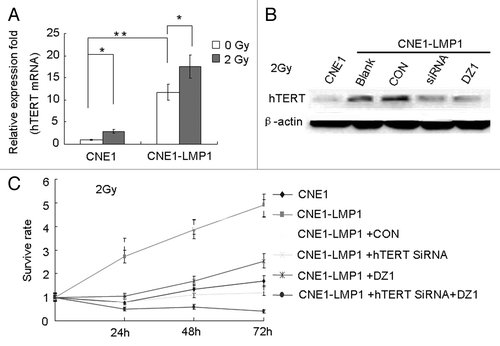
The proliferation of CNE1-LMP1 cells under irradiation, was analyzed by the MTS assay. As shown in , the survival rate of CNE1 cells was lower than that of the CNE1-LMP1 cells (P < 0.05). The proliferation rate of CNE1-LMP1 cells was significantly decreased after treatment with Dz1, and a similar effect of decreased cell proliferation was also observed after cells were treated with siRNA. The inhibition was more significant when a treatment with siRNA and Dz1 was combined (P < 0.01). This indicated that the LMP1 and hTERT expressions were related to the radiosensitivity of the NPC cells.
Inhibition of LMP1 and hTERT leads to radiosensitization of LMP1-positive NPC cells
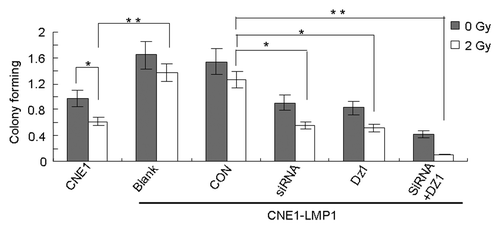
To provide further evidence for the role of LMP1 and hTERT in the regulation of cell growth under irradiation, a clonogenic assay was performed. CNE1-LMP1 cells were treated with Dz1 and hTERT-targeted siRNA and subjected to X-ray irradiation. As shown in , colony formation of CNE1 cells with or without irradiation is weaker than that of CNE1-LMP1 cells (P < 0.05), which indicated that the LMP1 could increase radioresistance in cells. When CNE1-LMP1 cells were exposed to irradiation at 2 Gy, it induced nearly 40% decrease in colony formation, irrespective of treatment with Dz1 or siRNA, compared with control cells, whereas only 20% decrease of colony formation occurred in CON-treated or untreated cells. In addition, a strong inhibition of colony formation was observed with a combined treatment of Dz1 and siRNA (P < 0.01). Together, the data indicated that inhibition of LMP1 and /or hTERT expression could lead to an increased radiosensitivity in LMP1 positive cells.
Figure 7. Inhibition of LMP1 and hTERT leads to radiosensitization of LMP1-positive NPC cells. CNE1-LMP1 cells were transfected with hTERT-targeted siRNA or Dz1. After 20 h, these cells were exposed to irradiation and then incubated for 2 weeks before fixation, staining and colony counting. Values are the means ± SD of 3 replicates, *P < 0.05, **P < 0.01 compared with the control.
Discussion
LMP1 hijacks cellular signaling pathways that are critical for NPC cell growth and survival, including some cascades that are also known to regulate hTERT expression and telomerase activity. LMP1 was shown to induce telomerase activity in NPC cells through NFκB activation, an effect that is c-Myc-dependent because mutagenesis of the c-Myc-responsive E box elements in the hTERT promoter inhibits LMP1 induced hTERT transactivation.Citation26,Citation27 Here, our data revealed that LMP1 enhances hTERT expression and phosphorylation through the Akt signaling pathway, and consequently increases the telomerase activity in NPC cells. It is well known that telomerase activation not only maintains the intracellular genetic stability but is also involved in the radioresistance of tumor cells.Citation16,Citation17,Citation28 The abnormally active telomerase in cancer cells has become one of the most interesting targets aimed at finding a cure for many different cancers.Citation29-Citation31 LMP1 expression in NPC cells may mimic the effects of growth factors by activating telomerase via Akt, thus contributing to cell radioresistance.
As a foreign antigen that constitutively activates multiple pathways, LMP1 represents a good therapeutic target in the treatment of EBV associated malignancies.Citation8-Citation10 In this report, we found that ectopic expression of LMP1, which resulted in hTERT activity in CNE1-LMP1 cells, rendered the cells more resistance against irradiation. Knockdown of LMP1 by Dz1 in LMP1-positive NPC cells induced a more radiosensitive phenotype. The ability of the DNAzyme to specifically cleave RNA with high efficiency under simulated physiological conditions has fueled expectations that this agent may have useful biological application as a gene inactivation strategy. DNAzymes have advantages over RNA molecules such as siRNA, which are more expensive to synthesize and are subject to ribonuclease degradation.Citation11,Citation32 In the present study, although the hTERT-targeted siRNA had a certain effect on cell radiosensitivity, the inhibitory effect of Dz1 was more obvious than that of the hTERT-targeted siRNA in cell survival and growth. This might be because Dz1 could affect cell proliferation and radioresistance through other signaling pathways.Citation15,Citation25 Nevertheless, the effect of the combined treatment with the hTERT-targeted siRNA and Dz1 was the strongest.
Therapeutic agents that are currently used to enhance radiosensitivity lack specificity for tumor cells and have toxic side effects for normal tissue, thereby limiting therapeutic usage. Cancer treatment could be improved by the development of drugs that target specific pathways deregulated in tumor cells.Citation33 In this study, we demonstrated that LMP1, which is expressed in over 75% of NPC cases,Citation2,Citation3 could upregulate hTERT expression and telomerase activity consequently resulting in radioresistance. As Dz1 targets the exogenous viral gene LMP1, which is absent in the normal human genome, thus the combined application of Dz1 and NPC radiation therapy will increase the cell radiosensitivity with reduced side effects.
Conclusions
The results of the present study demonstrated that hTERT activation is induced by LMP1 in NPC cells at the transcriptional and posttranscriptional levels through Akt-dependent pathways, which results in an increase of radiosensitivity in NPC cells. The findings also showed that Dz1 may be a potential radiotherapy sensitizer in NPC.
Materials and Methods
Cell culture
CNE1 is an EBV-LMP1-negative, low differentiated NPC cell line, and CNE1-LMP1 is a stable LMP1 integrated NPC cell line.Citation14 Cells were cultured in RPMI 1640 (Gibco-BRL), supplemented with 10% FBS (Invitrogen), 10 μg/ml streptomycin and 10 IU/ml penicillin, and incubated in a humidified atmosphere of 5% CO2 at 37 °C. The cells in logarithmic growth phase were used in all experiments.
DNAzyme and siRNA transfection
The Dz1 and the control oligonucleotides (CON) were synthesized and transfected as described.Citation14 hTERT-targeted siRNA (Thermo Scientific, M-003547-02) was transfected into cells using Lipofectamine 2000 (Invitrogen, P/N52887) following the manufacturer’s protocol.
Quantitative RT-PCR
Total RNA was isolated from cells with the TRIzol reagent (Invitrogen, 15596-026) according to the manufacturer’s instructions, followed by cDNA synthesis using the SuperScriptTM IIIRT (Invitrogen, 18080-051). Quantitative RT-PCR was performed with iTaq TM SYBR Green Supermix with ROX (Bio-Rad, 172-5850) using an ABI 7500 instrument. cDNA was subjected to PCR with primers specific for hTERT (sense: 5′-TCCACTCCCC ACATAGGAAT AGTC-3′ and anti-sense: 5′-TCCTTCTCAG GGTCTCCACC T-3′) and LMP1 (sense: 5′-CGTTATGAGT GACTGGACTG GA-3′ and anti-sense: 5′-TGAACAGCAC AATTCCAAGG-3′). The reaction was amplified as follows: 40 cycles of 95 °C for 15 s and 60 °C for 60 s followed by an extension at 72 °C for 10 min. PCR reaction with β-actin primers (sense: 5′-CATGTACGTT GCTATCCAGG C-3′; and anti-sense: 5′-CTCCTTAATG TCACGCACGA T-3′) was used as an internal control. Specificity of amplification products was confirmed by melting curve analysis. All experiments were repeated at least three times.
Western blot analysis
Cells were harvested and washed twice with ice-cold phosphate buffered saline (PBS), and lysed in lysis buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 2% SDS, 5 mM DTT, 10 mM PMSF, 1 mM Na3VO4, 1 mM NaF, 10% [vol./vol.] glycerol, protease inhibitor cocktail tablet [Roche, 11697498001]) for 30 min on ice and then centrifuged at 15 000 g for 10 min. The supernatant was collected from the whole cell lysates. Protein concentration was determined by the BCA Assay Reagent (Pierce, 23228). Then, 50 µg of the total protein from various cell preparations were analyzed by SDS PAGE and then electrotransferred onto nitrocellulose membranes. Rainbow molecular weight markers (Thermo Scientific, 26616) were used as standards. Blots were blocked with 5% non-fat dry milk in PBS with 0.05% Tween-20 for 2 h at RT, and then incubated with different primary antibodies overnight at 4 °C. After washing twice, the membranes were incubated with peroxidase-conjugated secondary antibodies for 1 h at RT and developed with Super-Signal West Pico chemiluminescent Substrate (Thermo Scientific, 34077). The study employed antibodies against LMP1 (M0897, DAKO), hTERT (sc-7214, Santa Cruz), p-hTERT against Ser-1125 (sc-130608, Santa Cruz), Akt (9272, Cell Signaling), p-Akt against Ser-473 (9271, Cell Signaling), and β-actin (sc-8432, Santa Cruz). Relative protein levels were quantified with the ImageJ software (NIH). Three independent experiments were conducted.
Analysis of telomerase activity
Telomerase activity was determined by using the Telomerase PCR ELISA kit (Roche, 12013789001) as recommended by the manufacturer. All solutions were included in the kit. Sample absorbance was measured with Model 550 Microplate Reader (Bio-Rad) at the wavelength of 450/690 nm within 30 min after addition of the stop reagent. Telomerase activity was determined in triplicate, and negative and positive controls were included in each experiment. An aliquot of each extract was heat inactivated for 10 min at 95 °C as a negative control.
Immunohistochemistry
Paraffin-embedded tissues were sectioned and stained for LMP1, phosphorylated Akt and hTERT, using a streptavidin–biotin technique, where biotin is coupled to peroxidase. A negative control was made for each slide using an irrelevant, isotype antibody. Quantitative expression of target molecular in tumor cells determined by staining intensity multiplied percentage of positive cells. Staining intensity of the tumor cells in the following rating: no color, yellow, brown, and tan, respectively, to give 0, 1, 2, and 3 points.
MTS assay
MTS cytotoxicity/proliferation assays were performed using the CellTiter 96 AQueous One Solution cell proliferation assay kit (Promega, G3581) according to manufacturer’s instructions. Cells were seeded in triplicate in a 96-well plate at 2.5 × 104 cells/ml, 100 μl per well. MTS reagent was added on days 1–3 for 4 h and absorbance was read at 490 nm. Blanks were subtracted from the values and plotted.
Colony-forming assay
Radiosensitivity was measured by colony-forming analysis following exposure to irradiation. Briefly, cells were transfected with Dz1 or hTERT-targeted siRNA. Twenty-four hours later, 100, 200, or 800 cells were plated in 6-well culture plates and cultured for another 24 h. These cells were irradiated with 2 Gy irradiation followed by further culturing for 14 d, and the number of surviving colonies (defined as a colony with >50 cells) was counted by staining with 0.0125% crystal violet (w/v in 75% ethanol; Sigma).
Statistical analysis
Statistical analysis was performed by the Student t test using SPSS15.0 software. (P < 0.05 was considered significant and P < 0.01 very significant). All data are expressed as the mean ± SD.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30873010 and 81072220), grants from the Postdoctoral Foundation of China (2011 M501302 and 2012T50712) and National High Technology Research and Development Program of China (2009AA02Z403).
References
- Lee AW, Sze WM, Au JS, Leung SF, Leung TW, Chua DT, Zee BC, Law SC, Teo PM, Tung SY, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys 2005; 61:1107 - 16; http://dx.doi.org/10.1016/j.ijrobp.2004.07.702; PMID: 15752890
- Eliopoulos AG, Young LS. LMP1 structure and signal transduction. Semin Cancer Biol 2001; 11:435 - 44; http://dx.doi.org/10.1006/scbi.2001.0410; PMID: 11669605
- Tsao SW, Tsang CM, Pang PS, Zhang G, Chen H, Lo KW. The biology of EBV infection in human epithelial cells. Semin Cancer Biol 2012; 22:137 - 43; http://dx.doi.org/10.1016/j.semcancer.2012.02.004; PMID: 22497025
- Meckes DG Jr., Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci U S A 2010; 107:20370 - 5; http://dx.doi.org/10.1073/pnas.1014194107; PMID: 21059916
- Zhao Y, Wang Y, Zeng S, Hu X. LMP1 expression is positively associated with metastasis of nasopharyngeal carcinoma: evidence from a meta-analysis. J Clin Pathol 2012; 65:41 - 5; http://dx.doi.org/10.1136/jclinpath-2011-200198; PMID: 22039283
- Chew MM, Gan SY, Khoo AS, Tan EL. Interleukins, laminin and Epstein - Barr virus latent membrane protein 1 (EBV LMP1) promote metastatic phenotype in nasopharyngeal carcinoma. BMC Cancer 2010; 10:574; http://dx.doi.org/10.1186/1471-2407-10-574; PMID: 20964870
- Shair KH, Schnegg CI, Raab-Traub N. EBV latent membrane protein 1 effects on plakoglobin, cell growth, and migration. Cancer Res 2008; 68:6997 - 7005; http://dx.doi.org/10.1158/0008-5472.CAN-08-1178; PMID: 18757414
- Hannigan A, Wilson JB. Evaluation of LMP1 of Epstein-Barr virus as a therapeutic target by its inhibition. Mol Cancer 2010; 9:184; http://dx.doi.org/10.1186/1476-4598-9-184; PMID: 20618963
- Mei YP, Zhou JM, Wang Y, Huang H, Deng R, Feng GK, Zeng YX, Zhu XF. Silencing of LMP1 induces cell cycle arrest and enhances chemosensitivity through inhibition of AKT signaling pathway in EBV-positive nasopharyngeal carcinoma cells. Cell Cycle 2007; 6:1379 - 85; http://dx.doi.org/10.4161/cc.6.11.4274; PMID: 17507800
- Li X, Liu X, Li CY, Ding Y, Chau D, Li G, Kung HF, Lin MC, Peng Y. Recombinant adeno-associated virus mediated RNA interference inhibits metastasis of nasopharyngeal cancer cells in vivo and in vitro by suppression of Epstein-Barr virus encoded LMP-1. Int J Oncol 2006; 29:595 - 603; PMID: 16865275
- Dass CR, Choong PF, Khachigian LM. DNAzyme technology and cancer therapy: cleave and let die. Mol Cancer Ther 2008; 7:243 - 51; http://dx.doi.org/10.1158/1535-7163.MCT-07-0510; PMID: 18281510
- Lu ZX, Ye M, Yan GR, Li Q, Tang M, Lee LM, Sun LQ, Cao Y. Effect of EBV LMP1 targeted DNAzymes on cell proliferation and apoptosis. Cancer Gene Ther 2005; 12:647 - 54; http://dx.doi.org/10.1038/sj.cgt.7700833; PMID: 15803142
- Wang Z, Luo F, Li L, Yang L, Hu D, Ma X, Lu Z, Sun L, Cao Y. STAT3 activation induced by Epstein-Barr virus latent membrane protein1 causes vascular endothelial growth factor expression and cellular invasiveness via JAK3 And ERK signaling. Eur J Cancer 2010; 46:2996 - 3006; http://dx.doi.org/10.1016/j.ejca.2010.07.008; PMID: 20709526
- Lu ZX, Ma XQ, Yang LF, Wang ZL, Zeng L, Li ZJ, Li XN, Tang M, Yi W, Gong JP, et al. DNAzymes targeted to EBV-encoded latent membrane protein-1 induce apoptosis and enhance radiosensitivity in nasopharyngeal carcinoma. Cancer Lett 2008; 265:226 - 38; http://dx.doi.org/10.1016/j.canlet.2008.02.019; PMID: 18353539
- Ma X, Yang L, Xiao L, Tang M, Liu L, Li Z, Deng M, Sun L, Cao Y. Down-regulation of EBV-LMP1 radio-sensitizes nasal pharyngeal carcinoma cells via NF-κB regulated ATM expression. PLoS One 2011; 6:e24647; http://dx.doi.org/10.1371/journal.pone.0024647; PMID: 22096476
- Ayouaz A, Raynaud C, Heride C, Revaud D, Sabatier L. Telomeres: hallmarks of radiosensitivity. Biochimie 2008; 90:60 - 72; http://dx.doi.org/10.1016/j.biochi.2007.09.011; PMID: 18006207
- Agarwal M, Pandita S, Hunt CR, Gupta A, Yue X, Khan S, Pandita RK, Pratt D, Shay JW, Taylor JS, et al. Inhibition of telomerase activity enhances hyperthermia-mediated radiosensitization. Cancer Res 2008; 68:3370 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-07-5831; PMID: 18451164
- Martínez P, Blasco MA. Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins. Nat Rev Cancer 2011; 11:161 - 76; http://dx.doi.org/10.1038/nrc3025; PMID: 21346783
- Sharma GG, Gupta A, Wang H, Scherthan H, Dhar S, Gandhi V, Iliakis G, Shay JW, Young CS, Pandita TK. hTERT associates with human telomeres and enhances genomic stability and DNA repair. Oncogene 2003; 22:131 - 46; http://dx.doi.org/10.1038/sj.onc.1206063; PMID: 12527915
- Daniel M, Peek GW, Tollefsbol TO. Regulation of the human catalytic subunit of telomerase (hTERT). Gene 2012; 498:135 - 46; http://dx.doi.org/10.1016/j.gene.2012.01.095; PMID: 22381618
- Chung J, Khadka P, Chung IK. Nuclear import of hTERT requires a bipartite nuclear localization signal and Akt-mediated phosphorylation. J Cell Sci 2012; 125:2684 - 97; http://dx.doi.org/10.1242/jcs.099267; PMID: 22366458
- Kang SS, Kwon T, Kwon DY, Do SI. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem 1999; 274:13085 - 90; http://dx.doi.org/10.1074/jbc.274.19.13085; PMID: 10224060
- Kimura A, Ohmichi M, Kawagoe J, Kyo S, Mabuchi S, Takahashi T, Ohshima C, Arimoto-Ishida E, Nishio Y, Inoue M, et al. Induction of hTERT expression and phosphorylation by estrogen via Akt cascade in human ovarian cancer cell lines. Oncogene 2004; 23:4505 - 15; http://dx.doi.org/10.1038/sj.onc.1207582; PMID: 15048073
- Terrin L, Dal Col J, Rampazzo E, Zancai P, Pedrotti M, Ammirabile G, Bergamin S, Rizzo S, Dolcetti R, De Rossi A. Latent membrane protein 1 of Epstein-Barr virus activates the hTERT promoter and enhances telomerase activity in B lymphocytes. J Virol 2008; 82:10175 - 87; http://dx.doi.org/10.1128/JVI.00321-08; PMID: 18684838
- Ding L, Li L, Yang J, Zhou S, Li W, Tang M, Shi Y, Yi W, Cao Y. Latent membrane protein 1 encoded by Epstein-Barr virus induces telomerase activity via p16INK4A/Rb/E2F1 and JNK signaling pathways. J Med Virol 2007; 79:1153 - 63; http://dx.doi.org/10.1002/jmv.20896; PMID: 17597480
- Lebel R, McDuff FO, Lavigne P, Grandbois M. Direct visualization of the binding of c-Myc/Max heterodimeric b-HLH-LZ to E-box sequences on the hTERT promoter. Biochemistry 2007; 46:10279 - 86; http://dx.doi.org/10.1021/bi700076m; PMID: 17705400
- Ding L, Li LL, Yang J, Tao YG, Ye M, Shi Y, Tang M, Yi W, Li XL, Gong JP, et al. Epstein-Barr virus encoded latent membrane protein 1 modulates nuclear translocation of telomerase reverse transcriptase protein by activating nuclear factor-kappaB p65 in human nasopharyngeal carcinoma cells. Int J Biochem Cell Biol 2005; 37:1881 - 9; http://dx.doi.org/10.1016/j.biocel.2005.04.012; PMID: 15967702
- Wesbuer S, Lanvers-Kaminsky C, Duran-Seuberth I, Bölling T, Schäfer KL, Braun Y, Willich N, Greve B. Association of telomerase activity with radio- and chemosensitivity of neuroblastomas. Radiat Oncol 2010; 5:66; http://dx.doi.org/10.1186/1748-717X-5-66; PMID: 20642823
- Dong X, Liu A, Zer C, Feng J, Zhen Z, Yang M, Zhong L. siRNA inhibition of telomerase enhances the anti-cancer effect of doxorubicin in breast cancer cells. BMC Cancer 2009; 9:133; http://dx.doi.org/10.1186/1471-2407-9-133; PMID: 19416503
- Li Y, Li M, Yao G, Geng N, Xie Y, Feng Y, Zhang P, Kong X, Xue J, Cheng S, et al. Telomerase inhibition strategies by siRNAs against either hTR or hTERT in oral squamous cell carcinoma. Cancer Gene Ther 2011; 18:318 - 25; http://dx.doi.org/10.1038/cgt.2010.81; PMID: 21233858
- Lü MH, Liao ZL, Zhao XY, Fan YH, Lin XL, Fang DC, Guo H, Yang SM. hTERT-based therapy: a universal anticancer approach (Review). Oncol Rep 2012; 28:1945 - 52; PMID: 22992764
- Cai H, Santiago FS, Prado-Lourenco L, Wang B, Patrikakis M, Davenport MP, Maghzal GJ, Stocker R, Parish CR, Chong BH, et al. DNAzyme targeting c-jun suppresses skin cancer growth. Sci Transl Med 2012; 4:39ra82; http://dx.doi.org/10.1126/scitranslmed.3003960; PMID: 22723462
- Zhang L, Yang L, Li JJ, Sun L. Potential use of nucleic acid-based agents in the sensitization of nasopharyngeal carcinoma to radiotherapy. Cancer Lett 2012; 323:1 - 10; http://dx.doi.org/10.1016/j.canlet.2012.03.030; PMID: 22484469
