Abstract
Background
Muscle invasive bladder carcinoma is an often lethal disease that requires aggressive treatment. Improved assays would contribute to better risk prediction and clinical management of this disease. A telomerase-based assay to detect circulating tumor cells (CTCs) may usefully fulfill this role.
Methods
Two patients (C1 and C2) were enrolled onto an IRB-approved bladder biomarker study before initiating post-operative radiation therapy (RT) for muscle invasive bladder carcinoma. Blood samples were taken at predefined intervals: before, during, and after RT and then retrospectively correlated with imaging studies and disease course.
Results
C1 began RT for positive resection margins on surgical pathology, at which time CTCs were undetectable and pelvic imaging demonstrated no evidence of disease. However, following the completion of treatment, the patient’s CTC count was found to have increased to 202 CTCs/mL, and MRI demonstrated new abdominal and pelvic masses consistent with progressive disease. C1 ultimately died of disease with distant and local failure. Conversely, C2 was found to have 632 CTCs/mL before the initiation of RT for positive surgical margins, although imaging demonstrated no visible masses. At the conclusion of RT, repeat imaging showed changes that were indeterminate for either tumor recurrence or post-radiation effects. However, the patient’s CTC count had dropped to 184 CTCs/mL. Furthermore, a second follow-up assay performed 6 months later revealed no detectable CTCs and repeat imaging showed complete resolution of worrisome imaging changes, thus excluding tumor progression.
Conclusions
To our knowledge this is the first report of a telomerase-based assay to identify CTCs in bladder cancer patients. Further studies are required to fully determine the ultimate clinical utility of this assay. However, the two patient vignettes described here illustrate how serial CTC assays may track the disease course and inform the management of bladder cancer patients undergoing adjuvant RT and potentially chemotherapy.
Introduction
Each year in the United States, urothelial bladder carcinoma is diagnosed in 70 000 individuals and causes death in 14 000.Citation1 A major difficulty in the management of patients with muscle-invasive bladder cancer is the limited ability to accurately classify the extent of disease within the bladder, within the pelvic lymph nodes, and systemically. Accurate assessment of disease extent is critical to optimize the use of definitive, adjunctive, and salvage treatment options, such as radical cystectomy, chemotherapy, and radiation. To that end, bladder circulating tumor cells (CTCs) have been investigated as a means of better informing diagnosis, staging, and prognostication and as a tool for post-treatment surveillance.
CTCs are biomarkers that may have the potential to address some of these needs. CTCs are cells that detach from the primary tumor and have the potential to be detected in the peripheral blood. The enumeration of CTCs has suggested prognostic significance of pretreatment counts in breast, colorectal, and prostate cancers.Citation2-Citation5 CTCs have also been explored in bladder carcinoma using epithelial cell adhesion molecule (EpCAM) and survivin-based techniques, finding a wide range (21% to 57%) of advanced stage bladder carcinoma patients with detectable CTC levels.Citation6-Citation12 Assays that rely on surface markers, however, may be susceptible to downregulation, such as that associated with epithelial–mesenchymal transition (EMT).
An alternative assay (hereafter referred to as “the assay”, ) relies on an adenoviral-based probe that expresses green fluorescent protein (GFP) with amplification of the GFP signal driven by the human telomerase promoter. Processing and enumeration relies on a three-step method as illustrated in . Telomerase is an enzyme that replenishes the ends of chromosomes to forestall senescence, and is upregulated in almost all tumor cells to help confer immortality. In contrast, telomerase is not expressed in the majority of normal cells, which are thus susceptible to senescence.Citation13,Citation14 This probe has been previously found to be effective in cells grown in culture from tumors such as non-small cell lung cancer, tongue squamous carcinoma, gastric cancer, prostate cancer, cervical adenocarcinoma, and mammary gland adenocarcinoma.Citation15,Citation16 The assay is currently under investigation at our institution in patients undergoing radiation therapy (RT) for a range of solid malignancies. Utilizing the assay, we have been able to routinely identify CTCs in the peripheral blood of bladder cancer patients. shows a representative CTC identified from a bladder cancer patient using the assay.
Figure 1. Overview of the telomerase-based CTC assay utilizing an adenoviral probe. The three-step process involves: (1) blood draw and CTC monolayer collection, (2) probe incubation and fluorescence imaging, and (3) assembly of tiled images, filtering and enumeration as highlighted in the flow diagram above.
Figure 2. Images of a representative bladder cancer-derived CTC. C2’s blood sample was incubated with the probe and then fixed and stained to allow for CTC verification. A representative microscopy image of an identified CTC is demonstrated from left to right: DAPI staining for cellular nuclei and identification of three cells in field of view, GFP staining indicating cell with active telomerase expression, anti-EpCAM staining indicating epithelial origin of cell, merge demonstrating probe identified bladder carcinoma CTC, and magnification of merge demonstrating GFP expression and co-staining of DAPI and anti-EpCAM. White arrow denotes the CTC in the field of view. DAPI, 4’,6-diamidino-2-phenylindole; GFP, green fluorescent protein; EpCAM, epithelial cell adhesion molecule; CTC, circulating tumor cell.

Results
Herein, we highlight the potential usefulness of the assay, especially employed serially, in two contrasting case studies of patients undergoing postoperative RT for advanced bladder cancer.
Case 1: patient C1
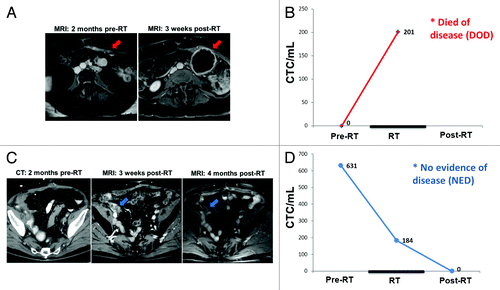
C1 is a 52-y-old woman with (pT3bN2M0) muscle invasive urothelial cell carcinoma of the bladder diagnosed in 2012 who initially presented with suprapubic pain and intermittent gross hematuria and was found to have a bladder mass on abdominopelvic CT (CT) imaging. She underwent cystoscopy with transurethral resection of the bladder tumor (TURBT). The tumor pathology was notable for high grade urothelial carcinoma with invasion into the muscularis propria and lymphovascular invasion. C1 subsequently received neoadjuvant chemotherapy (gemcitabine and cisplatin) but 2 mo later was found to have tumor progression within the bladder. She underwent a radical cystectomy and lymph node dissection, with pathology showing an invasive, high grade urothelial carcinoma, positive surgical margins at the dome of the bladder, and 3 of 29 positive lymph nodes. The patient was then enrolled on an IRB-approved clinical trial of adjuvant pelvic radiation which included CTC evaluation as a secondary aim. Prior to starting adjuvant radiation, C1’s CTC count was initially undetectable on the assay. C1 then received 28 fractions of pelvic radiation therapy (total dose of 5040 cGy) in early 2013. A repeat assay performed toward the end of the radiation course (at 4680 cGy of 5040 cGy) was, however, now markedly elevated at 202 CTCs/mL. Follow-up MRI scan performed approximately one month post-RT showed a new left abdominal fluid-filled peri-sacral mass suspicious for tumor dissemination and recurrence (see , red arrow, right panel). C1 was due to return for additional studies and consideration of new treatment, but passed away due to rapid progression of disease.
Figure 3. Serial imaging and corresponding CTC counts for bladder cancer patients C1 and C2, in relationship to radiation therapy (RT). (A) Axial image from C1’s abdomen/pelvis MRI scans at indicated time points. Red arrow indicates site of recurrence at 1 mo post-RT. (B) Graph of C1’s CTC counts at indicated time points demonstrating upward trend. (C) Axial image from C2’s abdomen/pelvis CT or MRI scans at indicated time points. Blue arrow shows area of MRI enhancement in right pelvis at 1 mo post-RT which resolved at 4 mo post-RT. (D) Graph of C2’s CTC counts at indicated time points demonstrating downward trend.
Case 2: patient C2
C2 is a 70-y-old man who initially presented with superficial bladder cancer that was treated with TURBT followed by 6 administrations of BCG vaccine. The patient was followed with serial cystoscopy until he was found to have a muscle-invasive recurrence (pT4aN0M0) in 2012. This was treated with a second TURBT followed by neoadjuvant gemcitabine and cisplatin chemotherapy, but his primary tumor progressed while on chemotherapy. Consequently, C2 underwent a radical cystectomy with pathology showing an invasive, high grade urothelial carcinoma and positive margins deep to the right ureteral orifice and the right seminal vesicle. He was enrolled on a trial of adjuvant pelvic radiation which included CTC evaluation. His pre-RT CTC count was 631 CTCs/mL. He received 28 fractions of RT, toward the end of which a repeat assay (at 3240 cGy of 5040 cGy) showed a substantial decrease to 184 CTCs/mL. Approximately 1 mo after completion of RT, pelvic MRI was interpreted by the radiologist as showing a soft tissue mass-like thickening at the junction of the ureters and ileal conduit, which was concerning for recurrence of bladder cancer (see , blue arrow, middle panel). The patient returned three months later for a repeat MRI (4 mo post-RT) and CTC assay. The repeat MRI fortunately demonstrated that the enhancement and thickening in the area of concern had decreased and was considered to be more likely due to resolving post-radiation changes. The repeat 4 mo post-RT CTC count was further decreased to 0.3 CTCs/mL, essentially equivalent to levels found in healthy volunteers. C2 is currently doing well, with no evidence of tumor recurrence at last follow-up approximately 8 mo after completing RT.
Discussion
There has been considerable interest in tracking the status of bladder cancer in patients through circulating biomarkers. For example, reverse transcriptase polymerase chain reaction (RT-PCR)-based assays to identify circulating bladder-carcinoma-specific mRNA encoding for cytokeratins (CK19, CK20), epidermal growth factor receptor (EGFR), and telomerase have been described.Citation17,Citation18 While these assays showed initial promise, issues regarding specificity and uncertainty regarding whether the mRNA is from tumor or normal tissue have impeded adoption for patients.Citation19 The detection of intact bladder carcinoma CTCs through the CellSearch platform therefore represented a major breakthrough.Citation10 This system incorporates immunomagnetic beads coated with anti-EpCAM antibodies to bind and magnetically precipitate individual epithelial cells, with reports of successful CTC detection in 44–57% of patients with metastatic bladder cancer and 18–30% of patients with advanced non-metastatic bladder cancer.Citation8-Citation10,Citation12,Citation20 It is possible that the detection rates in these groups of patients would be higher if not for epithelial–mesenchymal transition (EMT), which frequently occurs during the metastatic process and which results in the downregulation of surface markers such as EpCAM.Citation21
To minimize the risk of EMT interfering with detectability (which would result in “false negatives”), a CTC assay based on elevated telomerase has been developed. Telomerase is elevated in almost all tumor cells, but not in normal cells, and increased telomerase activity is also present throughout EMT, thus remaining biologically relevant even after metastasis.Citation22 These considerations may be favorable toward the detection of CTCs in advanced disease, or in patients in which the tumor has progressed despite treatment. Indeed, a study that compared the telomerase-based approach to the CellSearch method in patients with advanced disease found that the two techniques detected CTCs in patient groups that did not coincide.Citation23 While this may be due to the telomerase-based method detecting cancer cells that had undergone EMT, it may also be possible that normal cells expressing EpCAM were inadvertently detected. To our knowledge, our report is the first to show that the telomerase-based method may be able to track disease in patients with bladder cancer, particularly in the setting of advanced disease.
The integration of any CTC assay into standard clinical practice would ultimately depend on whether the assay is useful for patients and their providers. It remains to be demonstrated that CTC counts can contribute to accurate prognostication beyond standard measures such as tumor stage. In non-metastatic bladder cancer, two studies found detectable CTC levels correlated with tumor stage while in another study no correlation was found.Citation9,Citation20,Citation24 Nonetheless, a number of studies have suggested that the detection of CTCs, and higher CTC counts, in patients are associated with shorter median overall survival (156 vs 337 d for detectable and undetectable groups, respectively) and progression-free survival.Citation6,Citation20 The patient vignettes described here may illustrate another way CTCs assays may be potentially useful—to help clarify ambiguous radiographic findings or as a guide to whether additional therapies may be needed. Patient C2’s MRI scan soon after completion of RT showed soft tissue enhancement that would be cause for concern or even alarm. However, the CTC counts had fallen substantially during RT and fell further to near undetectable levels after completion of RT. Serial CTC assays may thus be invaluable for individual patients, providing useful “biologically relevant” information to complement standard studies to monitor disease status. Future studies are needed to confirm this and are currently ongoing.
In summary, this case study is the first report of a telomerase-based assay to identify CTCs serially in patients with bladder carcinoma. The CTC trends appeared to reflect the patients’ disease course in this case study. Additional prospective studies with large numbers will be required to establish whether CTC analysis could potentially serve a useful role in the management of muscle invasive bladder cancer patients.
Materials and Methods
Assay description and IRB-approved clinical trial overview
The assay has been previously summarized in . Briefly, the probe utilized in the assay consists of the human telomerase reverse transcriptase (hTERT) promoter element driving the expression of adenoviral E1A and E1B genes, followed by a CMV promoter driving green fluorescent (GFP) expression. The human bladder carcinoma cell line T24 was employed in validation experiments establishing the clinical usefulness of the assay. Patients described in this report were enrolled in an IRB-approved clinical trial of adjuvant pelvic radiation which included CTC biomarker evaluation as a secondary aim. Approximately 10 mL of whole blood was drawn from each patient, which was then processed via centrifugation through a density gradient to enrich for CTCs while removing red blood cells. The CTC-enriched layer was then exposed to the probe and incubated for 24 h. The samples were then imaged using a fluorescence microscope (Nikon Eclipse TE2000-U) via a computer-driven image processing and cell counting system (Image Pro Plus 7.0, Media Cybernetics). The computer software was set to filter and sort imaged cells by size and intensity, among other parameters, which together with manual visual confirmation ensured the counting of cells and exclusion of debris and other confounding objects. Samples were fixed by phosphate-buffered saline (PBS) rinse followed by formaldehyde fixation (4%, 15 min duration at room temperature), and an additional PBS rinse. Staining was performed using mounting medium containing DAPI (Vector Laboratories) and anti-EpCAM antibodies (1:2000 dilution; R&D Systems). This enabled a second method to distinguish white blood cells (which are DAPI+/EpCAM−) from bladder carcinoma CTCs (DAPI+/EPCAM+/GFP+).
Disclosure of Potential Conflicts of Interest
The University of Pennsylvania has submitted a patent based on a component of the technology presented in this manuscript.
Acknowledgments
We wish to thank the University of Pennsylvania Department of Radiation Oncology (DRO) for financial support of the Assay development and also DRO clinical research coordinators for their assistance. We acknowledge Oncolys Biopharma Inc. for contributing the Telomescan reagent used in this study.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59:225 - 49; http://dx.doi.org/10.3322/caac.20006; PMID: 19474385
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004; 351:781 - 91; http://dx.doi.org/10.1056/NEJMoa040766; PMID: 15317891
- Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26:3213 - 21; http://dx.doi.org/10.1200/JCO.2007.15.8923; PMID: 18591556
- Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res 2007; 13:7053 - 8; http://dx.doi.org/10.1158/1078-0432.CCR-07-1506; PMID: 18056182
- de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008; 14:6302 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-08-0872; PMID: 18829513
- Flaig TW, Wilson S, van Bokhoven A, Varella-Garcia M, Wolfe P, Maroni P, Genova EE, Morales D, Lucia MS. Detection of circulating tumor cells in metastatic and clinically localized urothelial carcinoma. Urology 2011; 78:863 - 7; http://dx.doi.org/10.1016/j.urology.2011.05.045; PMID: 21813167
- Gallagher DJ, Milowsky MI, Ishill N, Trout A, Boyle MG, Riches J, Fleisher M, Bajorin DF. Detection of circulating tumor cells in patients with urothelial cancer. Ann Oncol 2009; 20:305 - 8; http://dx.doi.org/10.1093/annonc/mdn627; PMID: 18836088
- Gazzaniga P, Gradilone A, de Berardinis E, Busetto GM, Raimondi C, Gandini O, Nicolazzo C, Petracca A, Vincenzi B, Farcomeni A, et al. Prognostic value of circulating tumor cells in nonmuscle invasive bladder cancer: a CellSearch analysis. Ann Oncol 2012; 23:2352 - 6; http://dx.doi.org/10.1093/annonc/mdr619; PMID: 22351740
- Guzzo TJ, McNeil BK, Bivalacqua TJ, Elliott DJ, Sokoll LJ, Schoenberg MP. The presence of circulating tumor cells does not predict extravesical disease in bladder cancer patients prior to radical cystectomy. Urol Oncol 2012; 30:44 - 8; http://dx.doi.org/10.1016/j.urolonc.2009.10.008; PMID: 20005748
- Naoe M, Ogawa Y, Morita J, Omori K, Takeshita K, Shichijyo T, Okumura T, Igarashi A, Yanaihara A, Iwamoto S, et al. Detection of circulating urothelial cancer cells in the blood using the CellSearch System. Cancer 2007; 109:1439 - 45; http://dx.doi.org/10.1002/cncr.22543; PMID: 17326057
- Small AC, Gong Y, Oh WK, Hall SJ, van Rijn CJ, Galsky MD. The emerging role of circulating tumor cell detection in genitourinary cancer. J Urol 2012; 188:21 - 6; http://dx.doi.org/10.1016/j.juro.2012.02.2558; PMID: 22578722
- Gradilone A, Petracca A, Nicolazzo C, Gianni W, Cortesi E, Naso G, Vincenzi B, Cristini C, De Berardinis E, Di Silverio F, et al. Prognostic significance of survivin-expressing circulating tumour cells in T1G3 bladder cancer. BJU Int 2010; 106:710 - 5; http://dx.doi.org/10.1111/j.1464-410X.2009.09130.x; PMID: 20128783
- Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer 1997; 33:787 - 91; http://dx.doi.org/10.1016/S0959-8049(97)00062-2; PMID: 9282118
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science 1994; 266:2011 - 5; http://dx.doi.org/10.1126/science.7605428; PMID: 7605428
- Kojima T, Hashimoto Y, Watanabe Y, Kagawa S, Uno F, Kuroda S, Tazawa H, Kyo S, Mizuguchi H, Urata Y, et al. A simple biological imaging system for detecting viable human circulating tumor cells. J Clin Invest 2009; 119:3172 - 81; http://dx.doi.org/10.1172/JCI38609; PMID: 19729837
- Maida Y, Kyo S, Sakaguchi J, Mizumoto Y, Hashimoto M, Mori N, Ikoma T, Nakamura M, Takakura M, Urata Y, et al. Diagnostic potential and limitation of imaging cancer cells in cytological samples using telomerase-specific replicative adenovirus. Int J Oncol 2009; 34:1549 - 56; PMID: 19424572
- Nezos A, Pissimisis N, Lembessis P, Sourla A, Dimopoulos P, Dimopoulos T, Tzelepis K, Koutsilieris M. Detection of circulating tumor cells in bladder cancer patients. Cancer Treat Rev 2009; 35:272 - 9; http://dx.doi.org/10.1016/j.ctrv.2008.11.003; PMID: 19103472
- Soria JC, Morat L, Durdux C, Housset M, Cortez A, Blaise R, Sabatier L. The molecular detection of circulating tumor cells in bladder cancer using telomerase activity. J Urol 2002; 167:352 - 6; http://dx.doi.org/10.1016/S0022-5347(05)65467-5; PMID: 11743355
- Msaouel P, Koutsilieris M. Diagnostic value of circulating tumor cell detection in bladder and urothelial cancer: systematic review and meta-analysis. BMC Cancer 2011; 11:336; http://dx.doi.org/10.1186/1471-2407-11-336; PMID: 21816094
- Rink M, Chun FK, Minner S, Friedrich M, Mauermann O, Heinzer H, Huland H, Fisch M, Pantel K, Riethdorf S. Detection of circulating tumour cells in peripheral blood of patients with advanced non-metastatic bladder cancer. BJU Int 2011; 107:1668 - 75; http://dx.doi.org/10.1111/j.1464-410X.2010.09562.x; PMID: 20735381
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646 - 74; http://dx.doi.org/10.1016/j.cell.2011.02.013; PMID: 21376230
- Liu Z, Li Q, Li K, Chen L, Li W, Hou M, Liu T, Yang J, Lindvall C, Björkholm M, et al. Telomerase reverse transcriptase promotes epithelial-mesenchymal transition and stem cell-like traits in cancer cells. Oncogene 2013; 32:4203 - 13; http://dx.doi.org/10.1038/onc.2012.441; PMID: 23045275
- Kim SJ, Masago A, Tamaki Y, Akazawa K, Tsukamoto F, Sato J, Ozawa T, Tsujino Y, Noguchi S. A novel approach using telomerase-specific replication-selective adenovirus for detection of circulating tumor cells in breast cancer patients. Breast Cancer Res Treat 2011; 128:765 - 73; http://dx.doi.org/10.1007/s10549-011-1603-2; PMID: 21630023
- Karl A, Tritschler S, Hofmann S, Stief CG, Schindlbeck C. Perioperative search for circulating tumor cells in patients undergoing radical cystectomy for bladder cancer. Eur J Med Res 2009; 14:487 - 90; http://dx.doi.org/10.1186/2047-783X-14-11-487; PMID: 19948444
