Abstract
Comment on: Smrž D, et al. Blood 2011; 118:6803–13
Mast cells are long-lived cells of hematopoietic origin that play a role in innate and acquired immunity but, when inappropriately activated, induce the symptomology associated with allergic diseases, including asthma and anaphylaxis.Citation1,Citation2 Mast cell progenitors originate in the bone marrow; from there they migrate into the circulation and eventually into tissues, where they become terminally differentiated.Citation3 These processes are controlled by arachidonic acid-derived molecules,Citation4 cytokines, chemokines and growth factors present in both the circulation and immediate tissue microenvironment.
The major factor regulating mast cell development is stem cell factor (SCF), which is primarily generated in stromal cells and which is the ligand for the mast cell growth factor receptor KIT.Citation5 In addition to regulating mast cell expansion and differentiation, SCF is critical for mast cell homeostasis by promoting mast cell survival. Somatic mutations in KIT, primarily the aspartic acid to valine substitution at position 816 (D816V), renders KIT hyper- and constitutively active, resulting in tissue infiltrates by clonal mast cells associated with the mast cell proliferative disorder, mastocytosis.Citation6 Nevertheless, it is thought that additional underlying defects also contribute to the dysregulated mast cell growth/survival associated with mast cell proliferative disorders.
As part of a study aimed at examining how phosphatidylinositol-3-kinase (PI3K)-dependent signaling pathways regulate mast cell physiology and function, supported by the intramural research program of NIAID within the National Institutes of Health, we observed a marked elevation in the expression and constitutive activation of mammalian target of rapamycin (mTOR) and its associated signaling in the neoplastic HMC-1.1, HMC.1.2 and LAD2 human mast cell lines. Of note, increased mTOR expression was observed irrespective of whether the D816V mutation was present (as in the HMC-1.2 cells) or not (as in the HMC-1.1 and LAD2 cells).Citation7 In addition, expression of mTOR mRNA was observed to be elevated in bone marrow mononuclear cells of patients with mastocytosis.Citation8
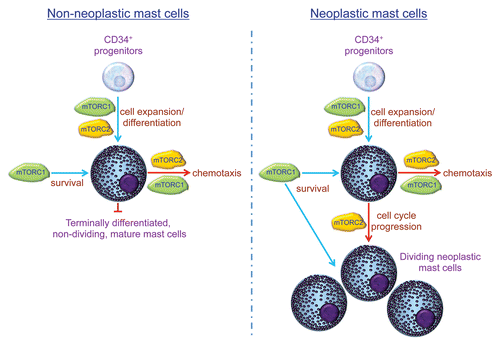
MTOR is a serine/threonine kinase that forms two distinct complexes by binding to associating proteins, including raptor, forming mTOR complex 1 (mTORC1), or rictor, forming mTOR complex 2 (mTORC2).Citation9 The mTORC1 complex primarily controls the activation of the downstream transcriptional regulators p70 S6 kinase and 4E-BP1, whereas mTORC2 primarily provides positive feedback regulation of AKT activation. Thus, these complexes regulate diverse cellular processes that impact cell homeostasis and function. Both raptor and rictor were observed to be elevated in neoplastic mast cells, implying a role for mTORC1 and/or mTORC2 in their dysregulated expansion.Citation7 We thus further investigated whether mTORC1 or mTORC2 preferentially controlled the homeostasis of neoplastic mast cells when compared with non-neoplastic terminally differentiated mast cells.
In our laboratory, in addition to studying human neoplastic mast cell lines, we are able to generate “normal” human mast cells from peripheral blood progenitors.Citation10 This allowed us to directly compare the relative roles of the mTOR complexes in developing terminally differentiated mature and neoplastic mast cells.Citation8 To this end, we adopted two approaches: (1) a pharmacological approach using inhibitors that selectively target mTORC1 (rapamycin) or both mTORC1 and mTORC2 (Torin1) and (2) a gene knockdown approach utilizing shRNA to downregulate the expression of raptor or rictor, respectively, blocking the function of mTORC1 and mTORC2. The combined results of these approaches revealed that mTORC1 and mTORC2 play different roles in the expansion and homeostasis of neoplastic and non-neoplastic mast cells. Whereas mTORC1 may contribute to mast cell survival, mTORC2 is critical for regulating mast cell division by controlling progression through the cell cycle. Thus, due to the terminally differentiated non-dividing nature of mature mast cells, once mast cells reach maturity, the function of mTORC2 in the homeostasis of these cells becomes largely redundant ().
This newly identified differential requirement for mTORC2 in the homeostasis of neoplastic and normal mature mast cells may allow the selective reduction of mast cell numbers associated with clonal mast cell disorders. In addition, as we have also demonstrated that mTORC2 contributes to the regulation of mast cell chemotaxis,Citation11 targeting mTORC2 may also help reduce a pathologic accumulation of mast cells in organs and tissues as well as at sites of allergic inflammation or tumor formation.
Comment on: Smrž D, et al. Blood 2011; 118:6803 - 6813
References
- Galli SJ, et al. Eur J Immunol 2010; 40:1843 - 1851; PMID: 20583030; http://dx.doi.org/10.1002/eji.201040559
- Brown JM, et al. Clin Exp Allergy 2008; 38:4 - 18; PMID: 18031566
- Metcalfe DD, et al. Physiol Rev 1997; 77:1033 - 1079; PMID: 9354811
- Boyce JA. Immunol Rev 2007; 217:168 - 185; PMID: 17498059; http://dx.doi.org/10.1111/j.1600-065X.2007.00512.x
- Jensen BM, et al. Br J Pharmacol 2008; 154:1572 - 1582; PMID: 18500355; http://dx.doi.org/10.1038/bjp.2008.204
- Metcalfe DD. Blood 2008; 112:946 - 956; PMID: 18684881; http://dx.doi.org/10.1182/blood-2007-11-078097
- Kim MS, et al. J Immunol 2008; 180:4586 - 4595; PMID: 18354181
- Smrž D, et al. Blood 2011; 118:6803 - 6813; PMID: 22053105; http://dx.doi.org/10.1182/blood-2011-06-359984
- Foster KG, et al. J Biol Chem 2010; 285:14071 - 14077; PMID: 20231296; http://dx.doi.org/10.1074/jbc.R109.094003
- Rådinger M, et al. Curr Protoc Immunol 2010; 7:7 - 37; PMID: 20814942; http://dx.doi.org/10.1002/0471142735.im0737s90
- Kuehn HS, et al. Biol Chem 2011; 286:391 - 402; PMID: 20980255; http://dx.doi.org/10.1074/jbc.M110.164772