Abstract
Zinc, an essential trace element, plays a critical role in cell signaling, and defect(s) in zinc homeostasis may contribute to adverse physiological and pathological conditions, including cancer. Zinc is present in healthy prostate at a very high concentration, where it is required for important prostatic functions. However, zinc levels are significantly diminished in cancerous tissue, and intracellular zinc level is inversely correlated with prostate cancer progression. During neoplastic transformation, zinc-accumulating, citrate-producing normal prostate cells are metabolically transformed to citrate oxidizing cells that lose the ability to accumulate zinc. Interestingly, zinc has been shown to function as chemopreventive agent against prostate cancer, albeit at high doses, which may lead to many adverse effects. Therefore, novel means to enhance bioaccumulation of sufficient zinc in prostate cells via increasing zinc transport could be useful against prostate cancer. On the basis of available evidence, we present a possibility that the grape antioxidant resveratrol, when given with zinc, may lead to retuning the zinc homeostasis in prostate, thereby abolishing or reversing malignancy. If experimentally verified in in vivo model(s) of prostate cancer, such as transgenic mouse models, this may lead to novel means toward management of prostate cancer and other conditions with compromised zinc homeostasis.
Introduction
Prostate cancer (PCa) is a major cancer of males, and it is estimated that 1 in 6 men will develop PCa during their lifetime in the USA. Although fatality rate due to PCa has decreased over the last decades, 10% of all cancer-related deaths in men were predicted to be due to PCa in 2013.Citation1 Therefore, there is still a pressing need to develop novel means to prevent, diagnose, and effectively treat PCa.
Zinc (Zn), the second most abundant trace element in the human body, has been shown to be essential for ~300 different cellular and physiological processes, including immune functions, protein synthesis, wound healing, and cell division as catalytic, structural, and regulatory element.Citation2,Citation3 A number of studies have shown that the loss of Zn from Zn-dependent enzymes or, Zn-finger proteins results into their compromised functions. Zn is an important cofactor of tumor suppressor protein p53, which is frequently mutated in cancers. Further, Zn supplementation has been shown to restore p53 active conformation to cells with p53 mutations and sensitize them to anticancer drugs.Citation4-Citation6 However, Morita and colleagues have suggested to exploit the use of Zn chelators for protecting against radiation-induced p53-dependent apoptosis to partially guard the damage of normal cells.Citation7 In addition to p53, several other transcription factors contain Zn finger DNA-binding motifs. Zn finger protein 521 (ZNF521), which contains 30 Zn fingers, has been shown to be overexpressed in human medulloblastomas and contribute to the clonogenic growth, migration, and tumorigenicity of medulloblastoma cells.Citation8 Importantly, Zn has also been shown to modulate the hypoxia-induced gene expression that influences tumor cell response to anticancer drugs.Citation9
Zn plays a critical role in a number of prostatic functions, including citrate production and sperm health. In the healthy prostate, Zn is required to establish and maintain a special metabolic situation, which is unique to the prostate gland and is characterized by the production and secretion of unusually high amounts of citrate. In prostate, high Zn concentrations inhibit mitochondrial-aconitase enzyme, resulting in a truncation of citric acid/Krebs cycle at the first step of citrate oxidation. This phenomenon leads to high citrate levels in the prostatic fluid, which is an important constituent of semen.Citation10 This is an energy inefficient process, and prostate cells spend a huge sum of energy to achieve this task.Citation10 In malignancy, prostate cells lose the ability to accumulate Zn, which allows them to save energy (by not making citrate) and utilize it for growth and spread of cancerous cells.Citation10-Citation12
It is conceivable that the progression of PCa may be halted if appropriate concentrations of Zn are available to prostatic tissue. However, a faulty transport of Zn into prostate cells leads to a dysregulated Zn homeostasis. The reason for this mis-regulation of Zn homeostasis in PCa is believed to be a malfunctioning of Zn transporter proteins. Being hydrophilic in nature, Zn ions are impermeable for cell membranes, and, therefore, shuttling of Zn across cellular membranes is achieved by Zn transporter proteins. The Zn transporter ZIP1 was the first member of this family to be connected with PCa progression and may be the major regulator of Zn transport in this organ.Citation13 However, recent studies have also suggested involvement of ZIP2, ZIP3, and ZIP4 in prostate malignancy.Citation14,Citation15 Interestingly, the grape antioxidant resveratrol has been shown to facilitate the accumulation of Zn in human prostate epithelial cells.Citation16 Therefore, it is conceivable that resveratrol may be useful in maintaining Zn homeostasis. In this perspective article, on the basis of available epidemiological and experimental studies, we are presenting evidence that resveratrol when given with Zn supplementation, could maintain and/or correct Zn homeostasis in prostate, thereby abolishing or reversing malignancy.
Zn in PCa Management: Promising Evidences from Epidemiological Data and In Vitro and In Vivo Studies
As discussed above, a high concentration of Zn is required for several prostatic functions, and the prostatic tissue contains more Zn than any other soft tissue in human body.Citation2 Interestingly, in the cancerous prostatic tissue, the Zn level is significantly diminished (~85% vs. normal tissue).Citation11,Citation12 Further, intracellular Zn levels have a strong inverse correlation with PCa progression.Citation10 A very interesting recent study has suggested an inverse association between soil Zn concentrations and PCa rates in the state of South Carolina.Citation17 Recently, Costello and Franklin suggested that the decrease in Zn uptake as well as downregulation of ZIP1 transporter are required events for prostate malignancy.Citation18 Furthermore, depleted prostatic Zn level due to compromised Zn transporters function as a PCa hallmark characteristics (reviewed in ref. Citation19).
The idea of targeting tissue Zn concentration to delay/reverse PCa progression seems obvious and has been pursued for many years using a variety of approaches. A number of epidemiological studies have assessed the relationship between Zn intake and the risk for PCa development in humans. Interestingly, while some studies have shown an association between high Zn intake an reduced PCa mortality,Citation20,Citation21 others found a contrary scenario, where Zn supplementation had either no effect or even led to higher risk for PCa.Citation22,Citation23 It is possible that these discrepancies in outcomes are due to variable Zn content in food and/or differences in study design; for example, some studies focused on Zn intake from diet or supplementation alone, whereas others assessed the effect of Zn intake from a combination of diet and supplementation. Costello and colleagues suggested that proclamations of an association of dietary/supplemental Zn and increased PCa are based on inconclusive and uncorroborated reports, and are in contrast to established clinical and experimental evidences that show the association of reduced Zn accumulation with PCa progression.Citation24 Further, the observed differences may also be due to compromised status of different Zn transporters at different stages of PCa progression and/or because of the different ethnic backgrounds of subjects under investigation.Citation19,Citation25 It will be important to mention here that the recommended daily allowance (RDA) for Zn is 11 mg/day for males and 8 mg/day for females.Citation26,Citation27
Similarly, a plethora of in vitro studies has suggested anti-proliferative efficacy of Zn in PCa cells. In one of the earliest in vitro studies evaluating the effect of Zn supplementation on PCa cells, Liang et al. found that Zn inhibited cell growth and induced cell cycle arrest and apoptosis in LNCaP and PC3 cells.Citation28 These findings were supported by studies from other laboratories. Uzzo and colleagues demonstrated that Zn inhibited NF-κB activation and sensitized PCa cells to cytotoxic agents.Citation29 Interestingly, Wong and Abubakar found that a continuous exposure of LNCaP cells to Zn resulted in an overexpression of certain cancer-promoting genes.Citation30
Zn has also been shown to inhibit PCa growth in vivo. Prasad and colleagues recently conducted a study to determine the effect of Zn supplementation on PCa development and progression in a transgenic adenocarcinoma of mouse prostate model (TRAMP mice).Citation31 Results from this study suggested that optimal Zn concentration is important in prostate carcinogenesis. The authors found that Zn-deficient diet (no Zn) as well as high-Zn (150 ppm Zn) diet enhances PCa tumor growth in TRAMP mice, whereas normal Zn diet (at 30 ppm Zn) was found to have a protective role.Citation31 This study concluded that optimal Zn concentration may have a protective role against PCa.
In another interesting study, in order to avoid the bioavailability issue, Shah et al. determined the effect of direct intra-tumoral injection of Zn into prostate tumors in an immunocompromised mouse model. The authors found that Zn treatments halted the growth of the prostate tumors and extended the survival of the animals.Citation32 This study provides a rationale that an appropriate amount of Zn bioavailable in prostatic tissue may be useful in PCa management.
Zn Transporter Proteins: The Control Panel of Zn Homeostasis
Maintaining an optimal intracellular Zn concentration is crucial for many cellular functions, especially because Zn serves as a catalytic or structural cofactor for a variety of different proteins. Although, a number of proteins may be involved in regulating cellular Zn homeostasis, the most prominent are 2 protein families of Zn transporters, solute carrier family 39 (SLC39) and solute carrier family 30 (SLC30), which work in opposite directionsCitation33 ( and ).
Table 1. Details about Zn transporter solute carrier family 39 (SLC39)
Table 2. Details about Zn transporter solute carrier family 30 (SLC30)
The SLC39 family, also called ZIP transporter proteins, consists of 14 members, functions as endogenous Zn uptake transporter, and is responsible for Zn import into the cytoplasm, either through the plasma membrane or out of intracellular organelles.Citation34,Citation35 Their counterparts, the 10 members SLC30 family, also known as ZnT transporter proteins, reduce the cytoplasmic Zn concentration by transporting it out of the cell or into organelles, thus preventing Zn toxicity. They are located in the cell membrane as well as in membranes of endoplasmic reticulum, mitochondria, Golgi, and vesicle.Citation36
Downregulation of ZIP1 has been shown as one reason for the reduced Zn bioaccumulation in prostatic tissue and is believed to be an early event during prostate carcinogenesis.Citation13,Citation37 Studies have also shown downregulation of ZIP2, ZIP3, and ZIP4 in malignant prostate cells.Citation14,Citation15 The status and role of ZIP5–ZIP14 are established in other organs or disease conditions ().Citation37-Citation51 However, their expression status and/or functional significance are not known in PCa. Similarly, limited information is available regarding the role of ZnT transporters in PCa. Modulations in ZnT1–ZnT4 and ZnT7 have been reported during prostate carcinogenesis; however, the role of ZnT5, ZnT6, and ZnT8–ZnT10 are not known ().Citation52-Citation62 The investigation on Zn transporters and Zn homeostasis during prostate carcinogenesis is still in infancy, and the possible involvement of other ZIPs and ZnTs in PCa development and/or progression cannot be ruled out. Further, an important biological question that needs to be answered is: how do ZIPs and ZnTs coordinate with each other to maintain Zn homeostasis in healthy prostate vs. PCa? It is not clear if the different forms of ZIPs and ZnTs have any functional redundancy?
Other Important Zn-Influencing Regulators: An Ongoing Investigation
Limited research has been done to identify the factors, other than Zn transporters, which influence the homeostasis and bioavailability of Zn in tissues and organs. The expression of ZIP1 has been shown to be influenced by the Zn finger transcription factor Ras responsive element binding protein-1 (RREB1). It is known that binding of RREB1 to the ZIP1 promoter inhibits its transcription and expression.Citation63,Citation64 RREB1 is activated by the RAS-RAF-MEK-ERK pathway, which is hyperactive in PCa. Overexpression of RREB1 contributes to the loss of ZIP1 expression and, thereby, depletion of intracellular Zn in PCa.Citation64,Citation65 Other Zn-influencing proteins are the 4 isoforms of metallothioneins (MT). Among these, MT1 and MT2 are ubiquitously expressed, whereas MT3 and MT4 are mainly found in the central nervous system. They function as major intracellular Zn-binding proteins, thereby regulating the available Zn content inside the cell.Citation66 Wei and colleaguesCitation67 demonstrated: (1) a significant downregulation of endogenous levels of MT1/2 in PC3 cells (95% reduction compared with the normal prostate cells) and in human adenocarcinoma tissues (73% MT1/2 negative) and (2) a moderate reduction of MT1/2 in BPH. Interestingly, in this study, Zn treatment was found to induce MT1/2 expression in PC3 and BPH cells, which corresponded with the restored cellular Zn level.Citation67 This study suggested the potential of MT1/2 as a candidate biomarker for PCa and the possible usefulness of Zn in PCa management.Citation67 Further, matrix metalloproteinases (MMPs), a large family of at least 20 Zn-dependent neutral endo-peptidases, have the ability to degrade all known components of extracellular matrix (ECM). Indeed, a critical characteristic that metastatic cancer cells acquire, is the ability to dissolve basement membranes and ECM. This degrading process is mediated largely by MMPs and modulations in one or more MMPs, and their influence on cancer prognosis has been reported in most cancers, including PCa.Citation68,Citation69
In an interesting recent study, Makhov and colleagues assessed the involvement of epigenetic processes in the disruption of Zn homeostasis in PCa.Citation70 In this study, the authors found an increase in ZIP1 and ZIP3 expression and Zn uptake by DU145 and LNCaP cells in response to 5-aza-2'-deoxycytidine.Citation70 This effect was attributed to demethylation of the promoter region of the activator protein (AP)-2α protein. The authors also found higher AP-2α promoter methylation in clinical samples of early-stage prostate adenocarcinoma when equated with adjacent non-malignant prostate tissue.Citation70
Resveratrol and PCa
Molecules derived from nature, including antioxidant agents that are easily accessible to human population via dietary means, have been highly valued by biomedical researchers. It may not be wrong to say that the naturally occurring dietary agents are somewhat optimized via evolution. Considering the vastness of nature, it is not unrealistic to think that the naturally occurring agents may interact with each other to influence their effectiveness. One such agent that received tremendous attention from biomedical researchers in the recent past is the grape ingredient trans-resveratrol (chemically known as trans-3,5,4'-trihydroxystilbene).Citation71 Resveratrol is a naturally occurring polyphenolic antioxidant compound present in grapes, berries, peanuts, and red wine and has shown to protect against various diseases, including cancer.Citation71,Citation72 Resveratrol has been shown to affect numerous cell-signaling molecules, and therefore is viewed as a multi-targeted agent for age-associated diseases, including aging.Citation73,Citation74 A number of in vitro and in vivo studies have suggested the anti-proliferative effect of resveratrol against PCa (reviewed in ref. Citation75). Previously we have demonstrated that (1) resveratrol imparts growth-inhibitory and pro-apoptotic effects against human PCa cells without affecting the normal prostate epithelial cells; and (2) the anti-proliferative effects of resveratrol against PCa cells are mediated via modulation of the PI3K/Akt pathway and Bcl-2 family of proteins.Citation76 Baptista and colleagues have shown that the resveratrol-mediated activation of SIRT1 leads to an abrupt decrease in histone variant H2A.Z, which plays a critical role in several biological processes as well as in prostate carcinogenesis.Citation77 Epidemiologic studies and laboratory observations suggest that excessive generation of reactive oxygen species impart oxidative damage to key regulatory biomolecules that affects the normal cellular functioning, leading to development and/or progression of cancers, including PCa. Yilmaz and colleagues have shown that the malondialdehyde levels were increased and the antioxidant activity and Zn levels decreased in PCa patients.Citation78 Resveratrol has been shown to inhibit PCa progression in transgenic mouseCitation79 and ratCitation80 models of PCa. These studies suggest that antioxidant resveratrol could be an excellent natural agent for the management of PCa. Thus, resveratrol-based combinatorial strategies with other agents are being increasingly appreciated for cancer management.Citation81 In this perspective article, we are presenting a case for combining resveratrol with Zn for the management of PCa.
Retuning of Zn Homeostasis by Resveratrol: A Novel Concept for Prostate Cancer Management
Resveratrol is a powerful antioxidant and anti-inflammatory agent that has been shown to have promise against a variety of cancers, including PCa. In addition to its cancer chemopreventive potential, resveratrol has also been shown to increase life span by improving cardiovascular health and protection against metabolic disorders. Recent investigations are exploring the possibility of resveratrol in conjunction with existing therapeutic modalities to enhance their response and limit their toxicity. We have recently reviewed the available scientific literature suggesting that resveratrol may be very useful when given in combination with other drugs or natural products, for chemoprevention as well as cancer treatment.Citation81
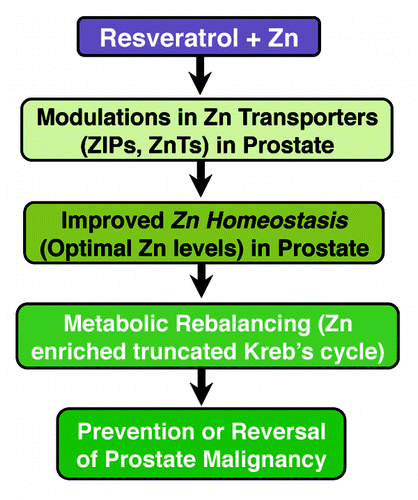
In a very interesting study, Zhang and colleagues determined the effect of resveratrol and Zn on the intracellular Zn status in normal human prostate epithelial cells. This study found that resveratrol and Zn co-treatment increases total cellular Zn and intracellular free labile Zn in normal human prostate epithelial cells.Citation16 In another study, an increase in plasma Zn level was reported in healthy adult rats administered with resveratrol.Citation82 These studies suggest that resveratrol supplementation may influence Zn homeostasis, possibly via enhancing intracellular Zn accumulation. Additionally, another recent study has also suggested that resveratrol can improve Zn’s antioxidant capacity.Citation83 Here, it’s important to point out that recent evidences, including clinical trials, suggest that resveratrol is safe up to a daily dose of 1 g, and it has been shown to reach tissues remote from the site of absorption.Citation84 Thus, based on available scientific literature, we are proposing that resveratrol when given with Zn supplementation would maintain and/or correct Zn homeostasis in prostate, thereby abolishing or reversing malignancy. A schematic representation of this perspective is provided in . In this scenario, resveratrol is expected to amend the circumstances that (1) trigger favorable modulations in Zn transporters (such as ZIPs and/or ZnTs) in the prostate, and/or (2) modulate the upstream effectors of Zn transporters such as RREB1, whose overexpression is known to contribute ZIP1 loss,Citation64,Citation65 and/or (3) correct epigenetic disruption of AP2α that leads to downregulation of ZIP1 and ZIP3,Citation70 and/or (4) modulate metallothioneins.Citation67 Thus, it is possible that resveratrol, when given with Zn, should be able to enhance its bioaccumulation in prostate to reduce PCa growth in vivo. If validated, it is conceivable that therapeutic approaches based on retuning of Zn could be developed for the management of PCa and other similar conditions with compromised Zn homeostasis. Additionally, continuous supplementation of more bioavailable Zn would be efficacious in maintaining a normal serum Zn level, and thereby easy uptake for prostate tissues. Tompkins and colleagues has shown better bioavailability of Zn from organic Zn yeast supplements than Zn gluconate salt in healthy volunteers.Citation85 Although bioavailability in prostate has not been tested, it is quite possible that an elevated serum Zn level could result in an enhanced Zn uptake by the malignant cells.
Summary and Conclusions
PCa is an age associated malignancy, and aging itself is related with low-grade inflammation and mild Zn deficiency. Further, inflammation believed to play a role in PCa progression. Kahmann and colleagues have described that moderate Zn supplementation balances immune response in the elderly by reducing spontaneous inflammatory cytokine release and by restoring T-cell functions.Citation86 Potential of Zn for PCa management is evident from epidemiological observations as well as in vitro and in vivo studies. Studies suggest that Zn depletion is a hallmark characteristic of metabolic transformation of healthy prostate to prostate malignancy, and Zn supplementation could abort prostate malignancy if able to reach the prostate. The clinical effects of Zn will not be seen without sufficient bioavailable Zn in prostatic tissues.Citation18,Citation87 Therefore, retuning of Zn homeostasis using resveratrol could be a novel strategy for PCa management. In this direction certain other dietary agents may also be helpful in enhancing the bioavailability of Zn in prostatic tissue. These include: (1) curcumin, an active ingredient of the Indian spice turmeric; (2) epigallocatechin- 3-gallate (EGCG), a polyphenolic antioxidant present in green tea; and (3) antioxidants present in grape seeds. Malhotra and colleagues have shown that combined supplementation of curcumin and resveratrol increases intra-tumoral Zn levels and controls inflammation by cox-2 and cell cycle arrest by p21 during lung carcinogenesis in mice.Citation88 Similalry, Quesada and colleagues have found that EGCG as well as grape-seed procyanidin extract (GSPE) enhance expression of Zn transporters ZIP1 and ZIP4 and inhibit the expression of Zn-binding metallothioneins and Zn exporter ZnT1 in hepatocarcinoma cell line HepG2.Citation89 Further, Sreenivasulu and colleagues have demonstrated that polyphenol-rich beverages such as red wine, red grape juice, and green tea or specific polyphenols enhance Zn uptake as well as metallothionein expression in Caco-2 cells.Citation90 Although none of the studies are performed in in vivo models of PCa, it is possible that these natural compounds may have abilities to influence Zn homeostasis and could be useful in combination with Zn for PCa management.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was partly supported by funding from the NIH (R21CA149560 and R21CA176867 to N.A.) and the Department of Defense (W81XWH-12-1-0105 to C.K.S.).
Author Contributions
Conception and design: C.K.S. and N.A. Writing, review, and/or revision of the manuscript: C.K.S., A.P., and N.A.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11 - 30; http://dx.doi.org/10.3322/caac.21166; PMID: 23335087
- Kelleher SL, McCormick NH, Velasquez V, Lopez V. Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv Nutr 2011; 2:101 - 11; http://dx.doi.org/10.3945/an.110.000232; PMID: 22332039
- Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol 2012; 86:521 - 34; http://dx.doi.org/10.1007/s00204-011-0775-1; PMID: 22071549
- Puca R, Nardinocchi L, Porru M, Simon AJ, Rechavi G, Leonetti C, Givol D, D’Orazi G. Restoring p53 active conformation by zinc increases the response of mutant p53 tumor cells to anticancer drugs. Cell Cycle 2011; 10:1679 - 89; http://dx.doi.org/10.4161/cc.10.10.15642; PMID: 21508668
- Norelli G, Bossi G. Zinc, a promising mineral for misfolded p53 reactivation. Cell Cycle 2011; 10:2415 - 6; http://dx.doi.org/10.4161/cc.10.15.15931; PMID: 21734452
- D’Orazi G, Givol D. p53 reactivation: the link to zinc. Cell Cycle 2012; 11:2581 - 2; http://dx.doi.org/10.4161/cc.21020; PMID: 22751437
- Morita A, Ariyasu S, Ohya S, Takahashi I, Wang B, Tanaka K, Uchida T, Okazaki H, Hanaya K, Enomoto A, et al. Evaluation of zinc (II) chelators for inhibiting p53-mediated apoptosis. Oncotarget 2013; 4:2439 - 50; PMID: 24280450
- Spina R, Filocamo G, Iaccino E, Scicchitano S, Lupia M, Chiarella E, Mega T, Bernaudo F, Pelaggi D, Mesuraca M, et al. Critical role of zinc finger protein 521 in the control of growth, clonogenicity and tumorigenic potential of medulloblastoma cells. Oncotarget 2013; 4:1280 - 92; PMID: 23907569
- Sheffer M, Simon AJ, Jacob-Hirsch J, Rechavi G, Domany E, Givol D, D’Orazi G. Genome-wide analysis discloses reversal of the hypoxia-induced changes of gene expression in colon cancer cells by zinc supplementation. Oncotarget 2011; 2:1191 - 202; PMID: 22202117
- Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer 2006; 5:17; http://dx.doi.org/10.1186/1476-4598-5-17; PMID: 16700911
- Costello LC, Franklin RB. Zinc is decreased in prostate cancer: an established relationship of prostate cancer!. J Biol Inorg Chem 2011; 16:3 - 8; http://dx.doi.org/10.1007/s00775-010-0736-9; PMID: 21140181
- Zaichick VYe, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol 1997; 29:565 - 74; http://dx.doi.org/10.1007/BF02552202; PMID: 9413764
- Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, Bagasra O, Costello LC. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer 2005; 4:32; http://dx.doi.org/10.1186/1476-4598-4-32; PMID: 16153295
- Desouki MM, Geradts J, Milon B, Franklin RB, Costello LC. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol Cancer 2007; 6:37; http://dx.doi.org/10.1186/1476-4598-6-37; PMID: 17550612
- Chen QG, Zhang Z, Yang Q, Shan GY, Yu XY, Kong CZ. The role of zinc transporter ZIP4 in prostate carcinoma. Urol Oncol 2012; 30:906 - 11; http://dx.doi.org/10.1016/j.urolonc.2010.11.010; PMID: 21803616
- Zhang JJ, Wu M, Schoene NW, Cheng W-H, Wang TTY, Alshatwi AA, Alsaif M, Lei KY. Effect of resveratrol and zinc on intracellular zinc status in normal human prostate epithelial cells. Am J Physiol Cell Physiol 2009; 297:C632 - 44; http://dx.doi.org/10.1152/ajpcell.00139.2009; PMID: 19553565
- Wagner SE, Burch JB, Hussey J, Temples T, Bolick-Aldrich S, Mosley-Broughton C, Liu Y, Hebert JR. Soil zinc content, groundwater usage, and prostate cancer incidence in South Carolina. Cancer Causes Control 2009; 20:345 - 53; http://dx.doi.org/10.1007/s10552-008-9248-0; PMID: 18949566
- Costello LC, Franklin RB. Cytotoxic/tumor suppressor role of zinc for the treatment of cancer: an enigma and an opportunity. Expert Rev Anticancer Ther 2012; 12:121 - 8; http://dx.doi.org/10.1586/era.11.190; PMID: 22149438
- Kolenko V, Teper E, Kutikov A, Uzzo R. Zinc and zinc transporters in prostate carcinogenesis. Nat Rev Urol 2013; 10:219 - 26; http://dx.doi.org/10.1038/nrurol.2013.43; PMID: 23478540
- Epstein MM, Kasperzyk JL, Andrén O, Giovannucci EL, Wolk A, Håkansson N, Andersson SO, Johansson JE, Fall K, Mucci LA. Dietary zinc and prostate cancer survival in a Swedish cohort. Am J Clin Nutr 2011; 93:586 - 93; http://dx.doi.org/10.3945/ajcn.110.004804; PMID: 21228268
- Gonzalez A, Peters U, Lampe JW, White E. Zinc intake from supplements and diet and prostate cancer. Nutr Cancer 2009; 61:206 - 15; http://dx.doi.org/10.1080/01635580802419749; PMID: 19235036
- Gallus S, Foschi R, Negri E, Talamini R, Franceschi S, Montella M, Ramazzotti V, Tavani A, Dal Maso L, La Vecchia C. Dietary zinc and prostate cancer risk: a case-control study from Italy. Eur Urol 2007; 52:1052 - 6; http://dx.doi.org/10.1016/j.eururo.2007.01.094; PMID: 17292532
- Leitzmann MF, Stampfer MJ, Wu K, Colditz GA, Willett WC, Giovannucci EL. Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst 2003; 95:1004 - 7; http://dx.doi.org/10.1093/jnci/95.13.1004; PMID: 12837837
- Costello LC, Franklin RB, Tan MT. A Critical Assessment of Epidemiology Studies Regarding Dietary/Supplemental Zinc and Prostate Cancer Risk. Open Urol Nephrol J 2008; 1; http://dx.doi.org/10.2174/1874303X00801010026; PMID: 24204440
- Rishi I, Baidouri H, Abbasi JA, Bullard-Dillard R, Kajdacsy-Balla A, Pestaner JP, Skacel M, Tubbs R, Bagasra O. Prostate cancer in African American men is associated with downregulation of zinc transporters. Appl Immunohistochem Mol Morphol 2003; 11:253 - 60; http://dx.doi.org/10.1097/00129039-200309000-00009; PMID: 12966353
- NIH. . The Office of Dietary Supplements (ODS) [ods.od.nih.gov/factsheets/Zinc-HealthProfessional/]
- Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc 2001; 101:294 - 301; http://dx.doi.org/10.1016/S0002-8223(01)00078-5; PMID: 11269606
- Liang J-H, Liu Y-Y, Franklin RB, Costello LC, Feng P. Inhibitrory effet of zinc on human prostatic carcinoma cell growth. Prostate 1999; 40:200 - 7
- Uzzo RG, Leavis P, Hatch W, Gabai VL, Dulin N, Zvartau N, Kolenko VM. Zinc inhibits nuclear factor-kappa B activation and sensitizes prostate cancer cells to cytotoxic agents. Clin Cancer Res 2002; 8:3579 - 83; PMID: 12429649
- Wong P-F, Abubakar S. Comparative transcriptional study of the effects of high intracellular zinc on prostate carcinoma cells. Oncol Rep 2010; 23:1501 - 16; PMID: 20428803
- Prasad AS, Mukhtar H, Beck FW, Adhami VM, Siddiqui IA, Din M, Hafeez BB, Kucuk O. Dietary zinc and prostate cancer in the TRAMP mouse model. J Med Food 2010; 13:70 - 6; http://dx.doi.org/10.1089/jmf.2009.0042; PMID: 20136438
- Shah MR, Kriedt CL, Lents NH, Hoyer MK, Jamaluddin N, Klein C, Baldassare J. Direct intra-tumoral injection of zinc-acetate halts tumor growth in a xenograft model of prostate cancer. J Exp Clin Cancer Res 2009; 28:84; http://dx.doi.org/10.1186/1756-9966-28-84; PMID: 19534805
- Murakami M, Hirano T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci 2008; 99:1515 - 22; http://dx.doi.org/10.1111/j.1349-7006.2008.00854.x; PMID: 18754861
- Franklin RB, Milon B, Feng P, Costello LC. Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer. Front Biosci 2005; 10:2230 - 9; http://dx.doi.org/10.2741/1692; PMID: 15970489
- Franklin RB, Ma J, Zou J, Guan Z, Kukoyi BI, Feng P, Costello LC. Human ZIP1 is a major zinc uptake transporter for the accumulation of zinc in prostate cells. J Inorg Biochem 2003; 96:435 - 42; http://dx.doi.org/10.1016/S0162-0134(03)00249-6; PMID: 12888280
- Huang L, Tepaamorndech S. The SLC30 family of zinc transporters - a review of current understanding of their biological and pathophysiological roles. Mol Aspects Med 2013; 34:548 - 60; http://dx.doi.org/10.1016/j.mam.2012.05.008; PMID: 23506888
- Johnson LA, Kanak MA, Kajdacsy-Balla A, Pestaner JP, Bagasra O. Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods 2010; 52:316 - 21; http://dx.doi.org/10.1016/j.ymeth.2010.08.004; PMID: 20705137
- Cui X, Zhang Y, Yang J, Sun X, Hagan JP, Guha S, Li M. ZIP4 confers resistance to zinc deficiency-induced apoptosis in pancreatic cancer. Cell Cycle 2014; 13:1180 - 6; http://dx.doi.org/10.4161/cc.28111; PMID: 24553114
- Cousins RJ. Gastrointestinal factors influencing zinc absorption and homeostasis. Int J Vitam Nutr Res 2010; 80:243 - 8; http://dx.doi.org/10.1024/0300-9831/a000030; PMID: 21462106
- Wang F, Kim BE, Petris MJ, Eide DJ. The mammalian Zip5 protein is a zinc transporter that localizes to the basolateral surface of polarized cells. J Biol Chem 2004; 279:51433 - 41; http://dx.doi.org/10.1074/jbc.M408361200; PMID: 15322118
- Lopez V, Kelleher SL. Zip6-attenuation promotes epithelial-to-mesenchymal transition in ductal breast tumor (T47D) cells. Exp Cell Res 2010; 316:366 - 75; http://dx.doi.org/10.1016/j.yexcr.2009.10.011; PMID: 19852955
- Huang L, Kirschke CP, Zhang Y, Yu YY. The ZIP7 gene (Slc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J Biol Chem 2005; 280:15456 - 63; http://dx.doi.org/10.1074/jbc.M412188200; PMID: 15705588
- Hogstrand C, Kille P, Nicholson RI, Taylor KM. Zinc transporters and cancer: a potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol Med 2009; 15:101 - 11; http://dx.doi.org/10.1016/j.molmed.2009.01.004; PMID: 19246244
- Wang CY, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B, Knutson MD. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J Biol Chem 2012; 287:34032 - 43; http://dx.doi.org/10.1074/jbc.M112.367284; PMID: 22898811
- Jenkitkasemwong S, Wang CY, Mackenzie B, Knutson MD. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 2012; 25:643 - 55; http://dx.doi.org/10.1007/s10534-012-9526-x; PMID: 22318508
- Taniguchi M, Fukunaka A, Hagihara M, Watanabe K, Kamino S, Kambe T, Enomoto S, Hiromura M. Essential role of the zinc transporter ZIP9/SLC39A9 in regulating the activations of Akt and Erk in B-cell receptor signaling pathway in DT40 cells. PLoS One 2013; 8:e58022; http://dx.doi.org/10.1371/journal.pone.0058022; PMID: 23505453
- Kagara N, Tanaka N, Noguchi S, Hirano T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci 2007; 98:692 - 7; http://dx.doi.org/10.1111/j.1349-7006.2007.00446.x; PMID: 17359283
- Yu Y, Wu A, Zhang Z, Yan G, Zhang F, Zhang L, Shen X, Hu R, Zhang Y, Zhang K, et al. Characterization of the GufA subfamily member SLC39A11/Zip11 as a zinc transporter. J Nutr Biochem 2013; 24:1697 - 708; http://dx.doi.org/10.1016/j.jnutbio.2013.02.010; PMID: 23643525
- Chowanadisai W, Graham DM, Keen CL, Rucker RB, Messerli MA. Neurulation and neurite extension require the zinc transporter ZIP12 (slc39a12). Proc Natl Acad Sci U S A 2013; 110:9903 - 8; http://dx.doi.org/10.1073/pnas.1222142110; PMID: 23716681
- Jeong J, Walker JM, Wang F, Park JG, Palmer AE, Giunta C, Rohrbach M, Steinmann B, Eide DJ. Promotion of vesicular zinc efflux by ZIP13 and its implications for spondylocheiro dysplastic Ehlers-Danlos syndrome. Proc Natl Acad Sci U S A 2012; 109:E3530 - 8; http://dx.doi.org/10.1073/pnas.1211775110; PMID: 23213233
- Franklin RB, Levy BA, Zou J, Hanna N, Desouki MM, Bagasra O, Johnson LA, Costello LC. ZIP14 zinc transporter downregulation and zinc depletion in the development and progression of hepatocellular cancer. J Gastrointest Cancer 2012; 43:249 - 57; http://dx.doi.org/10.1007/s12029-011-9269-x; PMID: 21373779
- Urani C, Melchioretto P, Gribaldo L. Regulation of metallothioneins and ZnT-1 transporter expression in human hepatoma cells HepG2 exposed to zinc and cadmium. Toxicol In Vitro 2010; 24:370 - 4; http://dx.doi.org/10.1016/j.tiv.2009.11.003; PMID: 19900532
- Hasumi M, Suzuki K, Matsui H, Koike H, Ito K, Yamanaka H. Regulation of metallothionein and zinc transporter expression in human prostate cancer cells and tissues. Cancer Lett 2003; 200:187 - 95; http://dx.doi.org/10.1016/S0304-3835(03)00441-5; PMID: 14568174
- Song Y, Elias V, Wong CP, Scrimgeour AG, Ho E. Zinc transporter expression profiles in the rat prostate following alterations in dietary zinc. Biometals 2010; 23:51 - 8; http://dx.doi.org/10.1007/s10534-009-9266-8; PMID: 19760107
- Smidt K, Rungby J. ZnT3: a zinc transporter active in several organs. Biometals 2012; 25:1 - 8; http://dx.doi.org/10.1007/s10534-011-9490-x; PMID: 21866305
- Beck FW, Prasad AS, Butler CE, Sakr WA, Kucuk O, Sarkar FH. Differential expression of hZnT-4 in human prostate tissues. Prostate 2004; 58:374 - 81; http://dx.doi.org/10.1002/pros.10344; PMID: 14968438
- Coneyworth LJ, Mathers JC, Ford D. Does promoter methylation of the SLC30A5 (ZnT5) zinc transporter gene contribute to the ageing-related decline in zinc status?. Proc Nutr Soc 2009; 68:142 - 7; http://dx.doi.org/10.1017/S0029665109001104; PMID: 19245740
- Huang L, Kirschke CP, Gitschier J. Functional characterization of a novel mammalian zinc transporter, ZnT6. J Biol Chem 2002; 277:26389 - 95; http://dx.doi.org/10.1074/jbc.M200462200; PMID: 11997387
- Tepaamorndech S, Huang L, Kirschke CP. A null-mutation in the Znt7 gene accelerates prostate tumor formation in a transgenic adenocarcinoma mouse prostate model. Cancer Lett 2011; 308:33 - 42; http://dx.doi.org/10.1016/j.canlet.2011.04.011; PMID: 21621325
- Lefebvre B, Vandewalle B, Balavoine AS, Queniat G, Moerman E, Vantyghem MC, Le Bacquer O, Gmyr V, Pawlowski V, Kerr-Conte J, et al. Regulation and functional effects of ZNT8 in human pancreatic islets. J Endocrinol 2012; 214:225 - 32; http://dx.doi.org/10.1530/JOE-12-0071; PMID: 22582094
- Overbeck S, Uciechowski P, Ackland ML, Ford D, Rink L. Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J Leukoc Biol 2008; 83:368 - 80; http://dx.doi.org/10.1189/jlb.0307148; PMID: 17971500
- Bosomworth HJ, Thornton JK, Coneyworth LJ, Ford D, Valentine RA. Efflux function, tissue-specific expression and intracellular trafficking of the Zn transporter ZnT10 indicate roles in adult Zn homeostasis. Metallomics 2012; 4:771 - 9; http://dx.doi.org/10.1039/c2mt20088k; PMID: 22706290
- Zhang S, Qian X, Redman C, Bliskovski V, Ramsay ES, Lowy DR, Mock BA. p16 INK4a gene promoter variation and differential binding of a repressor, the ras-responsive zinc-finger transcription factor, RREB. Oncogene 2003; 22:2285 - 95; http://dx.doi.org/10.1038/sj.onc.1206257; PMID: 12700664
- Milon BC, Agyapong A, Bautista R, Costello LC, Franklin RB. Ras responsive element binding protein-1 (RREB-1) down-regulates hZIP1 expression in prostate cancer cells. Prostate 2010; 70:288 - 96; PMID: 19802870
- Zou J, Milon BC, Desouki MM, Costello LC, Franklin RB. hZIP1 zinc transporter down-regulation in prostate cancer involves the overexpression of ras responsive element binding protein-1 (RREB-1). Prostate 2011; 71:1518 - 24; PMID: 21360563
- Vašák M, Meloni G. Chemistry and biology of mammalian metallothioneins. J Biol Inorg Chem 2011; 16:1067 - 78; http://dx.doi.org/10.1007/s00775-011-0799-2; PMID: 21647776
- Wei H, Desouki MM, Lin S, Xiao D, Franklin RB, Feng P. Differential expression of metallothioneins (MTs) 1, 2, and 3 in response to zinc treatment in human prostate normal and malignant cells and tissues. Mol Cancer 2008; 7:7; http://dx.doi.org/10.1186/1476-4598-7-7; PMID: 18208603
- Hadler-Olsen E, Winberg JO, Uhlin-Hansen L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol 2013; 34:2041 - 51; http://dx.doi.org/10.1007/s13277-013-0842-8; PMID: 23681802
- Ozden F, Saygin C, Uzunaslan D, Onal B, Durak H, Aki H. Expression of MMP-1, MMP-9 and TIMP-2 in prostate carcinoma and their influence on prognosis and survival. J Cancer Res Clin Oncol 2013; 139:1373 - 82; http://dx.doi.org/10.1007/s00432-013-1453-x; PMID: 23708302
- Makhov PB, Golovine KV, Kutikov A, Canter DJ, Rybko VA, Roshchin DA, Matveev VB, Uzzo RG, Kolenko VM. Reversal of epigenetic silencing of AP-2alpha results in increased zinc uptake in DU-145 and LNCaP prostate cancer cells. Carcinogenesis 2011; 32:1773 - 81; http://dx.doi.org/10.1093/carcin/bgr212; PMID: 21940908
- Cucciolla V, Borriello A, Oliva A, Galletti P, Zappia V, Della Ragione F. Resveratrol: from basic science to the clinic. Cell Cycle 2007; 6:2495 - 510; http://dx.doi.org/10.4161/cc.6.20.4815; PMID: 17726376
- Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal 2009; 11:2851 - 97; http://dx.doi.org/10.1089/ars.2008.2412; PMID: 19432534
- Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle 2008; 7:1020 - 35; http://dx.doi.org/10.4161/cc.7.8.5740; PMID: 18414053
- Valenzano DR, Cellerino A. Resveratrol and the pharmacology of aging: a new vertebrate model to validate an old molecule. Cell Cycle 2006; 5:1027 - 32; http://dx.doi.org/10.4161/cc.5.10.2739; PMID: 16687936
- Jasiński M, Jasińska L, Ogrodowczyk M. Resveratrol in prostate diseases - a short review. Cent European J Urol 2013; 66:144 - 9; PMID: 24579014
- Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther 2006; 5:1335 - 41; http://dx.doi.org/10.1158/1535-7163.MCT-05-0526; PMID: 16731767
- Baptista T, Graça I, Sousa EJ, Oliveira AI, Costa NR, Costa-Pinheiro P, Amado F, Henrique R, Jerónimo C. Regulation of histone H2A.Z expression is mediated by sirtuin 1 in prostate cancer. Oncotarget 2013; 4:1673 - 85; PMID: 24127549
- Yilmaz MI, Saglam K, Sonmez A, Gok DE, Basal S, Kilic S, Akay C, Kocar IH. Antioxidant system activation in prostate cancer. Biol Trace Elem Res 2004; 98:13 - 9; http://dx.doi.org/10.1385/BTER:98:1:13; PMID: 15051896
- Harper CE, Patel BB, Wang J, Arabshahi A, Eltoum IA, Lamartiniere CA. Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis 2007; 28:1946 - 53; http://dx.doi.org/10.1093/carcin/bgm144; PMID: 17675339
- Seeni A, Takahashi S, Takeshita K, Tang M, Sugiura S, Sato SY, Shirai T. Suppression of prostate cancer growth by resveratrol in the transgenic rat for adenocarcinoma of prostate (TRAP) model. Asian Pac J Cancer Prev 2008; 9:7 - 14; PMID: 18439064
- Singh CK, George J, Ahmad N. Resveratrol-based combinatorial strategies for cancer management. Ann N Y Acad Sci 2013; 1290:113 - 21; http://dx.doi.org/10.1111/nyas.12160; PMID: 23855473
- Kavas GO, Aribal-Kocatürk P, Büyükkağnici DI. Resveratrol: is there any effect on healthy subject?. Biol Trace Elem Res 2007; 118:250 - 4; http://dx.doi.org/10.1007/s12011-007-0033-9; PMID: 17916928
- Dias K, Nikolaou S. Does the combination of resveratrol with Al (III) and Zn (II) improve its antioxidant activity?. Nat Prod Commun 2011; 6:1673 - 6; PMID: 22224286
- Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res 2010; 70:9003 - 11; http://dx.doi.org/10.1158/0008-5472.CAN-10-2364; PMID: 20935227
- Tompkins TA, Renard NE, Kiuchi A. Clinical evaluation of the bioavailability of zinc-enriched yeast and zinc gluconate in healthy volunteers. Biol Trace Elem Res 2007; 120:28 - 35; http://dx.doi.org/10.1007/s12011-007-0072-2; PMID: 17916952
- Kahmann L, Uciechowski P, Warmuth S, Plümäkers B, Gressner AM, Malavolta M, Mocchegiani E, Rink L. Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res 2008; 11:227 - 37; http://dx.doi.org/10.1089/rej.2007.0613; PMID: 18279033
- Huang L, Kirschke CP, Zhang Y. Decreased intracellular zinc in human tumorigenic prostate epithelial cells: a possible role in prostate cancer progression. Cancer Cell Int 2006; 6:10; http://dx.doi.org/10.1186/1475-2867-6-10; PMID: 16579854
- Malhotra A, Nair P, Dhawan DK. Curcumin and resveratrol synergistically stimulate p21 and regulate cox-2 by maintaining adequate zinc levels during lung carcinogenesis. Eur J Cancer Prev 2011; 20:411 - 6; http://dx.doi.org/10.1097/CEJ.0b013e3283481d71; PMID: 21633290
- Quesada IM, Bustos M, Blay M, Pujadas G, Ardèvol A, Salvadó MJ, Bladé C, Arola L, Fernández-Larrea J. Dietary catechins and procyanidins modulate zinc homeostasis in human HepG2 cells. J Nutr Biochem 2011; 22:153 - 63; http://dx.doi.org/10.1016/j.jnutbio.2009.12.009; PMID: 20471814
- Sreenivasulu K, Raghu P, Nair KM. Polyphenol-rich beverages enhance zinc uptake and metallothionein expression in Caco-2 cells. J Food Sci 2010; 75:H123 - 8; http://dx.doi.org/10.1111/j.1750-3841.2010.01582.x; PMID: 20546406