Cells encounter up to 106 DNA damages per day, which can be induced by exogenous physical agents, spontaneous chemical reactions, and products of endogenous metabolism.Citation1 To cope with these threats, the DNA damage response (DDR) system is employed to detect and repair these damages. If massive DNA damages occur within cells which cannot be repaired properly or promptly, the DDR will cause apoptosis to avoid genomic instability. Accumulated DNA damages in somatic cells lead to tumorigenesis, while DNA damages in early embryos result in developmental failure or death.
Traditional methods for studying embryologic DNA damage are restricted to drug treatments or radiations. These approaches induce general DNA damages to the whole embryo, without the ability to distinguish the effect on individual blastomeres. With the potential to develop into a complete organism, each blastomere is affected by and responds to DNA damage independently. Thus, studying the DDR of individual blastomeres will advance our conventional understanding on embryo response against endogenous and exogenous threats. In this issue of Cell Cycle, by employing a laser microbeam, Wang et al. induced DNA damage to a specific single pronucleus of fertilized eggs or blastomere of early embryos and detected the DDR of the individual unit as well as the whole zygote/embryo.Citation2
As an efficient method to study DDR in somatic cells,Citation3 laser microbeam is a practical approach to generate DNA damage in early embryos. When targeting a single pronucleus within the fertilized egg, Wang et al. found that the DNA damage in either male or female pronucleus caused developmental failure to the blastocyst.Citation2 More interestingly, when the author induced DNA lesion to a single blastomere of 2-cell, 4-cell, or 8-cell embryos, the damaged blastomere ceased cleavage and failed to incorporate into the compacted morula, but instead underwent apoptosis at the blastocyst stage. This response was not caused by direct laser toxicity, since the status of γH2AX staining kept “strand” well, and the nuclear membrane of the blastomere stayed intact.Citation2 In principle, the response of cells against DNA damage includes cell arrest, DNA repair, senescence, and apoptosis.Citation4 Unlike somatic cells, blastomeres in early embryos do not repair damaged DNA, but proceed directly to apoptosis.Citation2 DNA repair will result in inevitable errors, since one of the major repair ways non-homologous end joining is error-prone.Citation5 To ensure genomic integrity, the embryo must sacrifice the broken blastomere, a potential risk to its own development. Although γH2AX, the surrogate marker of DNA double-strand breaks strictly localized to the cut line by the microbeam, Chk2 phosphorylation and the apoptosis marker caspase-3 were observed in the whole nucleus of the damaged blastomere, indicating the amplified signals and hypersensitivity upon DNA damage. As a consequence, the blastomere could not incorporate into the compacted morula ().Citation2 This finding is supported by an earlier work showing a novel surveillance mechanism for the elimination of cells damaged by ionizing radiation during mouse gastrulation, demonstrating that a hypersensitivity to apoptosis in the early mouse embryo is a cell fate-dependent manner to ensure genomic integrity.Citation6
Figure 1. The DNA damage response (DDR) of individual blastomere in early embryo. Laser microbeam is used to generate DNA breaks of a single blastomere by aiming at a specific region of the nucleus. DNA damages induce the activation of ATM, which phosphorylates H2AX and amplifies DNA damage signal. The active ATM further activates its downstream substrates, such as Chk2, the mediator of the DDR. Phosphorylated Chk2 causes a series of reactions and finally triggers apoptosis signals to the blastomere. The blastomere ceases cleavage and cannot incorporate into compacted morula.
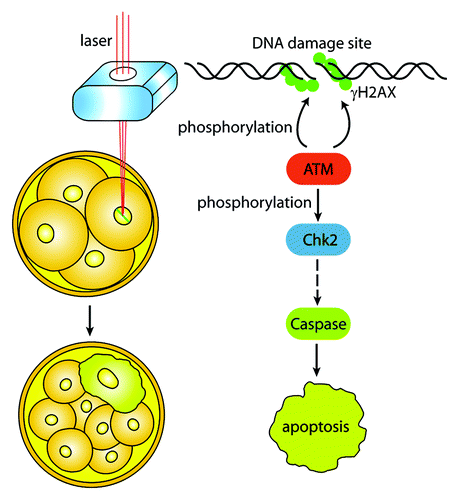
Although the embryos with one damaged blastomere could proceed to the blastocyst stage, the developmental capability is significantly decreased. Besides, when both blastomeres of the 2-cell embryos were cut by laser microbeam, apoptosis occurred 24 h earlier than when only one blastomere was cut,Citation2 suggesting a potential synergy between the individual blastomeres within the embryo. In the clinic, individual blastomeres are usually aspirated for genetic screening, and such manipulation may break the reciprocal symbiosis between the blastomeres. Besides, embryos cultured in vitro are exposed to amplified amounts of oxidative stress, one of the most serious hazards for embryogenesis.Citation7 The lack of antioxidants and the sources of reactive oxygen species in culture media will cause DNA damages to any individual blastomere. Therefore, further investigations are needed to assess the effects of such manipulations. In conclusion, the study by Wang et al.Citation2 reveals the elaborate response of an individual blastomere, the basic unit of the embryo, to DNA damage, and provides a practical approach for the researchers to study DDR of early embryos. As such, this work may have important research and clinical implications for embryogenesis and human reproduction.
References
- Ciccia A, et al. Mol Cell 2010; 40:179 - 204; http://dx.doi.org/10.1016/j.molcel.2010.09.019; PMID: 20965415
- Wang ZW, et al. Cell Cycle 2013; 12; PMID: 24036543
- Li M, et al. Cancer Cell 2013; 23:693 - 704; http://dx.doi.org/10.1016/j.ccr.2013.03.025; PMID: 23680151
- Harper JW, et al. Mol Cell 2007; 28:739 - 45; http://dx.doi.org/10.1016/j.molcel.2007.11.015; PMID: 18082599
- Lieber MR. Annu Rev Biochem 2010; 79:181 - 211; http://dx.doi.org/10.1146/annurev.biochem.052308.093131; PMID: 20192759
- Heyer BS, et al. Genes Dev 2000; 14:2072 - 84; PMID: 10950870
- Ruder EH, et al. Hum Reprod Update 2008; 14:345 - 57; http://dx.doi.org/10.1093/humupd/dmn011; PMID: 18535004