Abstract
The neurovascular unit—comprised of glia, pericytes, neurons and cerebrovasculature— is a dynamic interface that ensures physiological central nervous system (CNS) functioning. In disease dynamic remodeling of the neurovascular interface triggers a cascade of responses that determine the extent of CNS degeneration and repair. The dynamics of these processes can be adequately captured by imaging in vivo, which allows the study of cellular responses to environmental stimuli and cell-cell interactions in the living brain in real time. This perspective focuses on intravital imaging studies of the neurovascular unit in stroke, multiple sclerosis (MS) and Alzheimer disease (AD) models and discusses their potential for identifying novel therapeutic targets.
Introduction
The study of discreet processes underlying CNS function as they occur in real time in the living brain is a goal that has long fascinated neuroscientists. For many decades, the skull was a seemingly impenetrable vault, a black box preventing researchers from looking through it in the living mammal. The advent of two-photon laser scanning microscopy (TPLSM) enabled neuroscientists to record ongoing cellular processes in real time.Citation1 The first applications of intravital imaging to study the CNS set the stage for an innovative field within neuroscience research.Citation2-Citation5 In vivo imaging in the CNS became possible when the first transgenic mice expressing fluorescent proteins in different cell types of the brain were generated.Citation6 In recent years, the field has experienced an impressive growth by the introduction of novel imaging technologies, new surgical and technical methods and a wealth of sophisticated experimental approaches that have immensely expanded our insight on nervous system function in health and disease.Citation7-Citation9 The advantage of in vivo imaging is following the highly dynamic, time-dependent processes that are characteristic of CNS function in real time. The neurovascular unit, composed of cerebral blood vessels, glial cells and neurons, is at the center of precisely regulated and dynamic neuronal and vascular activities.Citation10 The features of this dynamic interface of complex, multiple cell-cell interactions render it a vulnerable target in CNS pathology,Citation10,Citation11 and an ideal site for in vivo imaging studies.
In this review, we will focus on intravital imaging using TPLSM as an indispensable experimental tool for studying real time alterations in the functioning of the neurovascular unit. We will discuss why and how the results of in vivo imaging of the neurovascular unit could provide critical information regarding the pathogenesis of several CNS diseases, including stroke, MS and AD, and how this approach could reveal novel and much needed therapeutic avenues.
Studying the Neurovascular Unit
The neurovascular unit initially served as a conceptual framework to describe the relationship between neuronal activity and cerebral blood flow (CBF), a mechanism known as functional hyperaemia.Citation10 Elevated neuronal activity is accompanied by increased blood supply, which accommodates higher neuronal metabolic demands and provides a means for the efflux of metabolic end products. The discovery that astrocytes, microglia, and recently also pericytes control vital functional processes in this tightly coupled, neuronal-CBF relationship expanded the perception of the neurovascular unit into one of a highly regulated apparatus functioning at a multi-cellular level.Citation10
Imaging the Elements of the Neurovascular Unit
The study of the neurovascular unit by histological means has identified the main cellular components and provided some crucial structural clarity into its functions.Citation11 Real-time in vivo imaging is required to capture the time-dependent activities and dynamic cell-cell interactions that take place within the complex cerebral network. It is the merit of such elegant and sophisticated studies that, for example, intricate functional contacts between dendritic spines—the major site of excitatory synaptic inputs in the brain—and astrocytic end feet have been revealed, showing that timely spine dynamics are dependent on interactions with astrocytes.Citation12 In addition, the idea that intercellular signaling within the brain is solely a neuronal privilege lost credence following in vivo imaging studies revealing communication between astrocytes in the form of Ca2+ waves that parallel neuronal activity.Citation8,Citation13 While the belief of astrocytes acting as a mere glue substance without functional roles was challenged as early as the end of the 19th century,Citation14 only recently in vivo imaging studies have provided strong support for the active role of astrocytes in neuronal health, stability, and transmission.
Some of the earliest in vivo imaging studies in the mouse cortex provided the first visual demonstrations of how complex and to this day unresolved processes as memory formation and learning could be taking place at the cellular level in the mammalian neocortex by following the same dendritic branches in the cortex over periods of time ranging from minutes to months.Citation15,Citation16 These studies identified the stochastic nature of new spine formation and revealed that some spines remain stable for prolonged periods of time while others exhibit a high turnover rate.Citation15,Citation16 By reimaging the exact same spines over time, neuronal activity was identified as a major regulator of spine turnover that can be modified by peripheral sensory stimulation of the corresponding input organs.Citation17 Similarly, in vivo imaging of microglial behavior in the intact cortex revealed that the resident immune cells of the CNS are far from “resting” at steady-state, but rather are continuously surveying the physiological brain parenchyma.Citation18,Citation19 In vivo imaging studies also detailed the nature of the physical interactions between microglial processes and dendritic spines in the brain of mice where both neurons and microglia were fluorescently labeled. These studies suggested that microglial process-dynamics might play a central role in regulating the fate of dendritic spines in the adult as well as the developing brain.Citation20-Citation22
Diseases of the neurovascular unit
As much as the tightly regulated functions of the neurovascular unit lie in the strictly regulated interplay between its cellular constituents, its vulnerability shares the very same origins. Failure of even one component can affect the system as a whole and can lead to overall malfunctioning. Stroke, MS and AD are neurological disorders that strongly impact the integrity of the neurovascular unit, whereas accumulating evidence suggests that neurovascular breakdown is an early pathological step, possibly crucial for disease initiation and progression.Citation10,Citation23 In light of these findings, there is an urgent need for tools that enable the study of the neurovascular unit in the context of disease, which could lead to the discovery of robust therapies and early disease biomarkers.Citation24 This section focuses on the impact of intravital imaging on deciphering the role of the neurovascular unit in stroke, MS and AD.
Stroke
Because cerebrovascular injury initiates cerebral impairment within a confined, chosen area of the brain, in vivo stroke models provide valuable tools for understanding the consequences of vascular disruption on cerebral functioning. Mouse and rat models of middle cerebral artery occlusion (MCAO) are based on the generation of an ischemic core causing neural cell loss as a result of mechanical vessel blockage, thereby mimicking the pathophysiological state as seen in stroke patients. In vivo imaging is a well-established method to measure subtle as well as large changes in CBF both in physiological and pathophysiological conditions.Citation25 Intravascular administration of fluorescently labeled dyes allows for visualization of blood vessels and red blood cells, the latter appearing as dark cellular shapes within a bright plasma background. This is the most commonly used approach to quantify CBF (), whereby the number of red blood cells passing through a given length of vessel within a specified time window functions as a measure of blood flow.Citation25 Altered CBF is a key phenomenon in MCAO which is partly the result of collapse of the lumenal space of microvessels followed by reorganization of the microvasculature in the stroke-affected area.Citation26 Another study employing in vivo imaging in combination with a fluorescent probe that detects activated factor XIII, revealed thrombotic events correlating with fibrin deposition within 1 to 24 h after MCAO, indicating early and time-dependent compromise of the blood-brain barrier (BBB) in stroke.Citation27 These studies exemplify how MCAO combined with intravital imaging has provided valuable understanding of the consequences of stroke on the cerebrovasculature and beyond.
Table 1. In vivo imaging tools for the study of the neurovascular unit
MCAO usually affects a very extensive area of the brain similar to a major artery occlusion in humans. However, there are also other, much more confined and probably more common incidents that result from the infarction of smaller diameter vessels or capillaries in humans. Using an in vivo stroke model that closely recapitulates such micro-occlusions, Lam and colleaguesCitation28 combined the infusion of fluorescently-labeled micro-emboli with in vivo two-photon microscopy to induce and study the dynamics of microvascular remodelling following stroke. This study revealed another highly unanticipated function within the neurovascular unit, whereby reorganization of the endothelial cells of the vascular wall resulted in extravasation of the emboli and restoration of the blood flow.Citation28 Furthermore, the extravasated emboli were taken up by neighboring microglia,Citation28 demonstrating a unique self-repair mechanism of the CNS that involves interactions between two cellular components working together at the neurovascular unit to restore blood circulation and prevent neuronal damage.
In vivo imaging of the pathological cascade downstream of the initial vascular insult has substantiated and clarified cellular events that occur in stroke, and importantly, are consistent with results of earlier histological approaches. Elegant electron microscopy work by Tagami and colleaguesCitation29 revealed stroke-induced degeneration of the enigmatic pericytes. In vivo imaging studies showed that stroke alters pericyte morphology, causing weakening of the BBB and entry of peripheral immune components including inflammatory T cells in the brain.Citation30,Citation31 Moreover, in vivo imaging showed that pericytes can unexpectedly contribute to stroke pathogenesis by intensifying cerebral ischemia via extensive microvascular constriction, leading to far-reaching and lasting reductions in CBF.Citation32
Similar to pericytes, astrocytes and recently also microglia were shown to negatively affect CBF following stroke.Citation33 This unexpected contributing role for astrocytes in stroke pathology occurs via a mechanism called spreading depression.Citation34 Spreading depression is characterized by a temporary inhibition of neuronal activity following neurological insult, such as stroke and traumatic brain injury. This inhibition spreads across parts of the cerebral cortex and originates from increased propagation of Ca2+ waves within astrocytes and neurons. The in vivo imaging studies of Chuquet and colleaguesCitation34 brought to light a synergy that exists between astrocytic and neuronal Ca2+ waves that leads to the detrimental halting of capillary CBF and results in ischemia. Astrocytes mediate signals between neurons and the cerebral vasculature to guarantee that elevated neuronal activity is paralleled by increased CBF, which facilitates the increased demand in oxygen supply. A disturbance in this tightly regulated mechanism can thus have dramatic effects on cerebral functioning.
The chronic occurrence of intracellular Ca2+ waves in neurons, as imaged in spreading depression, is not only detrimental for proper CBF, but affects neuronal integrity as well. Neuronal glutamate release is controlled by the intracellular Ca2+ concentration [Ca2+], and excessive increases in the intracellular Ca2+ pool causes neurons to release pathologic levels of glutamate, leading to neuronal swelling and breakdown.Citation35 Intravital imaging has provided further convincing evidence that stroke-induced ischemia alters dendrite morphology and affects dendritic spine dynamics within a time frame ranging from minutes to hours after the onset of CBF blockage.Citation36-Citation38 These imaging studies also yielded clarity into the mechanisms of neuronal plasticity in the recovery period after a stroke insult.Citation36 Such knowledge is essential for the advancement of stroke therapies, as they reveal the temporal response of the neurovascular unit and the time points at which therapeutic interventions might have a protective effect. With no adequate drug therapies available, the application of intravital imaging to the study of stroke pathogenesis could help to inform treatment strategies for this debilitating neurological disorder.
Multiple Sclerosis
The origins of MS, a devastating and often disabling neuroinflammatory disease, remain unknown. Some of the most prominent pathophysiological findings identified mostly in brain biopsies of MS patients are the formation of lesions in the white matter, characterized by extensive loss of myelin, axonal damage and inflammation, involving both resident and infiltrating immune cells in the CNS.Citation39 In addition to T cells, microglia,Citation40 peripheral monocytes,Citation41 and the blood protein fibrinogenCitation42,Citation43 contribute to the onset and progression of neuroinflammation. Although microglia and the disruption of the BBB are considered early events in MS pathology,Citation44 the sequence of events that lead to neuroinflammatory disease remains poorly understood. Repetitive in vivo imaging using two-photon microscopy in the animal model of MS—experimental autoimmune encephalomyelitis (EAE)—is uniquely positioned to answer these questions, since it allows the study of disease in the same animal over time. Indeed, in vivo imaging studies of T cell migration in EAE demonstrated formerly unknown interactions between autoimmune effector T cells and the cerebrovasculature. Such research, for example, revealed that T cells, after their attachment to the endothelium, explore the lumenal vessel wall in a bidirectional manner for multiple days before entering the CNS.Citation45 These imaging experiments have provided novel understanding of the migratory paths of T cells into the CNS and have expanded our understanding regarding how the physiological separation of the peripheral blood stream and the CNS is breached in neuroinflammation.
Intravital imaging has also increased our understanding of axonal pathology in neuroinflammation. Indeed, with the aid of in vivo two-photon imaging, Siffrin et al.Citation44 found an intriguing direct contact between myelin-specific Th17 and neuronal cells that was associated with extensive axonal damage. T cells induced changes in neuronal Ca2+ levels, suggesting that they participate in early stages of axonal damage. Intravital imaging was crucial in identifying “focal axonal degeneration” as a novel feature of immune-mediated axonal damage.Citation46 Nikić and colleagues,Citation46 using in vivo two-photon microscopy showed that axonal abnormalities start with focal swellings, and while some axons degenerate, in others swellings are reversible resulting in spontaneous recovery. Focal axonal degeneration was also observed in samples from human MS lesions.Citation46 Although these pioneering studies have identified novel mechanisms for T cells and axons, the contribution of glial cells and BBB leakage in the dynamic remodelling of the neurovascular interface in neuroinflammatory disease remains elusive. Technological advances of stable imaging in the spinal cord will further facilitate studies of repetitive imaging of multiple cell types within myelinated areas in anatomical areas accessible by two-photon microscopy.Citation47-Citation49 The availability of reporter transgenic mice and specific dyes for glia, T cells, and BBB leakage (), makes possible the simultaneous labeling of different components of the neurovascular unit for the study of neuroinflammatory disease.
Alzheimer Disease (AD)
Although AD has been traditionally considered a mere neuronal disease, cutting-edge studies have countered this view by identifying that breakdown of the neurovascular unit is central to the onset and progression of AD ().Citation10 One of the classic views in AD research is described by the amyloid hypothesis, according to which the pathological accumulation of amyloid β (Aβ)—a cellular cleavage product of the amyloid precursor protein APP—leads to the gradual formation of Aβ plaques over time,Citation10 and thus creating a toxic environment that causes neurodegeneration in the AD brain. Recently, the validity of the amyloid hypothesis has been strongly questioned and whether Aβ is indeed an instigator or an epiphenomenon in AD pathology remains a subject of active debate.Citation50 Nevertheless, the deposition of Aβ plaques occurs both in the brain parenchyma and on cerebral vessels, with the latter leading to cerebral amyloid angiopathy and causing a plethora of vessel pathologies including BBB breakdown, vascular stenoses and decreased CBF.Citation23 Aβ can therefore be regarded as one of the key instigators of neurovascular unit impairment in AD (). Excellent fluorescent Aβ dyes have been developed that make use of the characteristic fibrillar and β-sheet structure of Aβ within plaques and show highly specific binding. These dyes have allowed the application of intravital imaging to studies aimed at understanding the kinetics of Aβ deposition and evaluate the efficacy of anti-Aβ therapies in vivo.Citation51-Citation53 While in vivo imaging studies in AD animal models agree on the dynamics of individual plaque growth, different results have been reported on the growth kinetics of fibrillar amyloid deposits, ranging from within a day,Citation54 to gradually over weeksCitation55 to months.Citation56 Deposition kinetics of vascular Aβ have been shown to be strongly affected by the interaction of Aβ with the blood protein fibrinogen.Citation57 This interaction leads to both a decrease in lysis of fibrin clots and impaired vascular Aβ clearance inducing cerebral amyloid angiopathy, vascular Aβ deposits affecting the physiology of the cerebrovasculature.Citation57 The reciprocal character of the Aβ-fibrinogen interaction in AD was further supported by the results of in vivo imaging in a transgenic AD mouse model that demonstrated decreased fibrinolysis with blood clot formation paralleled by impaired CBF and cognitive functioning.Citation57 Such studies are demonstrative of the close relationship that exists between neuronal health and an intact, properly functioning neurovascular unit.
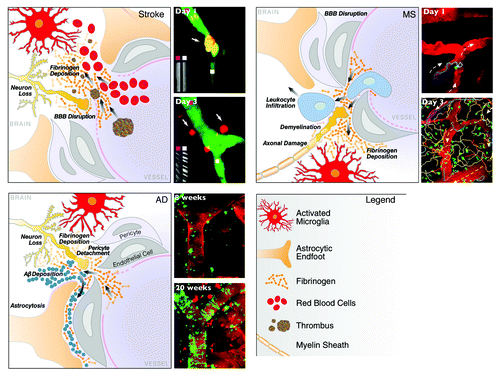
Figure 1. In vivo imaging of the neurovascular unit in stroke, MS and AD. Schematic representation of the dynamic alterations at the neurovascular unit in different neurologic diseases. Despite different origins, neuropathology and clinical disease progression, there are striking similarities in the cellular processes underlying breakdown of the neurovascular unit. Leakage of the BBB with entry of blood proteins, activation of microglia, astrocyte endfeet retraction, pericyte detachment, neuronal and axonal changes are key elements of cerebrovascular dysfunction. Repetitive in vivo imaging shows extravasation of micro-emboli causing alterations in CBF and restructuring of the microvasculature in stroke,Citation28 migratory paths of T cells after their attachment to the leptomeningeal vessels in a mouse model of MS,Citation45 and gradual progression of cerebral amyloid angiopathy over weeks as captured by time-lapse in vivo imaging in a transgenic mouse model of AD.Citation54 Images reproduced with permission.
A more careful evaluation of the relationship between amyloid plaque growth and the degree of neuritic dystrophy in their proximity seems to confirm a causal relationship between the two, however it would appear that the kinetics of amyloid deposition is a more critical determinant of neurotoxicity, possibly more so than the extent of the plaque growth itself.Citation58 Microglial activation influences Aβ plaque deposition by causing chronic cerebral inflammation, neuron loss and BBB breakdown. An example is the unexpected discovery that the microglial CX3CR1 chemokine receptor directly affects neuronal viability in mouse models of AD.Citation59 Another recent study by Liu and colleaguesCitation60 showed that microglia control brain Aβ concentrations and hence the degree of Aβ plaque formation in AD mouse models, opening the road for a potential new drug target in microglia. In vivo imaging studies in transgenic mouse lines with microglia-specific fluorescent protein expression () have thus elucidated how microglial activation contributes to AD-mediated cerebral inflammation. In addition to microglia, astrocyte activation is also a hallmark of AD pathology, especially around Aβ plaques, as first discovered by traditional histological approaches.Citation10 However, it had not been clearly established whether their activation is linked to detrimental effects on surrounding tissue. In some studies, astrogliosis was found to be indicative of Aβ plaque clearance.Citation61 Yet, Aβ-induced astrogliosis is also known to significantly impair astrocytic functions vital to neuronal integrity and CBF—including glucose uptake and lactate release—by triggering the retraction of astrocyte end feet from Aβ-affected blood vessels, thereby disturbing neurovascular coupling.Citation23 To date, in vivo imaging of astrocytes in AD models is focused mainly on understanding the function of these cells in the vicinity of Aβ plaques. These studies are based on imaging the intra- and intercellular astrocytic Ca2+ waves key to neuron-astrocyte communication, and have provided knowledge of the astrocytic activation state as exemplified by increased intracellular [Ca2+].Citation8,Citation13 The in vivo imaging techniques that are now available () enables a much-needed and better understanding of the fate and role of astrocytes in AD.
The extensive failure of glial cell and vascular function within the neurovascular unit is increasingly regarded as an initiating or at least largely contributing factor in AD-related neurodegeneration. Cognitive decline as a result of neurodegeneration is one of the symptomatic characteristics of AD. The availability of transgenic mouse lines with neuron-specific fluorescent protein expression () has allowed the study of the relationship between neuronal morphology/function and cognitive performance in vivo. Chronic loss of dendritic spines—the functional postsynaptic units critical for plasticity and ultimately cognitive function—and dendrites in the vicinity of Aβ plaques has been one the first discoveries revealed by in vivo imaging.Citation62,Citation63
These studies also demonstrated that a gradient exists in the degree of Aβ plaque toxicity on dendritic spine dynamics, whereby neuronal dendrites in close proximity to Aβ plaques (< 15 μm distance) display significantly less spine density than ones farther away.Citation64 Others have found similar results,Citation65 although the toxicity-to-distance ratio can differ significantly between the various transgenic AD mouse models. Besides dendritic spine loss, observations of neurite retraction and abnormal neurite morphology, including swelling and fragmentation in relation to Aβ plaque growth over time, have also come to light with intravital imaging.Citation63,Citation65 In vivo imaging is not only a powerful tool to study morphologic changes over time, but can also assess functional activity within the CNS. Combining structural and functional in vivo imaging, Busche and colleaguesCitation66 discovered that neurons in close proximity to Aβ plaques showed significantly increased activity. These findings offer crucial clues to help explain the phenomenon of altered neuronal firing patterns that can disturb neuronal function in AD.
Concluding Remarks
Research in neuroimmune and neurodegenerative disorders, including stroke, MS and AD has advanced tremendously our understanding of the mechanisms underlying disease pathology, onset and progression, as well as revealing potential drug targets. Unfortunately, the number of breakthroughs in actual drug discovery to treat these devastating diseases does not parallel the number of mechanistic findings of disease pathogenesis, perhaps due to the inherently costly and time-consuming features of CNS drug discovery. Intravital imaging has the potential to enhance translational efforts as it reveals novel and unanticipated features of disease pathogenesis and is instrumental in identifying the early events that trigger CNS disease.
Stroke and AD research in particular was traditionally approached from a mere neuron-centered point of view.Citation67 Given that these diseases cause dramatic neuron loss and decreased neuronal connectivity such focus is understandable. However, a more unifying view is emerging in which neurons are no longer acting alone, but instead act together with their counterparts within the dynamic environment of the neurovascular unit.Citation10 This view has evolved in large part thanks to the numerous intravital imaging studies discussed in this perspective, and will likely inform future CNS drug discovery efforts, as exemplified by the increasing use of in vivo imaging methods in drug discovery.Citation68 The potential of in vivo imaging to bridge the void between the outcomes of basic research and their translation to the clinic is also demonstrated by the increasing use of clinical imaging techniques in basic in vivo research and vice versa. Functional magnetic resonance imaging (fMRI) and positron-emission tomography (PET) studies of CBF and metabolic changes as a result of CNS pathology are examples of this interchangeable, translational character of clinical and in vivo imaging bringing to light formerly unknown similarities in (mal)functioning of brain regions between humans and rodents.Citation69
Stroke, MS and AD are characterized by unique molecular players, distinct disease onset and progression patterns, symptoms, and survival rates. Despite differences in mechanisms of disease pathogenesis, in vivo imaging studies in animal models have revealed compelling common alterations of the neurovascular unit in neuroimmune and neurodegenerative diseases (). In essence, the multifactorial character of these disorders and the substantial prevalence of co-morbidities could be explained by the similarities of neurovascular unit dysfunction. Furthering our knowledge and understanding of these intriguing parallels by employing a powerful tool like in vivo imaging could not only provide essential insight in these debilitating diseases, but perhaps also lead to the discovery of new targets for disease-modifying therapeutics or, ideally, cures. Given the contribution of the neurovascular unit to brain injury,Citation70 future in vivo imaging studies could thus also be instrumental for the understanding of the mechanisms at play in traumatic brain injury.
Since the neurovascular unit is composed of a multitude of cellular processes, its study and understanding require a multiform research approach. Although intravital imaging is a powerful tool for the study of the neurovascular unit, there remain significant technical challenges. Intravital imaging can be beset by sub-optimal signal-to-noise ratio due to poor resolution as a consequence of light scattering within the imaged tissue.Citation7 Moreover, brain movements caused by breathing patterns and beating of the heart can greatly disturb the quality of imaging, especially during continuous (time-lapse) imaging of small structures such as dendritic spines, microglial protrusions and astrocytic end feet.Citation9 Furthermore, while intravital imaging sheds exceptional light on structural and cellular changes, the molecular pathways and mechanisms underlying those alterations cannot be revealed using this technique. However, combining the power of in vivo imaging with other research tools can bring forth far-reaching knowledge of the neurovascular unit in CNS disease. The emerging advances in molecular imaging and the development of molecular probes, which can bind with high specificity to their targetCitation27,Citation51,Citation71,Citation72 bear great promise toward achieving enhanced understanding within the field.
| Abbreviations: | ||
| CNS | = | central nervous system |
| MS | = | multiple sclerosis |
| AD | = | Alzheimer disease |
| TPLSM | = | two-photon laser scanning microscopy |
| CBF | = | cerebral blood flow |
| MCAO | = | middle cerebral artery occlusion |
| BBB | = | blood-brain barrier |
| EAE | = | experimental autoimmune encephalomyelitis |
| Aβ | = | amyloid β |
| fMRI | = | functional magnetic resonance imaging |
| PET | = | positron-emission tomography |
Acknowledgments
We thank Anna Lisa Lucido for editorial assistance. M.M. was supported by a Swiss National Science Foundation Postdoctoral Fellowship, D.D. by the Nancy Davis Young Investigator Award and K.A. by NIH/NINDS grants NS051470, NS052189 and NS066361.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science 1990; 248:73 - 6; http://dx.doi.org/10.1126/science.2321027; PMID: 2321027
- Gan WB, Grutzendler J, Wong WT, Wong RO, Lichtman JW. Multicolor “DiOlistic” labeling of the nervous system using lipophilic dye combinations. Neuron 2000; 27:219 - 25; http://dx.doi.org/10.1016/S0896-6273(00)00031-3; PMID: 10985343
- Helmchen F, Svoboda K, Denk W, Tank DW. In vivo dendritic calcium dynamics in deep-layer cortical pyramidal neurons. Nat Neurosci 1999; 2:989 - 96; http://dx.doi.org/10.1038/14788; PMID: 10526338
- Lendvai B, Stern EA, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 2000; 404:876 - 81; http://dx.doi.org/10.1038/35009107; PMID: 10786794
- Svoboda K, Helmchen F, Denk W, Tank DW. Spread of dendritic excitation in layer 2/3 pyramidal neurons in rat barrel cortex in vivo. Nat Neurosci 1999; 2:65 - 73; http://dx.doi.org/10.1038/4569; PMID: 10195182
- Misgeld T, Kerschensteiner M. In vivo imaging of the diseased nervous system. Nat Rev Neurosci 2006; 7:449 - 63; http://dx.doi.org/10.1038/nrn1905; PMID: 16715054
- Hillman EM. Optical brain imaging in vivo: techniques and applications from animal to man. J Biomed Opt 2007; 12:051402; http://dx.doi.org/10.1117/1.2789693; PMID: 17994863
- Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods 2004; 1:31 - 7; http://dx.doi.org/10.1038/nmeth706; PMID: 15782150
- Yoder EJ. In Vivo Microscopy of the Mouse Brain Using Multiphoton Laser Scanning Techniques. Proc Soc Photo Opt Instrum Eng 2002; 4620:14 - 29; PMID: 20975841
- Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med 2010; 16:1370 - 1; http://dx.doi.org/10.1038/nm1210-1370; PMID: 21135839
- Baeten KM, Akassoglou K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol 2011; 71:1018 - 39; http://dx.doi.org/10.1002/dneu.20954; PMID: 21780303
- Barker AJ, Ullian EM. Astrocytes and synaptic plasticity. Neuroscientist 2010; 16:40 - 50; http://dx.doi.org/10.1177/1073858409339215; PMID: 20236948
- Takano T, Han X, Deane R, Zlokovic B, Nedergaard M. Two-photon imaging of astrocytic Ca2+ signaling and the microvasculature in experimental mice models of Alzheimer’s disease. Ann N Y Acad Sci 2007; 1097:40 - 50; http://dx.doi.org/10.1196/annals.1379.004; PMID: 17413008
- Andriezen WL. The neuroglia elements in the human brain. Br Med J 1893; 2:227 - 30; http://dx.doi.org/10.1136/bmj.2.1700.227; PMID: 20754383
- Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature 2002; 420:812 - 6; http://dx.doi.org/10.1038/nature01276; PMID: 12490949
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 2002; 420:788 - 94; http://dx.doi.org/10.1038/nature01273; PMID: 12490942
- Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol 2009; 71:261 - 82; http://dx.doi.org/10.1146/annurev.physiol.010908.163140; PMID: 19575680
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 2005; 8:752 - 8; http://dx.doi.org/10.1038/nn1472; PMID: 15895084
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005; 308:1314 - 8; http://dx.doi.org/10.1126/science.1110647; PMID: 15831717
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012; 74:691 - 705; http://dx.doi.org/10.1016/j.neuron.2012.03.026; PMID: 22632727
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol 2010; 8:e1000527; http://dx.doi.org/10.1371/journal.pbio.1000527; PMID: 21072242
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 2009; 29:3974 - 80; http://dx.doi.org/10.1523/JNEUROSCI.4363-08.2009; PMID: 19339593
- Merlini M, Meyer EP, Ulmann-Schuler A, Nitsch RM. Vascular β-amyloid and early astrocyte alterations impair cerebrovascular function and cerebral metabolism in transgenic arcAβ mice. Acta Neuropathol 2011; 122:293 - 311; http://dx.doi.org/10.1007/s00401-011-0834-y; PMID: 21688176
- Stanimirovic DB, Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence?. J Cereb Blood Flow Metab 2012; 32:1207 - 21; http://dx.doi.org/10.1038/jcbfm.2012.25; PMID: 22395208
- Helmchen F, Kleinfeld D. Chapter 10. In vivo measurements of blood flow and glial cell function with two-photon laser-scanning microscopy. Methods Enzymol 2008; 444:231 - 54; http://dx.doi.org/10.1016/S0076-6879(08)02810-3; PMID: 19007667
- Masamoto K, Tomita Y, Toriumi H, Aoki I, Unekawa M, Takuwa H, et al. Repeated longitudinal in vivo imaging of neuro-glio-vascular unit at the peripheral boundary of ischemia in mouse cerebral cortex. Neuroscience 2012; 212:190 - 200; http://dx.doi.org/10.1016/j.neuroscience.2012.03.034; PMID: 22516017
- Zhang ZG, Zhang L, Ding G, Jiang Q, Zhang RL, Zhang X, et al. A model of mini-embolic stroke offers measurements of the neurovascular unit response in the living mouse. Stroke 2005; 36:2701 - 4; http://dx.doi.org/10.1161/01.STR.0000190007.18897.e3; PMID: 16269633
- Lam CK, Yoo T, Hiner B, Liu Z, Grutzendler J. Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature 2010; 465:478 - 82; http://dx.doi.org/10.1038/nature09001; PMID: 20505729
- Tagami M, Nara Y, Kubota A, Fujino H, Yamori Y. Ultrastructural changes in cerebral pericytes and astrocytes of stroke-prone spontaneously hypertensive rats. Stroke 1990; 21:1064 - 71; http://dx.doi.org/10.1161/01.STR.21.7.1064; PMID: 2368108
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68:409 - 27; http://dx.doi.org/10.1016/j.neuron.2010.09.043; PMID: 21040844
- Fumagalli S, Coles JA, Ejlerskov P, Ortolano F, Bushell TJ, Brewer JM, et al. In vivo real-time multiphoton imaging of T lymphocytes in the mouse brain after experimental stroke. Stroke 2011; 42:1429 - 36; http://dx.doi.org/10.1161/STROKEAHA.110.603704; PMID: 21441145
- Fernández-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A 2010; 107:22290 - 5; http://dx.doi.org/10.1073/pnas.1011321108; PMID: 21135230
- Masuda T, Croom D, Hida H, Kirov SA. Capillary blood flow around microglial somata determines dynamics of microglial processes in ischemic conditions. Glia 2011; 59:1744 - 53; http://dx.doi.org/10.1002/glia.21220; PMID: 21800362
- Chuquet J, Hollender L, Nimchinsky EA. High-resolution in vivo imaging of the neurovascular unit during spreading depression. J Neurosci 2007; 27:4036 - 44; http://dx.doi.org/10.1523/JNEUROSCI.0721-07.2007; PMID: 17428981
- Tymianski M. Emerging mechanisms of disrupted cellular signaling in brain ischemia. Nat Neurosci 2011; 14:1369 - 73; http://dx.doi.org/10.1038/nn.2951; PMID: 22030547
- Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J Neurosci 2007; 27:4101 - 9; http://dx.doi.org/10.1523/JNEUROSCI.4295-06.2007; PMID: 17428988
- Johnston DG, Denizet M, Mostany R, Portera-Cailliau C. Chronic In Vivo Imaging Shows No Evidence of Dendritic Plasticity or Functional Remapping in the Contralesional Cortex after Stroke. Cereb Cortex 2012; In press http://dx.doi.org/10.1093/cercor/bhs092; PMID: 22499800
- Murphy TH, Li P, Betts K, Liu R. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J Neurosci 2008; 28:1756 - 72; http://dx.doi.org/10.1523/JNEUROSCI.5128-07.2008; PMID: 18272696
- Lassmann H. Axonal and neuronal pathology in multiple sclerosis: what have we learnt from animal models. Exp Neurol 2010; 225:2 - 8; http://dx.doi.org/10.1016/j.expneurol.2009.10.009; PMID: 19840788
- Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hövelmeyer N, et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med 2005; 11:146 - 52; http://dx.doi.org/10.1038/nm1177; PMID: 15665833
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci 2011; 14:1142 - 9; http://dx.doi.org/10.1038/nn.2887; PMID: 21804537
- Adams RA, Bauer J, Flick MJ, Sikorski SL, Nuriel T, Lassmann H, et al. The fibrin-derived gamma377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med 2007; 204:571 - 82; http://dx.doi.org/10.1084/jem.20061931; PMID: 17339406
- Adams RA, Schachtrup C, Davalos D, Tsigelny I, Akassoglou K. Fibrinogen signal transduction as a mediator and therapeutic target in inflammation: lessons from multiple sclerosis. Curr Med Chem 2007; 14:2925 - 36; http://dx.doi.org/10.2174/092986707782360015; PMID: 18045138
- Siffrin V, Radbruch H, Glumm R, Niesner R, Paterka M, Herz J, et al. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity 2010; 33:424 - 36; http://dx.doi.org/10.1016/j.immuni.2010.08.018; PMID: 20870176
- Bartholomäus I, Kawakami N, Odoardi F, Schläger C, Miljkovic D, Ellwart JW, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 2009; 462:94 - 8; http://dx.doi.org/10.1038/nature08478; PMID: 19829296
- Nikić I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM, et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med 2011; 17:495 - 9; http://dx.doi.org/10.1038/nm.2324; PMID: 21441916
- Davalos D, Lee JK, Smith WB, Brinkman B, Ellisman MH, Zheng B, et al. Stable in vivo imaging of densely populated glia, axons and blood vessels in the mouse spinal cord using two-photon microscopy. J Neurosci Methods 2008; 169:1 - 7; http://dx.doi.org/10.1016/j.jneumeth.2007.11.011; PMID: 18192022
- Davalos D, Akassoglou K. In vivo imaging of the mouse spinal cord using two-photon microscopy. J Vis Exp 2012; 59:e2760; PMID: 22258623
- Kim JV, Jiang N, Tadokoro CE, Liu L, Ransohoff RM, Lafaille JJ, et al. Two-photon laser scanning microscopy imaging of intact spinal cord and cerebral cortex reveals requirement for CXCR6 and neuroinflammation in immune cell infiltration of cortical injury sites. J Immunol Methods 2010; 352:89 - 100; http://dx.doi.org/10.1016/j.jim.2009.09.007; PMID: 19800886
- Pimplikar SW. Reassessing the amyloid cascade hypothesis of Alzheimer’s disease. Int J Biochem Cell Biol 2009; 41:1261 - 8; http://dx.doi.org/10.1016/j.biocel.2008.12.015; PMID: 19124085
- Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, et al. Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med 2001; 7:369 - 72; http://dx.doi.org/10.1038/85525; PMID: 11231639
- Burgold S, Bittner T, Dorostkar MM, Kieser D, Fuhrmann M, Mitteregger G, et al. In vivo multiphoton imaging reveals gradual growth of newborn amyloid plaques over weeks. Acta Neuropathol 2011; 121:327 - 35; http://dx.doi.org/10.1007/s00401-010-0787-6; PMID: 21136067
- Skoch J, Hickey GA, Kajdasz ST, Hyman BT, Bacskai BJ. In vivo imaging of amyloid-beta deposits in mouse brain with multiphoton microscopy. Methods Mol Biol 2005; 299:349 - 63; PMID: 15980616
- Dong J, Revilla-Sanchez R, Moss S, Haydon PG. Multiphoton in vivo imaging of amyloid in animal models of Alzheimer’s disease. Neuropharmacology 2010; 59:268 - 75; http://dx.doi.org/10.1016/j.neuropharm.2010.04.007; PMID: 20398680
- Yan P, Bero AW, Cirrito JR, Xiao Q, Hu X, Wang Y, et al. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J Neurosci 2009; 29:10706 - 14; http://dx.doi.org/10.1523/JNEUROSCI.2637-09.2009; PMID: 19710322
- Hefendehl JK, Wegenast-Braun BM, Liebig C, Eicke D, Milford D, Calhoun ME, et al. Long-term in vivo imaging of β-amyloid plaque appearance and growth in a mouse model of cerebral β-amyloidosis. J Neurosci 2011; 31:624 - 9; http://dx.doi.org/10.1523/JNEUROSCI.5147-10.2011; PMID: 21228171
- Cortes-Canteli M, Paul J, Norris EH, Bronstein R, Ahn HJ, Zamolodchikov D, et al. Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer’s disease. Neuron 2010; 66:695 - 709; http://dx.doi.org/10.1016/j.neuron.2010.05.014; PMID: 20547128
- Condello C, Schain A, Grutzendler J. Multicolor time-stamp reveals the dynamics and toxicity of amyloid deposition. Sci Rep 2011; 1:19; http://dx.doi.org/10.1038/srep00019; PMID: 22355538
- Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, et al. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat Neurosci 2010; 13:411 - 3; http://dx.doi.org/10.1038/nn.2511; PMID: 20305648
- Liu Z, Condello C, Schain A, Harb R, Grutzendler J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis. J Neurosci 2010; 30:17091 - 101; http://dx.doi.org/10.1523/JNEUROSCI.4403-10.2010; PMID: 21159979
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, et al. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med 2003; 9:453 - 7; http://dx.doi.org/10.1038/nm838; PMID: 12612547
- Spires-Jones TL, Mielke ML, Rozkalne A, Meyer-Luehmann M, de Calignon A, Bacskai BJ, et al. Passive immunotherapy rapidly increases structural plasticity in a mouse model of Alzheimer disease. Neurobiol Dis 2009; 33:213 - 20; http://dx.doi.org/10.1016/j.nbd.2008.10.011; PMID: 19028582
- Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci 2004; 7:1181 - 3; http://dx.doi.org/10.1038/nn1335; PMID: 15475950
- Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci 2005; 25:7278 - 87; http://dx.doi.org/10.1523/JNEUROSCI.1879-05.2005; PMID: 16079410
- Bittner T, Fuhrmann M, Burgold S, Ochs SM, Hoffmann N, Mitteregger G, et al. Multiple events lead to dendritic spine loss in triple transgenic Alzheimer’s disease mice. PLoS One 2010; 5:e15477; http://dx.doi.org/10.1371/journal.pone.0015477; PMID: 21103384
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 2008; 321:1686 - 9; http://dx.doi.org/10.1126/science.1162844; PMID: 18802001
- de la Torre JC. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol 2004; 3:184 - 90; http://dx.doi.org/10.1016/S1474-4422(04)00683-0; PMID: 14980533
- Bullen A. Microscopic imaging techniques for drug discovery. Nat Rev Drug Discov 2008; 7:54 - 67; http://dx.doi.org/10.1038/nrd2446; PMID: 18079755
- Borsook D, Becerra L, Hargreaves R. A role for fMRI in optimizing CNS drug development. Nat Rev Drug Discov 2006; 5:411 - 24; http://dx.doi.org/10.1038/nrd2027; PMID: 16604100
- Sashindranath M, Sales E, Daglas M, Freeman R, Samson AL, Cops EJ, et al. The tissue-type plasminogen activator-plasminogen activator inhibitor 1 complex promotes neurovascular injury in brain trauma: evidence from mice and humans. Brain 2012; In press; http://dx.doi.org/10.1093/brain/aws178; PMID: 22822039
- Lee DK, Nahrendorf M, Schellingerhout D, Kim DE. Will molecular optical imaging have clinically important roles in stroke management, and how?. J Clin Neurol 2010; 6:10 - 8; http://dx.doi.org/10.3988/jcn.2010.6.1.10; PMID: 20386638
- Chen B, Friedman B, Whitney MA, Winkle JA, Lei IF, Olson ES, et al. Thrombin activity associated with neuronal damage during acute focal ischemia. J Neurosci 2012; 32:7622 - 31; http://dx.doi.org/10.1523/JNEUROSCI.0369-12.2012; PMID: 22649241
- Nolte C, Matyash M, Pivneva T, Schipke CG, Ohlemeyer C, Hanisch UK, et al. GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia 2001; 33:72 - 86; http://dx.doi.org/10.1002/1098-1136(20010101)33:1<72::AID-GLIA1007>3.0.CO;2-A; PMID: 11169793
- Guo F, Maeda Y, Ma J, Delgado M, Sohn J, Miers L, et al. Macroglial plasticity and the origins of reactive astroglia in experimental autoimmune encephalomyelitis. J Neurosci 2011; 31:11914 - 28; http://dx.doi.org/10.1523/JNEUROSCI.1759-11.2011; PMID: 21849552
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 2000; 20:4106 - 14; http://dx.doi.org/10.1128/MCB.20.11.4106-4114.2000; PMID: 10805752
- Hirase H, Creso J, Singleton M, Barthó P, Buzsáki G. Two-photon imaging of brain pericytes in vivo using dextran-conjugated dyes. Glia 2004; 46:95 - 100; http://dx.doi.org/10.1002/glia.10295; PMID: 14999817
- Yokota T, Kawakami Y, Nagai Y, Ma JX, Tsai JY, Kincade PW, et al. Bone marrow lacks a transplantable progenitor for smooth muscle type alpha-actin-expressing cells. Stem Cells 2006; 24:13 - 22; http://dx.doi.org/10.1634/stemcells.2004-0346; PMID: 16099999
- Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, et al. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med 2012; 209:1219 - 34; http://dx.doi.org/10.1084/jem.20111622; PMID: 22615129
- Motoike T, Loughna S, Perens E, Roman BL, Liao W, Chau TC, et al. Universal GFP reporter for the study of vascular development. Genesis 2000; 28:75 - 81; http://dx.doi.org/10.1002/1526-968X(200010)28:2<75::AID-GENE50>3.0.CO;2-S; PMID: 11064424
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 2000; 28:41 - 51; http://dx.doi.org/10.1016/S0896-6273(00)00084-2; PMID: 11086982