The p53 tumor suppressor gene plays a critical role in maintaining tissue homeostasis through both transcriptionally dependent and independent mechanisms. The response of p53 is dictated by the type of stress (genotoxic, oncogenic, hypoxia, metabolic, etc.) through a complex and incompletely understood number of post-translational modifications. Wild type p53 promotes apoptosis, cell cycle arrest and senescence in response to growth-restrictive conditions, including glucose deprivation.Citation1,Citation2 Under such conditions, nuclear p53 has also been reported to stimulate autophagy through the transcriptional activation of genes that comprise the autophagic network, such as ULK1 and DRAM1, and modulators of autophagy, such as Sestrin2.Citation3-Citation6 Unlike nuclear p53, cytoplasmic p53 appears to inhibit autophagy through mechanisms that are not well understood, but appear to involve localization of p53 to the endoplasmic reticulum (ER) and binding to FIP200, the human ortholog of yeast Atg17.Citation7 In fact, some cancer-associated forms of mutant p53 found predominantly in the cytoplasm can also inhibit autophagy.Citation8 This negative regulation of autophagy by p53 has been proposed to act as rheostat to prevent an excessive amount of autophagy from occurring. Thus, the role of p53 in autophagy is complex and may be dependent on the autophagic stimulus and the mutational status of p53 in the cell.
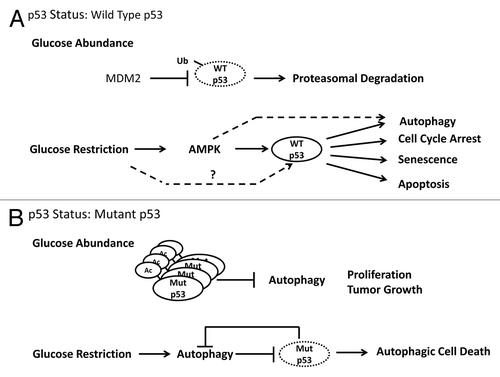
Studies describing the role p53 in the response to metabolic stress have focused primarily on the wild-type version of the protein. However, the vast majority of human tumors express high levels of mutant p53 protein that have acquired novel tumor-promoting functions distinct from those of wild type p53.Citation9 To date, little is known about how mutant versions of p53 respond to growth-restrictive conditions, including the absence of glucose. In an elegant paper by Rodriguez et al.,Citation10 the authors explored the effects of glucose restriction on the stability of a number of common cancer-associated p53 mutants and found that glucose deprivation resulted in degradation of mutant p53 protein levels. Interestingly, the negative regulation of p53 by glucose restriction was specific to mutant p53, since glucose restriction had a stabilizing effect on wild type p53. Surprisingly, the decreased levels of mutant p53 proteins were associated with rapid deacetylation and degradation through an autophagy-dependent but proteasome-independent process. Through the use of a constitutive acetylation-mimetic mutant p53, the authors demonstrated that autophagic degradation of p53 was dependent on the acetylation status of the protein. A major consequence of mutant p53 degradation in tumor cells after glucose deprivation is the loss of a critical check on the autophagic process that results in increased autophagy and leads to cell death (). Importantly, wild type p53 has been previously demonstrated to protect cells from glucose deprivation through induction of a reversible G1/S phase cell cycle arrest, suggesting that normal tissues will respond to glucose shortage differently than tumors harboring mutant p53.Citation1
Figure 1. Glucose deprivation leads to autophagic degradation of mutant p53. (A) Wild type p53 is degraded by the proteasome under basal conditions but can be activated in an AMPK-dependent manner after glucose deprivation. Activated wild type p53 can induce a variety of cellular responses to glucose deprivation including autophagy, cell cycle arrest, senescence and apoptosis. (B) Cancer-associated p53 mutants are constitutively expressed at high levels and inhibit basal autophagy. Glucose deprivation leads to rapid de-acetylation of mutant p53 and subsequent degradation through autophagy. Because mutant p53 suppresses autophagy, its degradation leads to a feedforward autophagic loop that results in cell death.
The authors also provided evidence that mutant p53A135V knock-in mice fed a low-carbohydrate diet expressed reduced levels of the mutated transgene compared with mice fed on a normal or high-carbohydrate diet. Importantly the low-carbohydrate diet had no effect on wild-type p53 levels in multiple tissues studied. Critically, the authors also demonstrated that a low-glucose diet inhibited the tumor-forming ability of cells that possess mutant forms of p53, and that this was dependent on acetylation status of the mutant p53 protein. Taken together, these findings strongly indicate that some tumor-promoting forms of mutant p53 can be targeted for autophagic degradation through glucose restriction. These exciting results could be tested in the clinic by randomizing patients with tumors that harbor similar p53 mutations to a glucose-restrictive, low-carbohydrate diet compared with a normal diet. The expectation of such studies would be that the tumors of patients on a glucose-restrictive diet would see their tumors regress or grow more slowly than those on an unrestrictive glucose diet. However, the next step in this saga will be to see what combinations of chemotherapy or targeted therapy will be more effective against mutant p53 tumors that are glucose-restrictive.
References
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 2005; 18:283 - 93; http://dx.doi.org/10.1016/j.molcel.2005.03.027; PMID: 15866171
- Zhao Y, Coloff JL, Ferguson EC, Jacobs SR, Cui K, Rathmell JC. Glucose metabolism attenuates p53 and Puma-dependent cell death upon growth factor deprivation. J Biol Chem 2008; 283:36344 - 53; http://dx.doi.org/10.1074/jbc.M803580200; PMID: 18990690
- Gao W, Shen Z, Shang L, Wang X. Upregulation of human autophagy-initiation kinase ULK1 by tumor suppressor p53 contributes to DNA-damage-induced cell death. Cell Death Differ 2011; 18:1598 - 607; http://dx.doi.org/10.1038/cdd.2011.33; PMID: 21475306
- Mah LY, O’Prey J, Baudot AD, Hoekstra A, Ryan KM. DRAM-1 encodes multiple isoforms that regulate autophagy. Autophagy 2012; 8:18 - 28; http://dx.doi.org/10.4161/auto.8.1.18077; PMID: 22082963
- Maiuri MC, Malik SA, Morselli E, Kepp O, Criollo A, Mouchel PL, et al. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle 2009; 8:1571 - 6; http://dx.doi.org/10.4161/cc.8.10.8498; PMID: 19377293
- Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci USA 2005; 102:8204 - 9; http://dx.doi.org/10.1073/pnas.0502857102; PMID: 15928081
- Morselli E, Shen S, Ruckenstuhl C, Bauer MA, Mariño G, Galluzzi L, et al. p53 inhibits autophagy by interacting with the human ortholog of yeast Atg17, RB1CC1/FIP200. Cell Cycle 2011; 10:2763 - 9; http://dx.doi.org/10.4161/cc.10.16.16868; PMID: 21775823
- Morselli E, Tasdemir E, Maiuri MC, Galluzzi L, Kepp O, Criollo A, et al. Mutant p53 protein localized in the cytoplasm inhibits autophagy. Cell Cycle 2008; 7:3056 - 61; http://dx.doi.org/10.4161/cc.7.19.6751; PMID: 18818522
- Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer 2009; 9:701 - 13; PMID: 19693097
- Rodriguez OC, Choudhury S, Kolukula V, Vietsch EE, Catania J, Preet A, et al. Dietary downregulation of mutant p53 levels via glucose restriction: mechanisms and implications for tumor therapy. Cell Cycle 2012; 11:4436 - 46; http://dx.doi.org/10.4161/cc.22778; PMID: 23151455