Abstract
Phthalates are a group of chemicals used to make plastics softer and more flexible. They are used in hundreds of products, such as vinyl flooring, adhesives, detergents, lubricating oils, automotive plastics, plastic clothes (raincoats) and personal-care products (soaps, shampoos, hair sprays and nail polishes). People are exposed to phthalates by eating and drinking foods that have been in contact with containers and products containing phthalates. To a lesser extent, exposure can occur from breathing in air that contains vapors or dust contaminated with phthalate particles. Once phthalates enter a person's body, they are converted into breakdown products that pass out quickly in urine. However, biomonitoring studies that measure urine metabolites in humans show widespread exposure to phthalates. Phthalates have been labeled as potential endocrine disrupting chemicals (EDCs) because studies have demonstrated that they interfere with hormones of the reproductive system. Recently, we reported for the first time concentrations of phthalates in United States food and used these finding to estimate dietary phthalate intake.1 The estimated intakes for individual phthalates in our study were more than an order of magnitude lower than EPA Reference Doses (RfDs), but more studies are needed to evaluate cumulative exposure to phthalates as byproducts of food consumption.
Introduction
Phthalate esters are added to plastics to provide cheaper, lighter, stronger and safer products and consumer goods. Plastics can be designed to keep our foods fresh, provide therapeutic benefits through timed-release pharmaceuticals and prevent electronics and other household items from starting or spreading fires.Citation2,Citation3 Phthalates are not chemically bound to their parent polymers and therefore readily leach, migrate or off-gas from plastics, particularly when products are exposed to high temperatures. Following absorption into the body, phthalates are rapidly hydrolyzed to the corresponding monoesters with metabolites excreted in urine and feces. In humans, phthalates are eliminated mostly within hours, with excretion complete by a day or two.Citation4
In animal studies, phthalates have been identified as EDCs primarily because of their effects on the male reproductive system.Citation5 Studies have shown a wide-range of effects in males termed the phthalate syndrome, which includes infertility, decreased sperm count, cryptorchidism (undescended testes), hypospadias (malformation of the penis in which the urethra does not open at the tip of the organ) and other reproductive tract malformations. These findings led the National Toxicology Program (NTP) to convene an expert panel in 2000 to examine the experimental evidence surrounding phthalate reproductive and developmental toxicity and to investigate the extent of human exposure. The panel concluded that there was “concern” about possible exposures to the most common phthalate, di-2-ethylhexyl phthalate (DEPH), in healthy infants, and “serious concern” for exposures in very sick infants, due to potential leaching from medical equipment.Citation6 These concerns prompted the European Union to ban certain phthalates from cosmetics and children’s products.
Shortly after, a study in young girls showed possible associations between premature breast development and early phthalate exposure.Citation7 Then a study in women linked shortened pregnancy with phthalate exposure.Citation8 Average gestational age at birth was significantly shorter in 65 newborns with detectable mono-(2-ethylhexyl) phthalate (MEHP) in cord serum compared with 19 newborns with MEHP-negative cord serum.Citation8 In 2005 the NTP re-evaluated the scientific literature on DEHP and considered these new studies. However, this panel found “insufficient evidence” that phthalate exposure during pregnancy, childhood or adulthood causes adverse health effects.Citation9 In May 2005 Swan et al. reported an association between phthalates and decreased anogenital distance (AGD)—the space between the anus and the genitalia—among male infants.Citation10 Reduced AGD is a developmental end point reflective of alterations in anti-androgenic exposure and has been tied to reduced sperm quality in young men.Citation11 Phthalate exposure was assessed by measuring metabolites in the mothers' urine during pregnancy. Swan reported that those babies whose mothers were exposed to higher levels of four phthalate metabolites—[mono-n-butyl phthalate (MBP), monobenzyl phthalate (MBzP), monoethyl phthalate (MEP) and mono-isobutyl phthalate (MiBP)]—had shorter anogenital indexes and were more likely to have small genitalia and partially undescended testicles. The associations between three metabolites of DEHP measured in the study and the anogenital index were not statistically significant. The panel concluded that the Swan study was useful for the evaluation process, but the data were insufficient to show that prenatal exposure to DEHP was harmful to male infants.Citation9
Phthalate metabolism
Upon ingestion, phthalates normally follow a two-phase metabolic process. During the first phase, the diester phthalate is hydrolyzed into a monoester phthalate in the intestine, liver and kidney. Normally, this step would lead to detoxification, but in vivo studies have shown that diester phthalates become more bioactive when they are hydrolyzed to monoester phthalates.Citation12 Short-chain phthalates are mainly excreted in urine as monoester phthalates, while the more long-chain phthalates undergo several biotransformations, including further hydroxylation, oxidation and phase II conjugation before they are excreted in urine and feces.Citation13
Endocrine disrupting properties of phthalates
Phthalates have been implicated as EDCs primarily due to their effects on the reproductive system. Studies have reported that exposure to phthalates can cause damage to sperm,Citation14 early puberty in females,Citation15 anomalies of reproductive tract,Citation16 adverse outcomes of pregnancyCitation17,Citation18 and liver tumors in rodents.Citation19 Various phthalates and their metabolites have documented biochemical activity as PPAR activators, thyroid hormone axis antagonists or as antiandrogens.Citation20 Stahlhut et al. found positive associations for waist circumference, BMI and measures of insulin resistance with several urinary phthalate levels in an analysis of data from the National Health and Nutrition Examination Survey (NHANES).Citation21 Generally, short chain phthalates (those with ester chains of four to six carbon atoms) have been shown to have more potent effects on the male reproductive system than longer chain phthalates. However, recent human studies show exposure to multiple phthalates. Thus, risk assessment strategies need to consider combined effects of phthalates as mixtures.Citation22,Citation23
Phthalate exposure levels in humans
The ubiquitous use of phthalate in plastics, personal care products and food packaging results in widespread exposure to the general population. Ingestion, inhalation, intravenous injection and skin absorption are potential pathways of exposure. Human exposure to phthalates can occur as a result of direct contact or through the leaching of phthalates from one product into another, as may occur with food packaging or intravenous fluids, or by general contamination of the ambient environment. In the fourth NHANES report, CDC showed that the majority of people in the USA have detectable concentrations of several phthalate monoesters in urine [(monoethyl phthalate (MEP), monoethyl-hexyl phthalate (MEHP), monobutyl phthalate (MBP) and monobenzyl phthalate (MBzP)], reflecting widespread exposure to the parent compounds among the general population.Citation24 The study also reported that adult women have higher levels of urinary metabolites than men for phthalates that are used in soaps, body washes, shampoos, cosmetics and similar personal care products. Fatty foods such as milk, butter, oils and meats are thought to be the main exposure source for certain phthalates such as DEHP, dibutyl phthalate (DBP) and diisobutyl phthalate (DIBP) because they are lipophilic.Citation25
Phthalates can migrate into food during production, packaging and preparation. Food exposures have been estimated primarily based on duplicate diet composite studies in which phthalates were measured in samples of all food and drink consumed by participants.Citation26 Duplicate diet studies provide the best analysis of overall dietary exposure assessment because they capture exposures from food as prepared and eaten. However, these studies are costly and usually test only a small number of individuals and, thus, may not be representative of the larger population in that country. Broader studies analyzing individual food items for phthalates, total diet studies, as well as market basket studies are needed. Some surveys have been conducted in Europe, Canada and China.Citation25,Citation27-Citation29
Phthalates are difficult to measure in food because of their ubiquitous occurrence in many products including many lab-based materials. To our knowledge, our study is the first to measure phthalate concentrations in foods from the US. In this study, we sampled 72 commonly consumed foods from supermarkets in New York for nine specific phthalate esters shown in .Citation1 We detected phthalates in various food types in ranges from zero to 100%. Despite this wide variance, every food group in our study had at least some detectable level of phthalates. For example, DEHP was detected in 74% of food samples, including all seven infant food samples, whereas dicyclohexyl phthalate (DCHP) was detected in only 6% of food samples. Other phthalate esters were detected at the following frequencies: DEP (57%), DiBP (55%), benzyl butyl phthalate (BBzP) (54%), dimethyl Phthalate (DMP) (37%), DBP (31%) ().Citation1 Estimated intakes of all phthalates were fairly comparable between adults and infants on a body weight basis, with the exception of DEHP, which was more than 2-fold higher in babies than adults.Citation1
Figure 1. Structural formulas of nine phthalate esters sampled in food.Citation1
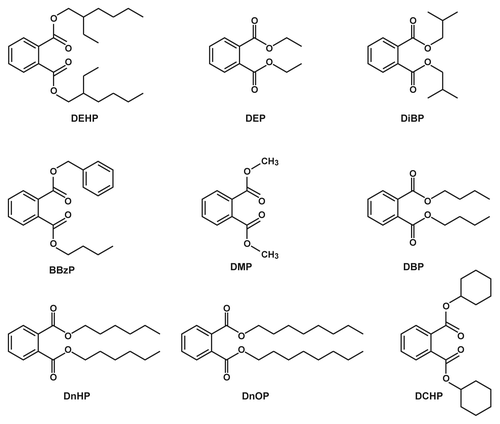
Table 1. Detection frequencies of phthalates in sampled food
Several studies have estimated phthalate intakes based on measured concentrations in media and consumption rates. Most of these studies have reported higher levels of phthalates because they included non-dietary intakes, and because concentrations measured in food surveys were often higher than measured in our food samples. For example, in a study conducted in Europe, Wormuth et al. (2006) estimated total phthalate intakes that accounted for food and non-food pathways of exposure.Citation25 They reported that total intakes from all sources reported by for DEHP, DBP, BBzP and DiBP were approximately 3.0, 4.0, 0.4 and 0.6 µg/kg-day, respectively, which is considerably higher than our US estimates of 0.7, 0.2, 0.1 and 0.02 µg/kg-day based only on our food survey ( and ).Citation1
Table 2. Food group consumption rate and sample content
Table 3. Phthalate concentrations in food groups (ng/g wet weight)
Conclusions
Our data suggest that dietary intakes of individual phthalates are much less than currently published RfD benchmarks. The current published oral RfD for DEHP set by EPA in 1986, based primarily on studies in guinea pigs and rats conducted in 1953,Citation30 is 20 µg/kg-day,Citation31,Citation32 whereas our estimate of total dietary intake is only 0.7 µg/kg-day.Citation1 However, if individual phthalates operate along similar modes of actions, then cumulative exposure estimates, from all food sources, may exceed the RfD. To date, EPA has not published RfDs for the other individual phthalates measured in our study or possible mixtures of phthalates, but they are undertaking a comprehensive Phthalate Action Plan.Citation33 The FDA regulates phthalates in food contact substances (such as plastic wrap), cosmetics, pharmaceuticals and medical devices. FDA announced in June 2008 that, in tandem with its review of the safety of bisphenol A (BPA) in such products, it is conducting a comprehensive inventory of regulated products that contain phthalates.Citation34
Our study suggests that previous phthalate exposure assessments may be comparatively higher due to consideration of total exposure patterns, and/or differences in the food samples measured to determine average concentrations. While our study focused only on food exposures, the use of phthalates in many consumer products has been recognized as important sources for human exposure. Our study measured a limited sample of foods purchased from grocery stores in only one geographic location, and did not evaluate foods as packaged, processed or served in homes or restaurants. Further representative surveys of phthalates in US food are needed, as is research on the toxicity of phthalate mixtures in food and from other sources. Future studies should also focus on the influence of packaging as well as the preparation of foods.
References
- Schecter A, Lorber M, Guo Y, Wu Q, Yun SH, Kannan K, et al. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ Health Perspect 2013; 121:473 - 94, e1-4; PMID: 23461894
- Andrady AL, Neal MA. Applications and societal benefits of plastics. Philos Trans R Soc Lond B Biol Sci 2009; 364:1977 - 84; http://dx.doi.org/10.1098/rstb.2008.0304; PMID: 19528050
- Thompson RC, Moore CJ, vom Saal FS, Swan SH. Plastics, the environment and human health: current consensus and future trends. Philos Trans R Soc Lond B Biol Sci 2009; 364:2153 - 66; http://dx.doi.org/10.1098/rstb.2009.0053; PMID: 19528062
- Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci 2009; 364:2063 - 78; http://dx.doi.org/10.1098/rstb.2008.0208; PMID: 19528056
- Foster PM, Cattley RC, Mylchreest E. Effects of di-n-butyl phthalate (DBP) on male reproductive development in the rat: implications for human risk assessment. Food Chem Toxicol 2000; 38:Suppl S97 - 9; http://dx.doi.org/10.1016/S0278-6915(99)00128-3; PMID: 10717378
- Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, et al. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol 2002; 16:529 - 653; http://dx.doi.org/10.1016/S0890-6238(02)00032-1; PMID: 12406494
- Colón I, Caro D, Bourdony CJ, Rosario O. Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environ Health Perspect 2000; 108:895 - 900; http://dx.doi.org/10.1289/ehp.00108895; PMID: 11017896
- Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, et al. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect 2003; 111:1783 - 5; http://dx.doi.org/10.1289/ehp.6202; PMID: 14594632
- Kavlock R, Barr D, Boekelheide K, Breslin W, Breysse P, Chapin R, et al. NTP-CERHR Expert Panel Update on the Reproductive and Developmental Toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol 2006; 22:291 - 399; http://dx.doi.org/10.1016/j.reprotox.2006.04.007; PMID: 17068859
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al, Study for Future Families Research Team. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 2005; 113:1056 - 61; http://dx.doi.org/10.1289/ehp.8100; PMID: 16079079
- Mendiola J, Stahlhut RW, Jørgensen N, Liu F, Swan SH. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environ Health Perspect 2011; 119:958 - 63; http://dx.doi.org/10.1289/ehp.1103421; PMID: 21377950
- Heindel JJ, Powell CJ. Phthalate ester effects on rat Sertoli cell function in vitro: effects of phthalate side chain and age of animal. Toxicol Appl Pharmacol 1992; 115:116 - 23; http://dx.doi.org/10.1016/0041-008X(92)90374-2; PMID: 1321518
- Frederiksen H, Skakkebaek NE, Andersson AM. Metabolism of phthalates in humans. Mol Nutr Food Res 2007; 51:899 - 911; http://dx.doi.org/10.1002/mnfr.200600243; PMID: 17604388
- Rozati R, Reddy PP, Reddanna P, Mujtaba R. Role of environmental estrogens in the deterioration of male factor fertility. Fertil Steril 2002; 78:1187 - 94; http://dx.doi.org/10.1016/S0015-0282(02)04389-3; PMID: 12477510
- Wolff MS, Teitelbaum SL, Pinney SM, Windham G, Liao L, Biro F, et al, Breast Cancer and Environment Research Centers. Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates, and phenols and pubertal stages in girls. Environ Health Perspect 2010; 118:1039 - 46; http://dx.doi.org/10.1289/ehp.0901690; PMID: 20308033
- Desdoits-Lethimonier C, Albert O, Le Bizec B, Perdu E, Zalko D, Courant F, et al. Human testis steroidogenesis is inhibited by phthalates. Hum Reprod 2012; 27:1451 - 9; http://dx.doi.org/10.1093/humrep/des069; PMID: 22402212
- Latini G, Del Vecchio A, Massaro M, Verrotti A, DE Felice C. In utero exposure to phthalates and fetal development. Curr Med Chem 2006; 13:2527 - 34; http://dx.doi.org/10.2174/092986706778201666; PMID: 17017909
- Whyatt RM, Adibi JJ, Calafat AM, Camann DE, Rauh V, Bhat HK, et al. Prenatal di(2-ethylhexyl)phthalate exposure and length of gestation among an inner-city cohort. Pediatrics 2009; 124:e1213 - 20; http://dx.doi.org/10.1542/peds.2009-0325; PMID: 19948620
- Kaufmann W, Deckardt K, McKee RH, Butala JH, Bahnemann R. Tumor induction in mouse liver: di-isononyl phthalate acts via peroxisome proliferation. Regul Toxicol Pharmacol 2002; 36:175 - 83; http://dx.doi.org/10.1006/rtph.2002.1575; PMID: 12460752
- Boberg J, Metzdorff S, Wortziger R, Axelstad M, Brokken L, Vinggaard AM, et al. Impact of diisobutyl phthalate and other PPAR agonists on steroidogenesis and plasma insulin and leptin levels in fetal rats. Toxicology 2008; 250:75 - 81; http://dx.doi.org/10.1016/j.tox.2008.05.020; PMID: 18602967
- Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect 2007; 115:876 - 82; http://dx.doi.org/10.1289/ehp.9882; PMID: 17589594
- Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res 2008; 108:177 - 84; http://dx.doi.org/10.1016/j.envres.2008.08.007; PMID: 18949837
- Parlett LE, Calafat AM, Swan SH. Women’s exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol 2013; 23:197 - 206; http://dx.doi.org/10.1038/jes.2012.105; PMID: 23168567
- CDC. Fourth Report on Human Exposure to Environmental Chemicals. 2009;
- Wormuth M, Scheringer M, Vollenweider M, Hungerbühler K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans?. Risk Anal 2006; 26:803 - 24; http://dx.doi.org/10.1111/j.1539-6924.2006.00770.x; PMID: 16834635
- Fromme H, Gruber L, Schlummer M, Wolz G, Böhmer S, Angerer J, et al. Intake of phthalates and di(2-ethylhexyl)adipate: results of the Integrated Exposure Assessment Survey based on duplicate diet samples and biomonitoring data. Environ Int 2007; 33:1012 - 20; http://dx.doi.org/10.1016/j.envint.2007.05.006; PMID: 17610953
- Fierens T, Servaes K, Van Holderbeke M, Geerts L, De Henauw S, Sioen I, et al. Analysis of phthalates in food products and packaging materials sold on the Belgian market. Food Chem Toxicol 2012; 50:2575 - 83; http://dx.doi.org/10.1016/j.fct.2012.04.029; PMID: 22554646
- Guo Y, Kannan K. Challenges encountered in the analysis of phthalate esters in foodstuffs and other biological matrices. Anal Bioanal Chem 2012; 404:2539 - 54; http://dx.doi.org/10.1007/s00216-012-5999-2; PMID: 22535438
- Page BD, Lacroix GM. The occurrence of phthalate ester and di-2-ethylhexyl adipate plasticizers in Canadian packaging and food sampled in 1985-1989: a survey. Food Addit Contam 1995; 12:129 - 51; http://dx.doi.org/10.1080/02652039509374287; PMID: 7758627
- Carpenter CP, Weil CS, Smyth HF Jr.. Chronic oral toxicity of di-(2-ethylhexyl) phthalate of rats, guinea pigs, and dogs. AMA Arch Ind Hyg Occup Med 1953; 8:219 - 26; PMID: 13079323
- EPA. Di(2-ethylhexyl)phthalate (DEHP) (CASRN 117-81-7). 1997;
- EPA. Phthalates TEACH Chemical Summary. 2007;
- EPA. Phthalates Action Plan. 2012;
- CRS. Bisphenol A (BPA) in Plastics and Possible Human Health Effects. 2010;