Abstract
The development of bispecific antibodies has attracted substantial interest, and many different formats have been described. Those specifically containing an Fc part are mostly tetravalent, such as stabilized IgG-scFv fusions or dual-variable domain (DVD) IgGs. However, although they exhibit IgG-like properties and technical developability, these formats differ in size and geometry from classical IgG antibodies. Thus, considerable efforts focus on bispecific heterodimeric IgG antibodies that more closely mimic natural IgG molecules. The inherent chain association problem encountered when producing bispecific heterodimeric IgG antibodies can be overcome by several methods. While technologies like knobs-into-holes (KiH) combined with a common light chain or the CrossMab technology enforce the correct chain association, other approaches, e.g., the dual-acting Fab (DAF) IgGs, do not rely on a heterodimeric Fc part. This review discusses the state of the art in bispecific heterodimeric IgG antibodies, with an emphasis on recent progress.
Introduction
In the field of recombinant antibody technology, bispecific antibodies have attracted substantial interestCitation1-Citation4 due to potential advantages summarized in . From the perspective of their development as biopharmaceuticals, bispecific antibodies should clearly differentiate from the respective monotherapies and combination therapies. It can be envisioned that this is the case for all applications where targeting or specificity for a specific tissue or broad neutralization, e.g., of viruses such as HIV,Citation5 is required. Similarly, bispecific antibodies are required to mediate transport phenomena, e.g., through the blood-brain-barrier,Citation6 or effector cell recruitment.Citation7 Furthermore, considerations such as pharmacoeconomics, convenience or an advantageous clinical development path may favor the choice of a bispecific antibody over the respective combination or co-formulation; in particular, in the likely case that future treatment regimens require combination of more than two targeted biologics at the same time.
Table 1. Overview of (potential) advantages of bispecific heterodimeric antibodies over classical IgG antibodies and their combination
Preliminary efforts to design and engineer bispecific antibodies focused primarily on non-Fc-containing bispecific antibody formats such as scFv-based or diabody formats. Concomitant advances in antibody engineering and, importantly, antibody expression and purification greatly diminished the technical challenges associated with the production of Fc-containing bispecific antibodies. Encouraging clinical data, including the recent approval of the EpCAM/CD3 mouse/rat chimeric bispecific antibody catumaxomab (Trion)Citation8 and promising clinical data from studies of the CD19/CD3 scFv bispecific T cell engager (BiTE) blinatumomab (Amgen) in B-lineage acute lymphoblastic leukemia (ALL)Citation7 further fostered a renewed interest.Citation9 Until recently, bispecific antibodies in clinical trials almost exclusively belonged to the class of effector cell recruiters. The first dual-targeting bispecific non-effector cell recruiters have recently entered clinical trials, e.g., (1) MM-111, a half-life enhanced bispecific human serum albumin/human epidermal growth factor receptor EGFR-HER3 scFv fusion (Merrimack);Citation10 (2) SAR156597, a bispecific IL-4/IL-14 DVD-IgG (Sanofi); (3) CVX-241, a bispecific tetravalent CovX body against vascular endothelial growth factor (VEGF) and Angiopoietin-2 (CovX/Pfizer).Citation11 As judged by publications and patents, numerous bispecific antibodies are likely to enter clinical trials during the coming years.
In the field of Fc-containing tetravalent bispecific antibodies, several antibody formats such as stabilized IgG-scFv fusionsCitation12-Citation16 or dual-variable domain DVD-IgCitation17,Citation18 and others have been described. However, although these bispecific antibody formats exhibit IgG-like properties and can be technically developed, they differ in size and geometry from conventional IgG antibodies. Thus, there is continued interest in bispecific antibodies that are as close as possible to the natural IgG design.
This review focuses on the status and recent progress in the field of bispecific heterodimeric IgG antibodies. Several approaches to overcome the chain association problem in the generation of these antibodies, including the KiH technology that allows the generation of defined bispecific heterodimeric IgG antibodies when combined with a common light chain approach or the CrossMab technology, and alternative approaches that do not rely on heterodimeric Fc, such as dual-acting Fab (DAF)-IgGs, are discussed in detail.
The Chain Association Issue and the Quadroma Approach
Bispecific Fab2 fragments can be made from two different Fab2 fragments using biochemical techniques such as reduction and selective re-oxidation.Citation19,Citation20 Similarly, full-length bispecific IgG antibodies can be generated from two IgG antibodies by reduction/oxidation followed by affinity chromatography using the respective antigens.Citation19,Citation20 However, these approaches do not allow the large scale supply of bispecific antibodies in qualities required for clinical trials; thus, ideally, the desired bispecific heterodimeric IgG antibody is produced in one cell line. illustrates the basic challenge in generating bispecific heterodimeric IgG antibodies from 4 antibody chains (2 different heavy and 2 different light chains) in one expression cell line, the so-called chain association issue. In , the homo- and heterodimerization interfaces between the individual antibody chains are schematically shown. Use of different chains for the left and the right arm of the antibody will lead to mixtures; the two heavy chains are able to associate in four different combinations, and each of those can associate in a stochastic manner with the light chains, resulting in 24 (total of 16) possible chain combinations, or 10 different antibodies of which only one corresponds to the desired functional bispecific antibody.Citation21 The difficulties in isolating this desired bispecific antibody out of complex mixtures and the inherent poor yield of a maximum of 12.5% () make the production of a bispecific antibody in one expression cell line extremely challenging and disadvantageous. Nevertheless, bispecific antibodies have been generated via this approach where two hybridomas of different specificities are fused together to form a “quadroma” cell line that secretes the two antibodies in a mixture that includes the desired bispecific antibody.Citation22,Citation23
Figure 1. (A) Schematic depiction of the homo- and heterodimerization interfaces between light- and heavy-chain domains leading to mixtures when expressed simultaneously. (B) Chain association issue when co-expressing two different antibody heavy and light chains in one cell line, assuming random chain association (Quadroma). In total, 24 = 16 combinations are possible. Of those, 6 are identical; thus, a purely statistical association leads to 6 tetramers that occur twice (each 12.5% yield) and 4 tetramers that occur once (each 6.25%). The desired bispecific antibody makes up statistically 12.5% of the total yield. (C) Light chain association issue when co-expressing two different antibody light chains in one cell line, assuming random chain association. Heavy chain heterodimerization is enforced using KiH technology. The desired bispecific antibody makes up statistically 25% of the total yield.
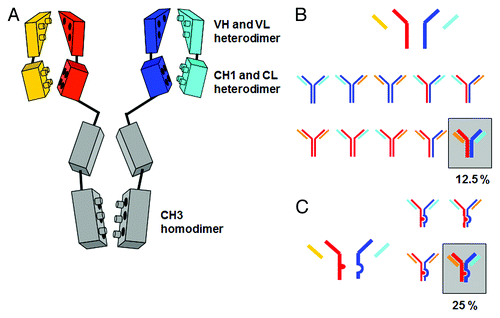
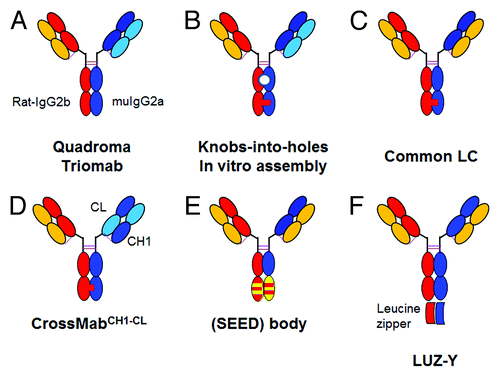
Lindhofer et al. successfully managed the challenge of isolating the desired bispecific quadroma-derived antibody from this mixture with specifications allowing sufficient supply for marketing. For this purpose, they selected the expression of mouse IgG2a and rat IgG2b chimeric antibodies from chimeric mouse/rat quadroma cells ().Citation24 Aside from the strong immunogenicity, which is a major drawback, the use of mouse and rat antibodies has three major technical advantages: (1) Preferential intra-species heavy-light chain pairing resulting in a reduction of mispairing; (2) efficient heterologous heavy chain pairing; and (3) different affinities of the selected mouse and rat IgG isotypes for protein A, which allows a relatively straightforward isolation of the bispecific antibody product by a combination of protein A affinity and ion exchange chromatography.Citation24 Using this technology, catumaxomab, a bispecific antibody recognizing EpCAM and CD3, was produced and clinically developed.Citation8 Due to the retained effector function of the murine/rat Fc part, these antibodies are also termed trifunctional antibodies or Triomabs®.Citation25 Catumaxomab was approved in 2009 in the European Union for the intraperitoneal treatment of patients with malignant ascites, and thereby became the first approved bispecific antibody.Citation8,Citation26 Due to severe infusion reactions as a consequence of FcγR co-activation, catumaxomab cannot be administered via the systemic intravenous route. Furthermore, repeated dosing is impossible because of the highly immunogenic nature of the murine/rat chimeric antibody, and manufacturing from quadromas does not allow large scale supply and may not fulfill specifications for diseases other than late-stage cancer. Taken together, the quadroma/Triomab approach may be feasible for special applications, but suffers from several drawbacks requiring significant optimization by employing state-of-the-art antibody engineering technologies such as humanization and others explained below.
Figure 2. Overview of bispecific heterodimeric IgG antibodies with heterodimeric Fc-region. (A) Quadroma approach with isolation of the desired bispecific Triomab. (B) KiH approach with two different light chains based on in vitro assembly; the white circle indicates the lack of glycosylation due to expression in E. coli. (C) KiH approach with common light chain. (D) CrossMabCH1-CL based on KiH approach in combination with light chain crossover. (E) (SEED)body approach based on strand exchange between IgG and IgA CH3 domains. (F) LUZ-Y with C-terminal fusion of a leucine zipper to the heavy chain to ensure HC heterodimerization and common light chain. The leucine zipper can subsequently be cleaved off proteolytically. For a more detailed description, refer to the text.
Enforcing Correct Heavy Chain Heterodimerization
To overcome the chain association issue and enforce the correct association of the two different heavy chains, in the late 1990s Carter et al. from Genentech invented an elegant approach termed “knobs-into-holes” (KiH).Citation27-Citation31 Basically, the concept relies on modifications of the interface between the two CH3 domains where most interactions occur. A bulky residue is introduced into the CH3 domain of one antibody heavy chain and acts similarly to a key. In the other heavy chain, a “hole” is formed that is able to accommodate this bulky residue, mimicking a lock. The resulting heterodimeric Fc-part can be further stabilized by artificial disulfide bridges. During the process of optimizing the heterodimerization interface, various rational designs, including steric complementarity, KiH, disulfide bonds and salt bridges juxtaposing oppositely charged residues on either side of the CH3 domain, were evaluated and ultimately optimized using a phage display library.Citation27,Citation28,Citation30,Citation31 Correct heavy chain association with heterodimerization yields above 97% can be achieved by introducing six mutations: S354C, T366W in the “knob” heavy chain and Y349C, T366S, L368A, Y407V in the “hole” heavy chain ().Citation27 While hole-hole homodimers may occur, knob-knob homodimers typically are not observed; however, approximately equal expression of both chains is a prerequisite to obtain a homogeneous bispecific antibody product. Thus, good product quality with low hole-hole dimer content can be enhanced by selecting appropriate and stable expression clones. In addition, hole-hole dimers can either be depleted by selective purification procedures or by methods explained below. Notably, all KiH mutations are buried within the CH3 domains and not “visible” to the immune system. In line with this observation, MetMab, a one-armed antibody against cMet produced in E. coli carrying the KiH mutations described aboveCitation32,Citation33 has recently successfully passed Phase 2 clinical trials with promising signs of efficacy in patients with high cMet expression without evidence of immunogenicity different from classical IgG antibodies.Citation34 In addition, properties of antibodies with KiH mutations such as (thermal) stability, FcγR binding and effector functions (e.g., ADCC, FcRn binding) and pharmacokinetic (PK) behavior are not affected. Similar approaches based on charged residues with ionic interactions (compare ) or steric complementarity () have recently been described. Igawa and Tsunoda from Chugai and Gunasekaran et al. from Amgen chose to alter the charge polarity in the CH3 interface so that co-expression of electrostatically matched Fc domains support favorable attractive interactions and heterodimer formation while retaining the hydrophobic core, whereas unfavorable repulsive charge interactions suppress homodimerization.Citation35,Citation36 In 2006, Igawa and Tsunoda identified 3 negatively charged residues in the CH3 domain of one chain that pair with three positively charged residues in the CH3 domain of the other chain. These specific charged residue pairs are: E356-K439, E357-K370, D399-K409 and vice versa. By introducing at least two of the following three mutations in chain A: E356K, E357K and D399K, as well as K370E, K409D, K439E in chain B, alone or in combination with newly identified disulfide bridges, they were able to favor very efficient heterodimerization while suppressing homodimerization at the same time (see Figure 27 in ref. Citation33). This work was not broadly recognized because it was published in a Japanese patent application, and only recently Gunasekaran et al. described the use of these species conserved pairs of oppositely charged residues in the CH3-CH3 interface as rationalized on available crystal structures: E356–K439, E357–K370, D399–K409 and K392–D399. Of these, the K409–D399 pair in particular is structurally conserved and buried. Subsequently, they introduced respective mutations switching the charged residue polarity in chain A from K409 to D409 and in chain B from D399 to K399. Taking the symmetry of the homodimeric CH3-CH3 domain into consideration, this results in repulsive interactions in the case of homodimerization and a stabilizing ionic interaction for the heterodimer.Citation36 This approach theoretically suppresses formation of both possible homodimers (), whereas in the case of KiH, an excess of the hole chain may still lead to the observation of hole-hole dimers. Using a suitable combination of mutations, they were ultimately able to achieve a high degree of heterodimerization by introduction of K409D–K392D in chain A and D399K–E356K in chain B with nearly the same expression yields.Citation36 However, although theoretically advantageous, the use of charged residue pairs did not appear to result in higher heterodimerization yield and purity of the respective heterodimeric proteins/antibodies compared with the KiH approach combined with disulfide bridge, and can also result in significant decrease of antibody productivity.Citation35,Citation36 Clearly, combining the two approaches may result in an even higher rate of heterodimerization, but may not be desirable in terms of production yields and in efforts to minimize the number of “non-human” mutations in therapeutic antibodies.
Figure 3. Enforcing correct heavy chain association by CH3-CH3 interface modification. Structural model of heterodimeric Fc (one CH3 domain as black line, the other as gray line) with (A) KiH mutations and S-S stabilization (1 knob mutation, blue; 3 hole mutations, red; one disulfide bridge, yellow).Citation25 (B) charged residues located at the CH3-CH3 interface (Glu, Asp, red; Lys blue) that can typically be used to enforce heterodimerization by appropriate exchange as shown by Amgen and Chugai. (C) optimal variant (4 mutations, purple and magenta) obtained by Xencor. (D) schematic representation of (from left) the desired heterodimer and the two unwanted homodimers obtained by the KiH method (top) or use of electrostatic steering (bottom).
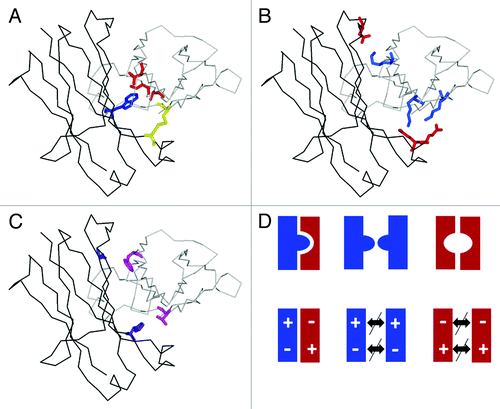
Moore et al. from Xencor defined 41 variant pairs based on combining structural calculations and sequence information that were subsequently screened for maximal heterodimerization.Citation37 Here, the combination of S364H, F405A (HA) on chain A and Y349T, T394F on chain B (TF) () resulted in heterodimer formation up to 89% and was comparable to the KiH approach without disulfide stabilization and electrostatic steering approach in their hands.Citation37
Davis et al. from Merck Serono followed an approach that is based on the fact that the CH3 domains of human IgG and IgA do not bind to each other. Structurally-related β-strand segments were exchanged to yield asymmetric CH3 domains that were used to generate heterodimeric IgG-like antibodies.Citation38 They termed the resulting bispecific antibodies strand-exchange engineered domain (SEEDbodies; ) and demonstrated that the properties of a native Fc part are retained while gaining almost only heterodimeric products if one of the chains is expressed in slight excess. The concept can be exploited for various IgG-like formats and was assessed in more detail for derivatives of C225, a chimeric anti-epidermal growth factor receptor antibody that was clinically developed as cetuximab (Erbitux®).Citation39 The aforementioned approaches of generating bispecific antibodies by introducing changes in the Fc interface region were also addressed by Zymeworks. The company developed a modeling platform called AzymetricTM to generate heterodimeric IgG1 antibodies, but details of this platform are not accessible yet.
In the early 1990s, generation of bispecific antibodies using leucine zippers was described.Citation40,Citation41 Those constructs did not include an Fc, and only recently this concept was adapted by Christensen, Wranik et al. from Genentech by fusion of heterodimeric coiled-coil regions at the C-terminus of antibody heavy chains to allow for specific generation of heterodimeric bispecific antibodies ().Citation42 The heterodimerization moiety can be proteolytically cleaved either during the antibody secretion process or as part of the antibody purification process with proteases such as furin.Citation42An overview of approaches to enforce correct heavy chain association is given in .
Enforcing Correct Light Chain Association
While the issue of random heavy chain association is addressed by the methods described above, it is also essential to ensure and enforce correct light chain association. Otherwise, even in the presence of KiH, a mixture of undesired antibodies and the desired bispecific antibody is obtained because of random light chain association (). Much progress in achieving correct heavy chain association has been made over the recent years, but progress in enforcing correct light chain association has actually hampered progress in the field of bispecific heterodimeric IgG antibodies. This is partly due to the fact that, from a structural point of view, modification of the heterodimeric VL-CL/VH-CH1 interface to enforce correct association in a bispecifc antibody is more difficult to achieve and requires more modifications of the interface than for the homodimeric CH3/CH3 interaction (). Typically, the specificity of antibodies is governed by the complementarity-determining regions (CDRs) residing in the heavy chain, particularly by the heavy chain CDR3; thus the most obvious approach to circumvent the chain association issue is the use of a common light chain ().Citation27 This approach requires identification of two antibodies that recognize their target using the same light chain by phage display or immunization of common light chain transgenic animals.Citation43,Citation44 A notable example of applying the common light chain approach to generating bispecific antibodies was recently described.Citation45 In this work, Jackman et al. from Genentech generated bispecific antibodies using a common light chain together with separate expression of half antibodies in E. coli (see below) that cross-link FcεRI with the inhibitory receptor FcγRIIb to inhibit the high affinity IgE receptor FcεRI on mast cells and basophils. This approach may be useful for the therapy of asthma and other allergic diseases.Citation45 Although the common light chain concept is straightforward, few examples have been described so far, and no common light chain antibody has progressed toward the clinic. This may have to do with the fact that one cannot easily rely on pre-existing and validated antibodies, but rather one must identify novel antibody pairs that allow a common light chain approach. It is unclear whether this is possible for any arbitrary pair of antigens because the diversity found in natural antibodies is reduced when a common light chain is being used.
Therefore, development of novel approaches allowing the generic conversion of pre-existing antibodies into bispecific heterodimeric IgG antibodies with correct light chain association are still of high importance. Similar to the KiH CH3 domain approach, efforts have been undertaken to investigate asymmetric light chain-heavy chain interactions that might ultimately lead to full bispecific IgGs. Zhu et al. introduced several sterically complementary mutations, as well as disulfide bridges, in the two VL/VH interfaces of diabody variants. When the mutations VL Y87A:F98M and VH V37F:L45W were introduced into the anti-p185HER2 VL/VH interface, a heterodimeric diabody was recovered with > 90% yield while maintaining overall yield and affinity compared with the parental diabody.Citation29 Researchers from Chugai have similarly designed bispecific diabodies by introduction of mutations into the VH-VL interfaces (mainly conversion of Q39 in VH and Q38 in VL to charged residues) to foster correct light chain association.Citation35,Citation46 Although these approaches were able to induce correct heavy-light chain pairing in diabody systems, it is unclear whether they are sufficient to abolish or sufficiently inhibit mispairing to a degree required for bispecific heterodimeric IgG antibodies.
We recently developed the CrossMab approach at Roche as a possibility to enforce correct light chain pairing in bispecific heterodimeric IgG antibodies when combined with the KiH technologyCitation47,Citation48 () and generate bispecific antibodies from existing antibodies in a generic fashion. In this format, one arm of the intended bispecific antibody is left untouched. In the second arm, the whole Fab region, the VH-VL or the CH1-CL domains are exchanged by domain crossover between the heavy and light chain Fab domains (). As a consequence, the newly formed “crossed” light chain does not associate with the heavy chain Fab region of the the other arm of the bispecific antibody any longer. Thus, the correct “light chain” association can be enforced by this minimal change in domain arrangement.Citation47 Early theoretical considerations predicted certain side products for the CrossMabFab and the CrossMabVH-VL, whereas no theoretical side products were expected for the CrossMabCH1-CL. Indeed, for the CrossMabFab, a non-functional heavy chain dimer as well as an unproductive Fab fragment could be observed, whereas for the CrossMabVH-VL a Bence-Jones-like side product was formed.
Figure 4. CrossMab principle. Starting from a conventional IgG antibody correct chain association in a bispecific heterodimeric antibody can be achieved using the KiH technology to enforce correct heavy chain heterodimerization in combination with domain crossover of light chain domains to enforce correct light chain association. The three possible light chain domain crossovers are depicted: Fab domain crossover on the left, VH-VL domain crossover on the right and CH1-CL crossover on the top.
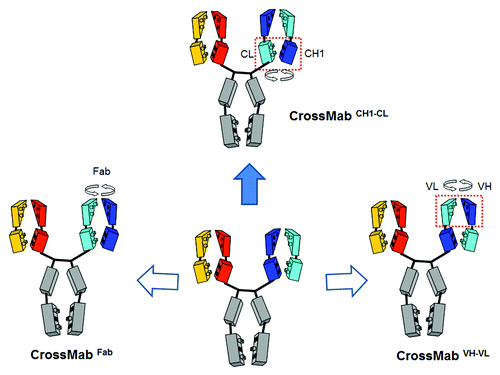
As predicted, the CrossMabCH1-CL showed the best side product profile and purity after transient expression.Citation47 The sequences were designed to ensure a Fab domain with domain crossover (CrossFab) with unaltered stability and three-dimensional structure. In line with this, stability and expression yields for monospecific CrossMabs were comparable to those of the parental unmodified IgG antibodies (unpublished data). We also did not see differences in expression rates, binding, or stability due to the different linkers tested. Furthermore, retrospective analysis predicts that the chosen linkers do not contain T cell neo-epitopes, so that no immunogenic potential of the CrossMab approach is expected. Using this approach, the three possible bispecific CrossMab variants against Angiopoietin-2 and VEGF-A were generated based on two existing antibodies, bevacizumab and the Ang-2 selective antibody LC06,Citation47,Citation49 with the goal of blocking the two angiogenic factors VEGF-A and Ang-2 simultaneously. Surface plasmon resonance and cellular studies showed that the two different arms of all three Ang-2/VEGF CrossMabs retained their antigen binding affinity for VEGF-A and Ang-2, and interfered with the respective receptor interaction, VEGF-induced human umbilical vein endothelial cell proliferation and Ang-2-induced Tie2-phosphorylation similarly compared with the parental antibodies.Citation47
Based on its superior theoretical and effective side product profile, the Ang-2/VEGF CrossMabCH1-CL was selected for subsequent experiments. This molecule showed potent tumor growth inhibition and anti-angiogenic activity in subcutaneous Colo205 xenograft tumors and superior efficacy compared with bevacizumab treatment.Citation47 In the VEGF-induced cornea pocket assay, the Ang-2/VEGF CrossMabCH1-CL resulted in complete shutdown of angiogenesis. A similar effect regarding superiority to bevacizumab treatment was observed in this model.Citation47 Furthermore, the Ang-2/VEGF CrossMabCH1-CL shows retained FcγRIIIa and FcRn binding affinity, and its PK properties in mouse and cynomolgus monkeys are comparable to those of standard IgG1 antibodies (unpublished data). The CrossMab approach works both in transient HEK293 and stable Chinese hamster ovary (CHO) cell expression systems; however, to obtain optimal product quality with very few side products relating to the KiH or CrossMab approach, the selection of an appropriate CHO clone that expresses the four antibody chains in a stable and equal manner is beneficial. Therefore, an early product assessment, e.g., via sandwich ELISA, RP-HPLC, or CE-SDS, during clone selection is performed. According to this procedure, we were able to generate CHO clones expressing an Ang-2/VEGF CrossMabCH1-CL with volumetric productivities in the range of several g per liter, similar to standard IgG processes, that can subsequently be purified and formulated according to established DSP processes. Thermal stability and long-term stability were found to be comparable to that of the parental antibodies (unpublished data). Furthermore, X-ray crystallographic analysis of an isolated CrossFab confirmed molecular modeling assumptions and showed that the structure of the CrossFab lies within the conformational space occupied by Fab fragments of conventional IgG antibodies (unpublished data). Taken together, the domain exchange or CrossMab approach is suitable to solve the challenge of correct light chain assembly in bispecific antibodies, together with the KiH technology with a stabilizing disulfide bridge, on a manufacturing scale. The charm of the CrossMab approach is that it can be applied in a more or less generic manner to pairs of existing antibodies without changing the basic antibody architecture and without the need for identification of a common light chain as shown by the inclusion of bevacizumab and LC06 in the Ang-2/VEGF CrossMab. It should also be mentioned that the domain crossover principle is not limited to the 1+1 format; it can equally well be applied to other formats such as tetravalent IgG-Fab fusions in which correct light chain association is desired.
Bispecific Heterodimeric IgG Antibodies Without Enforced Heavy Chain Heterodimerization
In the previous section, we summarized methods that foster heterodimerization of heavy chains by systematic modification of the two CH3 domain interfaces. Enrichment of the desired heterodimeric product can also be achieved by protein engineering that does not affect the heavy chain dimerization per se, but relies on established purification methods. In analogy to the Triomab approach in which different affinities of mouse and rat IgG isotypes for protein A are used to isolate the desired heterodimer, residues derived from IgG3, which is known not to bind protein A, can be inserted into one of the IgG1 CH3 domains in order to abrogate protein A interaction. This results in a mixture of bispecific antibodies with two, one or no protein A binding heavy chains that can be subsequently chromatographically separated. Davis et al. from Regeneron chose these known mutations combined with a common light chain approach to generate bispecific heterodimeric antibodies ().Citation44,Citation50 A similar approach was applied by Igawa and Tsunoda from Chugai who, by mutation in the variable region of the heavy chains, introduced a difference in the isoelectric point between the two chains of the two antibodies that can be used for the chromatographic isolation of the desired bispecific antibody by ion exchange chromatography.Citation51
Figure 5. Overview of bispecific heterodimeric IgG antibodies. (A) Common light chain bispecific antibody with H435R Y436F mutation to allow depletion of hole-hole antibodies. (B) Duobody approach based on IgG4 Fab arm exchange with in vitro assembly. (C) Bispecific heterodimeric IgG1/IgG2 with EEE/RRR mutations with in vitro assembly. (D) Dual-acting Fab (DAF) IgG or pan-specific monoclonal antibodies. (E) MabCitation2 approach with introduction of additional binding sites in the Fc region of an IgG antibody. (F) CovX body approach with fusion of a bispecific binding peptide into the active center of a catalytically active IgG antibody. The bispecific binding peptide is indicated on the top. For a more detailed description, refer to the text.
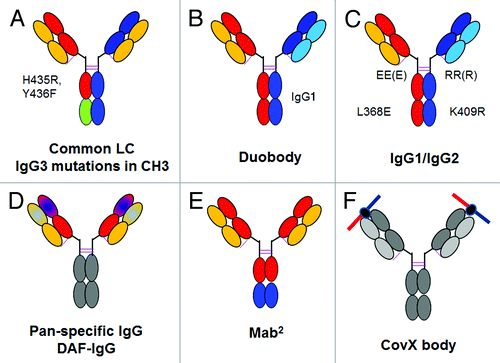
Bispecific Antibody Production by In Vitro Assembly of IgG Heterodimers
Recently, an alternative, more or less generally applicable, approach that combines the KiH methodology with separate expression of half antibodies was described by Jackman et al. from Genentech.Citation45 Here, the two heavy chains with either knob or hole mutation(s) are expressed with their corresponding light chains as half antibodies in separate E. coli cultures and isolated. The half antibodies are subsequently isolated from E. coli periplasm and the bispecific antibody is then formed from the two half antibodies by in vitro assembly (). This approach allows the correct light chain association during expression of the half antibody without need for crossover or other approaches by avoiding co-expression of the four different antibody chains in one cell line. The subsequent correct heavy chain heterodimerization is achieved in vitro via the KiH technology. One obvious restriction of this approach is the subsequent lack of glycosylation due to expression in E. coli, so that this approach cannot be applied in a fully generic manner, e.g., to IgG1 antibodies where effector function such as ADCC is desired. Furthermore, not all antibodies are compatible with E. coli expression. Nevertheless, using this approach Yu et al. designed a bispecific antibody that binds with low affinity to the transferrin receptor (TfR) and with high affinity to the enzyme β-secretase (BACE1). They could show that the bispecific TfR/BACE1 antibody showed enhanced penetration of the blood-brain-barrier and accumulated in the mouse brain.Citation52 In an accompanying paper, Atwal et al. showed that treatment with anti-BACE1 antibodies resulted in sustained reduction of amyloid-β peptide plaques in the peripheral and central nervous system,Citation6 making the bispecific TfR/BACE1 antibody a promising candidate to improve antibody uptake into the brain for Alzheimer disease therapy, which cannot be achieved with a conventional classical monospecific BACE1 antibody.
A different technology to obtain bispecific heterodimeric antibodies is based on the fact that IgG4 undergoes a “Fab arm exchange” in vitro and in vivo.Citation53-Citation55 The two arms of an immunoglobulin are held together by hydrophobic and electrostatic forces between the two CH3 domains and two disulfide bonds in the hinge region. In the case of IgG4, the affinity between the CH3 domains is reduced compared with IgG1. As a consequence, reduction and subsequent re-oxidation of a mixture of two different individual IgG4 antibodies leads to separation and recombination of half antibodies, which results in a mixture of the two original homodimeric antibodies (statistically 25% each) and a heterodimeric bispecific molecule (50%). The intermediate half antibodies consist of a heavy chain and its correct light chain partner; the light chain mispairing issue does not occur. Schuurman and colleagues from Genmab combine this IgG4 property with a number of modifications in the two different CH3 interfaces in order to shift the equilibrium in an in vitro process based on two individually produced and isolated monospecific antibodies toward the desired heterodimeric Duobody ().Citation56 Similarily, Strop and colleagues from Rinat/Pfizer have combined mutations in the hinge region as well as in the CH3 domain (K409R and L368E) of human antibodies of the IgG1 and IgG2 isotype to form the desired full-length bispecific antibodies when mixed together under appropriate redox conditions with high yields.Citation57 Specifically, they expressed two different antibodies, e.g., directed against CD20 and CD3, one containing L368E and the hinge mutations 221E, 228E for IgG1 or 223E, 225E, 228E for IgG2 (EEE) and the second containing K409R and the hinge mutations 221R, 228R for IgG1 or 223R, 225R, 228R for IgG2 (RRR) (). Subsequently, the two antibodies were purified and mixed together in equimolar ratio in the presence of a mild reducing agent. Subsequent experiments demonstrated high and IgG-like stability, unaffected FcγR and FcRn affinity, as well as retained PK properties, the anticipated cytotoxic effects in vitro and potent in vivo B cell depletion.Citation57
Pan-Specific and Dual Acting Fab (DAF) IgG Antibodies
The terms dual-, multi- or pan-specific refer to monoclonal antibodies that specifically recognize more than one target without being unspecific ().Citation58 In fact, promiscuous binding and broad specificity with high affinity, as opposed to the typically-assumed high specificity and selectivity of monoclonal antibodies, is known in inflammatory diseases from natural proteins such as viral chemokine inhibitors vCCICitation59 or the smallpox cytokine response modifier CrmD.Citation60 M. Kosco-Vilbois, N. Fischer et al. from Novimmune exploited this observation for the generation of so-called “pan-specific” antibodies that recognize multiple mediators of inflammation and to address redundancy of biological systems.Citation58,Citation61 Using extensive phage display selection and affinity maturation campaigns. they were able to identify antibodies that recognize and inhibit, for example, both CXCR3 ligands CXCL9 and CXCL10, while not interfering with CXCL11.Citation58,Citation61 Epitope mapping confirmed that the pan-specificity is mediated by structural mimicry.Citation61
In this context, it is important to distinguish between (1) antibodies recognizing a common conserved sequence or an identical structural epitope that is found in several members of a protein family such as the HER proteins or angiopoietins (structural identity); (2) antibodies recognizing two different sequences, e.g., on chemokine receptor ligands that have similar conformations (structural mimicry); and (3) antibodies that can recognize two distinct epitopes in a different binding conformation and orientation with separate parts of their CDRs.Citation58 Bostrom, Fuh et al. from Genentech have generated antibodies that fulfill the latter definition. Based on a rational approach, they produced antibodies with dual specificity, called dual-acting-Fab (DAF)-IgGs or two-in-one antibodies.Citation62,Citation63 The DAF concept requires antibodies that primarily bind antigens via their heavy chain CDRs as starting material. Such antibodies can be identified by screening antigen A against a heavy chain CDR-restricted library. In a second step, antibodies specific for the second antigen B with retained binding affinity for antigen A are selected from these candidates with constant heavy chain(s) by light chain shuffling. The obtained candidates are then subjected to several rounds of affinity maturation until the antibody fulfills the specifications. Bostrom et al. initially described the methodology for a proof-of-concept HER2 and VEGF-A binding DAF that was based on a trastuzumab backbone.Citation62-Citation64 MEHD7945A, an EGFR/HER3 specific DAF IgG1 antibody is currently in Phase 1 clinical studies.Citation65 In both cases, structures of the corresponding Fabs in complex with each of the antigens have been established.Citation63,Citation65 They nicely illustrate how the CDRs of the DAF-IgG can accommodate binding sites for two completely different antigens (). In contrast to the other formats, the DAF approach combines bispecificity with all typical advantages of classical IgG antibodies such as low risk of immunogenicity. However, whether such a DAF can exist at all depends strongly on the structural properties of the complex between the intended antibody and the two antigens. It may thus be impossible to identify an ideal dual specific candidate that exhibits all desired properties such as target binding, epitope recognition, signaling inhibition; more experience is required to judge the general applicability. Since recognition of both antigens can occur on each arm of the antibody in a random fashion, five potential binding modes (A-no binding, A-A, A-B, B-B, and B-no binding) are conceivable for the DAF. In contrast to other bispecific formats, this directly affects the active antibody dose for the less prominent antigen. It can therefore be estimated that DAF antibodies have more complex pharmacodynamics. Depending on the therapeutic application, whether a DAF or a conventional bispecific antibody may be the preferred format must be considered on a case by case basis. For example, the ambiguity of mono- or bivalent antigen binding precludes the possibility of using combinations that require monovalency. For example, an EpCAM/CD3 or CD19/CD3 DAF would not be feasible as it would very likely result in cross-linking of CD3 and subsequent unspecific T cell activation. Based on these structural and therapeutic limitations, dual acting Fab antibodies contribute to the tool set of bispecific antibodies, but this format is not the solution to all problems.
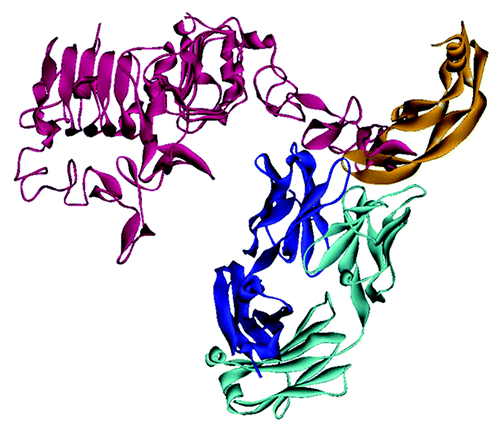
Figure 6. HER2- and VEGF-binding dual acting antibody (DAF)Citation62,Citation63 shown as superposition of the complex structures with HER2 (PDB:3bdy) and with VEGF-A (PDB:3be1). The picture illustrates that HER2 (red) interacts mainly with the heavy chain of the antibody (dark blue), whereas VEGF-A (orange) interacts almost exclusively with the light chain (light blue). There are no significant interactions within the unrelated pairs HC-VEGF and LC-HER2. The Fab of 3be1 has been omitted for clarity since both Fabs exhibit an almost identical structure.
Bispecific tetravalent IgG fusions/conjugates
Although this review focuses on bispecific heterodimeric antibodies, we introduce here two more concepts that rely on a generic IgG format. The Fcab (Fc antigen binding) approach exploits three loop regions in the IgG CH3 domain for generation of an antibody-like library. The overall structural integrity of the Ig-fold is retained and the concept bears similarity to the CDRs found in the IgG variable region. Each CH3 domain is able to bind one antigen, thereby yielding bivalency. Yeast display was used by Rücker et al. to generate HER2 specific Fcabs that retain all properties of a regular Fc domain.Citation66 Bispecificity is obtained by the integration of Fcab within a regular IgG format, which results in antibodies that are termed Mab2 ().
An alternative approach developed by Doppalpaudi et al. from CovX Research uses a generic catalytic IgG antibody as a scaffold for attachment of various binding peptides identified by phage display.Citation11 The scaffold antibody catalyzes an aldolase reaction that leads to a covalent modification of a nucleophilic heavy chain lysine located in a hydrophobic pocket on each of the two Fab arms. Although the PK properties do not match those of conventional IgG antibodies (e.g., an Ang-2 CovX body has a β half-life of 72 to 110 h)Citation67 this approach contributes favorable PK properties to peptides. Obviously this approach can be used to attach mono- and bispecific peptides, allowing development of several CovX bodies including CVX-241, which can bind simultaneously to Ang-2 and VEGF-A through a bispecific binding peptide. As the conjugated peptides recognize both proteins, it results in a bispecific “tetravalent” peptide-antibody fusion ().Citation11 It should be emphasized that the CovX body, although a bispecific molecule, is based on a monospecific IgG-peptide fusion that differs systematically from the other formats described in this article. CVX-241 was evaluated in Phase 1 clinical studies, but recently discontinued as the molecule’s half-life was found to be shorter than expected, most likely due to instability, or potential immunogenicity of the incorporated peptides.
Outlook
In recent years, the field of antibody engineering has made great progress. It benefits from advancements in antibody selection methods such as phage and ribosome display, implementation of rapid sequencing, cloning and gene synthesis methods, superior transient and stable expression systems, as well as advanced purification and analytical methods. The vast “zoo” of published and patented bispecific antibody formats ( and resulting from these efforts impressively illustrate the high degree of modularity of recombinant antibodies. It is surprising even to people familiar with the field how many novel and ingenious approaches have been designed or are still unexplored. It is somewhat surprising that these efforts have largely been undertaken by researchers from biopharmaceutical companies, and it will be interesting to see these technologies being exploited in an academic setting. However, the impressive zoo of bispecific antibody formats whether bivalent or tetravalent, also raises a number of questions. Which features are required for an ideal bispecific antibody? How will these bispecific antibodies and formats differentiate and perform in clinical trials? Will there be a kind of dominant format in the future, or will we have 20 different bispecific antibody platforms in clinical trials, and ultimately on the market, in some years from now? We are convinced that bispecific heterodimeric IgG antibodies will play an important role throughout the coming years because they mimic the evolutionary design of classical bivalent IgG antibodies with a functional Fc-part. Nevertheless, custom-made bispecific antibodies with additional features not covered by the classical IgG format will still be required for specific purposes. Obviously, bispecific antibodies are ready to progress to multiple clinical trials and ultimately advance medical science within the next 5–10 y.
NOTE: Author, please cite in the text.
Table 2. Overview of approaches to enforce correct heavy chain association in the heavy chain CH3 domains of heavy chain 1 (HC1) and heavy chain 2 (HC2)
| Abbreviations: | ||
| CDR | = | complementarity-determining region |
| DAF | = | dual acting Fab |
| DVD | = | dual variable domain |
| KiH | = | knobs-into-holes |
Acknowledgments
The authors want to thank the numerous colleagues in Large Molecule Research and Discovery Oncology, Roche Pharma Research and Early Development (pRED) contributing to the progress in the field of bispecific CrossMab antibodies throughout the last years.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed. All authors are employees of Roche Glycart AG or Roche Diagnostics GmbH.
References
- Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol 2010; 10:345 - 52; http://dx.doi.org/10.1038/nri2747; PMID: 20414207
- Carter PJ. Introduction to current and future protein therapeutics: a protein engineering perspective. Exp Cell Res 2011; 317:1261 - 9; http://dx.doi.org/10.1016/j.yexcr.2011.02.013; PMID: 21371474
- Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol 2010; 10:301 - 16; http://dx.doi.org/10.1038/nri2761; PMID: 20414204
- Fischer N, Léger O. Bispecific antibodies: molecules that enable novel therapeutic strategies. Pathobiology 2007; 74:3 - 14; http://dx.doi.org/10.1159/000101046; PMID: 17496428
- Mouquet H, Warncke M, Scheid JF, Seaman MS, Nussenzweig MC. Enhanced HIV-1 neutralization by antibody heteroligation. Proc Natl Acad Sci U S A 2012; 109:875 - 80; http://dx.doi.org/10.1073/pnas.1120059109; PMID: 22219363
- Atwal JK, Chen Y, Chiu C, Mortensen DL, Meilandt WJ, Liu Y, et al. A therapeutic antibody targeting BACE1 inhibits amyloid-β production in vivo. Sci Transl Med 2011; 3:84ra43; http://dx.doi.org/10.1126/scitranslmed.3002254; PMID: 21613622
- Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011; 29:2493 - 8; http://dx.doi.org/10.1200/JCO.2010.32.7270; PMID: 21576633
- Linke R, Klein A, Seimetz D. Catumaxomab: clinical development and future directions. MAbs 2010; 2:129 - 36; http://dx.doi.org/10.4161/mabs.2.2.11221; PMID: 20190561
- Holmes D. Buy buy bispecific antibodies. Nat Rev Drug Discov 2011; 10:798 - 800; http://dx.doi.org/10.1038/nrd3581; PMID: 22037028
- McDonagh CF, Huhalov A, Harms BD, Adams S, Paragas V, Oyama S, et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther 2012; 11:582 - 93; http://dx.doi.org/10.1158/1535-7163.MCT-11-0820; PMID: 22248472
- Doppalapudi VR, Huang J, Liu D, Jin P, Liu B, Li L, et al. Chemical generation of bispecific antibodies. Proc Natl Acad Sci U S A 2010; 107:22611 - 6; http://dx.doi.org/10.1073/pnas.1016478108; PMID: 21149738
- Miller BR, Demarest SJ, Lugovskoy A, Huang F, Wu X, Snyder WB, et al. Stability engineering of scFvs for the development of bispecific and multivalent antibodies. Protein Eng Des Sel 2010; 23:549 - 57; http://dx.doi.org/10.1093/protein/gzq028; PMID: 20457695
- Demarest SJ, Glaser SM. Antibody therapeutics, antibody engineering, and the merits of protein stability. Curr Opin Drug Discov Devel 2008; 11:675 - 87; PMID: 18729019
- Fitzgerald J, Lugovskoy A. Rational engineering of antibody therapeutics targeting multiple oncogene pathways. MAbs 2011; 3:299 - 309; http://dx.doi.org/10.4161/mabs.3.3.15299; PMID: 21393992
- Metz S, Haas AK, Daub K, Croasdale R, Stracke J, Lau W, et al. Bispecific digoxigenin-binding antibodies for targeted payload delivery. Proc Natl Acad Sci U S A 2011; 108:8194 - 9; http://dx.doi.org/10.1073/pnas.1018565108; PMID: 21536919
- Schanzer J, Jekle A, Nezu J, Lochner A, Croasdale R, Dioszegi M, et al. Development of tetravalent, bispecific CCR5 antibodies with antiviral activity against CCR5 monoclonal antibody-resistant HIV-1 strains. Antimicrob Agents Chemother 2011; 55:2369 - 78; http://dx.doi.org/10.1128/AAC.00215-10; PMID: 21300827
- Digiammarino EL, Harlan JE, Walter KA, Ladror US, Edalji RP, Hutchins CW, et al. Ligand association rates to the inner-variable-domain of a dual-variable-domain immunoglobulin are significantly impacted by linker design. MAbs 2011; 3:487 - 94; http://dx.doi.org/10.4161/mabs.3.5.16326; PMID: 21814039
- Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol 2007; 25:1290 - 7; http://dx.doi.org/10.1038/nbt1345; PMID: 17934452
- Herrmann T, Grosse-Hovest L, Otz T, Krammer PH, Rammensee HG, Jung G. Construction of optimized bispecific antibodies for selective activation of the death receptor CD95. Cancer Res 2008; 68:1221 - 7; http://dx.doi.org/10.1158/0008-5472.CAN-07-6175; PMID: 18281499
- Carlring J, De Leenheer E, Heath AW. A novel redox method for rapid production of functional bi-specific antibodies for use in early pilot studies. PLoS One 2011; 6:e22533; http://dx.doi.org/10.1371/journal.pone.0022533; PMID: 21811628
- De Lau WB, Heije K, Neefjes JJ, Oosterwegel M, Rozemuller E, Bast BJ. Absence of preferential homologous H/L chain association in hybrid hybridomas. J Immunol 1991; 146:906 - 14; PMID: 1899099
- Ménard S, Canevari S, Colnaghi MI. Hybrid antibodies in cancer diagnosis and therapy. Int J Biol Markers 1989; 4:131 - 4; PMID: 2693537
- Renner C, Jung W, Sahin U, Denfeld R, Pohl C, Trümper L, et al. Cure of xenografted human tumors by bispecific monoclonal antibodies and human T cells. Science 1994; 264:833 - 5; http://dx.doi.org/10.1126/science.8171337; PMID: 8171337
- Lindhofer H, Mocikat R, Steipe B, Thierfelder S. Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies. J Immunol 1995; 155:219 - 25; PMID: 7602098
- Zeidler R, Reisbach G, Wollenberg B, Lang S, Chaubal S, Schmitt B, et al. Simultaneous activation of T cells and accessory cells by a new class of intact bispecific antibody results in efficient tumor cell killing. J Immunol 1999; 163:1246 - 52; PMID: 10415020
- Chelius D, Ruf P, Gruber P, Plöscher M, Liedtke R, Gansberger E, et al. Structural and functional characterization of the trifunctional antibody catumaxomab. MAbs 2010; 2:309 - 19; http://dx.doi.org/10.4161/mabs.2.3.11791; PMID: 20418662
- Carter P. Bispecific human IgG by design. J Immunol Methods 2001; 248:7 - 15; http://dx.doi.org/10.1016/S0022-1759(00)00339-2; PMID: 11223065
- Merchant AM, Zhu Z, Yuan JQ, Goddard A, Adams CW, Presta LG, et al. An efficient route to human bispecific IgG. Nat Biotechnol 1998; 16:677 - 81; http://dx.doi.org/10.1038/nbt0798-677; PMID: 9661204
- Zhu Z, Presta LG, Zapata G, Carter P. Remodeling domain interfaces to enhance heterodimer formation. Protein Sci 1997; 6:781 - 8; http://dx.doi.org/10.1002/pro.5560060404; PMID: 9098887
- Ridgway JB, Presta LG, Carter P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng 1996; 9:617 - 21; http://dx.doi.org/10.1093/protein/9.7.617; PMID: 8844834
- Atwell S, Ridgway JB, Wells JA, Carter P. Stable heterodimers from remodeling the domain interface of a homodimer using a phage display library. J Mol Biol 1997; 270:26 - 35; http://dx.doi.org/10.1006/jmbi.1997.1116; PMID: 9231898
- Jin H, Yang R, Zheng Z, Romero M, Ross J, Bou-Reslan H, et al. MetMAb, the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic pancreatic tumor growth and improves survival. Cancer Res 2008; 68:4360 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-07-5960; PMID: 18519697
- Martens T, Schmidt NO, Eckerich C, Fillbrandt R, Merchant M, Schwall R, et al. A novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivo. Clin Cancer Res 2006; 12:6144 - 52; http://dx.doi.org/10.1158/1078-0432.CCR-05-1418; PMID: 17062691
- Surati M, Patel P, Peterson A, Salgia R. Role of MetMAb (OA-5D5) in c-MET active lung malignancies. Expert Opin Biol Ther 2011; 11:1655 - 62; http://dx.doi.org/10.1517/14712598.2011.626762; PMID: 22047509
- Igawa T, Tsunoda H. Methods for producing polypeptides by regulating polypeptide association. WO2006106905. 2006.
- Gunasekaran K, Pentony M, Shen M, Garrett L, Forte C, Woodward A, et al. Enhancing antibody Fc heterodimer formation through electrostatic steering effects: applications to bispecific molecules and monovalent IgG. J Biol Chem 2010; 285:19637 - 46; http://dx.doi.org/10.1074/jbc.M110.117382; PMID: 20400508
- Moore GL, Bautista C, Pong E, Nguyen DH, Jacinto J, Eivazi A, et al. A novel bispecific antibody format enables simultaneous bivalent and monovalent co-engagement of distinct target antigens. MAbs 2011; 3:546 - 57; http://dx.doi.org/10.4161/mabs.3.6.18123; PMID: 22123055
- Davis JH, Aperlo C, Li Y, Kurosawa E, Lan Y, Lo KM, et al. SEEDbodies: fusion proteins based on strand-exchange engineered domain (SEED) CH3 heterodimers in an Fc analogue platform for asymmetric binders or immunofusions and bispecific antibodies. Protein Eng Des Sel 2010; 23:195 - 202; http://dx.doi.org/10.1093/protein/gzp094; PMID: 20299542
- Muda M, Gross AW, Dawson JP, He C, Kurosawa E, Schweickhardt R, et al. Therapeutic assessment of SEED: a new engineered antibody platform designed to generate mono- and bispecific antibodies. Protein Eng Des Sel 2011; 24:447 - 54; http://dx.doi.org/10.1093/protein/gzq123; PMID: 21498564
- Pack P, Kujau M, Schroeckh V, Knüpfer U, Wenderoth R, Riesenberg D, et al. Improved bivalent miniantibodies, with identical avidity as whole antibodies, produced by high cell density fermentation of Escherichia coli. Biotechnology (N Y) 1993; 11:1271 - 7; PMID: 7764189
- Kostelny SA, Cole MS, Tso JY. Formation of a bispecific antibody by the use of leucine zippers. J Immunol 1992; 148:1547 - 53; PMID: 1531669
- Christensen EH, Eaton DL, Vendel AC, Wranik B. Coiled coil and/or tether containing protein complexes and uses thereof. WO2011034605. 2011.
- Houtzager E, Pinto RD, Logtenberg T, Throsby M, Kramer RA, De Kruif CA. Antibody producing non-mammals. WO2009157771. 2009.
- McWhirter J, Macdonald L, Stevens S, Davis S, Buckler DR, Murphy AJ. Common light chain mouse. WO2011097603. 2011.
- Jackman J, Chen Y, Huang A, Moffat B, Scheer JM, Leong SR, et al. Development of a two-part strategy to identify a therapeutic human bispecific antibody that inhibits IgE receptor signaling. J Biol Chem 2010; 285:20850 - 9; http://dx.doi.org/10.1074/jbc.M110.113910; PMID: 20444694
- Igawa T, Tsunoda H, Kikuchi Y, Yoshida M, Tanaka M, Koga A, et al. VH/VL interface engineering to promote selective expression and inhibit conformational isomerization of thrombopoietin receptor agonist single-chain diabody. Protein Eng Des Sel 2010; 23:667 - 77; http://dx.doi.org/10.1093/protein/gzq034; PMID: 20576629
- Schaefer W, Regula JT, Bähner M, Schanzer J, Croasdale R, Dürr H, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A 2011; 108:11187 - 92; http://dx.doi.org/10.1073/pnas.1019002108; PMID: 21690412
- Cain C. Crossing over to bispecificity. SciBX 2011; 4:1 - 4
- Rennel ES, Regula JT, Harper SJ, Thomas M, Klein C, Bates DO. A human neutralizing antibody specific to Ang-2 inhibits ocular angiogenesis. Microcirculation 2011; 18:598 - 607; http://dx.doi.org/10.1111/j.1549-8719.2011.00120.x; PMID: 21851472
- Davis S, Smith E, Macdonald D, Olson KL. Readily isolated bispecific antibodies with native immunoglobulin format. WO2010151792. 2011.
- Igawa T, Tsunoda H. Antibody Modification Method For Purifying Bispecific Antibody. WO2007114325. 2011.
- Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, Lu Y, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med 2011; 3:84ra44; http://dx.doi.org/10.1126/scitranslmed.3002230; PMID: 21613623
- Stubenrauch K, Wessels U, Regula JT, Kettenberger H, Schleypen J, Kohnert U. Impact of molecular processing in the hinge region of therapeutic IgG4 antibodies on disposition profiles in cynomolgus monkeys. Drug Metab Dispos 2010; 38:84 - 91; http://dx.doi.org/10.1124/dmd.109.029751; PMID: 19850673
- Labrijn AF, Buijsse AO, van den Bremer ET, Verwilligen AY, Bleeker WK, Thorpe SJ, et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol 2009; 27:767 - 71; http://dx.doi.org/10.1038/nbt.1553; PMID: 19620983
- van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martínez-Martínez P, Vermeulen E, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007; 317:1554 - 7; http://dx.doi.org/10.1126/science.1144603; PMID: 17872445
- Schuurman J, Vink T, Van De Winkel J, Labrijn AF, Aalberse R, Van Der Neut Kolfschoten M et al. Heterodimeric Antibody Fc-Containing Proteins And Methods For Production Thereof. WO2011131746. 2011.
- Strop P, Ho WH, Boustany LM, Abdiche YN, Lindquist KC, Farias SE, et al. Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair. J Mol Biol 2012; 420:204 - 19; http://dx.doi.org/10.1016/j.jmb.2012.04.020; PMID: 22543237
- Fagète S, Fischer N. Smarter drugs: a focus on pan-specific monoclonal antibodies. BioDrugs 2011; 25:357 - 64; http://dx.doi.org/10.2165/11594690-000000000-00000; PMID: 22050338
- Buatois V, Fagète S, Magistrelli G, Chatel L, Fischer N, Kosco-Vilbois MH, et al. Pan-CC chemokine neutralization restricts splenocyte egress and reduces inflammation in a model of arthritis. J Immunol 2010; 185:2544 - 54; http://dx.doi.org/10.4049/jimmunol.1000182; PMID: 20644170
- Alejo A, Ruiz-Argüello MB, Ho Y, Smith VP, Saraiva M, Alcami A. A chemokine-binding domain in the tumor necrosis factor receptor from variola (smallpox) virus. Proc Natl Acad Sci U S A 2006; 103:5995 - 6000; http://dx.doi.org/10.1073/pnas.0510462103; PMID: 16581912
- Fagète S, Rousseau F, Magistrelli G, Gueneau F, Ravn U, Kosco-Vilbois MH, et al. Dual specificity of anti-CXCL10-CXCL9 antibodies is governed by structural mimicry. J Biol Chem 2012; 287:1458 - 67; http://dx.doi.org/10.1074/jbc.M111.253658; PMID: 22041899
- Bostrom J, Haber L, Koenig P, Kelley RF, Fuh G. High affinity antigen recognition of the dual specific variants of herceptin is entropy-driven in spite of structural plasticity. PLoS One 2011; 6:e17887; http://dx.doi.org/10.1371/journal.pone.0017887; PMID: 21526167
- Bostrom J, Yu SF, Kan D, Appleton BA, Lee CV, Billeci K, et al. Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site. Science 2009; 323:1610 - 4; http://dx.doi.org/10.1126/science.1165480; PMID: 19299620
- Bostrom J, Lee CV, Haber L, Fuh G. Improving antibody binding affinity and specificity for therapeutic development. [xiii.] Methods Mol Biol 2009; 525:353 - 76, xiii; http://dx.doi.org/10.1007/978-1-59745-554-1_19; PMID: 19252851
- Schaefer G, Haber L, Crocker LM, Shia S, Shao L, Dowbenko D, et al. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell 2011; 20:472 - 86; http://dx.doi.org/10.1016/j.ccr.2011.09.003; PMID: 22014573
- Wozniak-Knopp G, Bartl S, Bauer A, Mostageer M, Woisetschläger M, Antes B, et al. Introducing antigen-binding sites in structural loops of immunoglobulin constant domains: Fc fragments with engineered HER2/neu-binding sites and antibody properties. Protein Eng Des Sel 2010; 23:289 - 97; http://dx.doi.org/10.1093/protein/gzq005; PMID: 20150180
- Huang H, Lai JY, Do J, Liu D, Li L, Del Rosario J, et al. Specifically targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing monocyte infiltration, and tumor growth. Clin Cancer Res 2011; 17:1001 - 11; http://dx.doi.org/10.1158/1078-0432.CCR-10-2317; PMID: 21233403