Abstract
The pro-inflammatory cytokine interleukin (IL)-1β is a clinical target in many conditions involving dysregulation of the immune system; therapeutics that block IL-1β have been approved to treat diseases such as rheumatoid arthritis (RA), neonatal onset multisystem inflammatory diseases, cryopyrin-associated periodic syndromes, active systemic juvenile idiopathic arthritis. Here, we report the generation and engineering of a new fully human antibody that binds tightly to IL-1β with a neutralization potency more than 10 times higher than that of the marketed antibody canakinumab. After affinity maturation, the derived antibody shows a >30-fold increased affinity to human IL-1β compared with its parent antibody. This anti-human IL-1β IgG also cross-reacts with mouse and monkey IL-1β, hence facilitating preclinical development. In a number of mouse models, this antibody efficiently reduced or abolished signs of disease associated with IL-1β pathology. Due to its high affinity for the cytokine and its potency both in vitro and in vivo, we propose that this novel fully human anti-IL-1β monoclonal antibody is a promising therapeutic candidate and a potential alternative to the current therapeutic arsenal.
Introduction
Interleukin (IL)-1β is a potent cytokine that drives both the acute and chronic phases of the inflammatory response and plays an essential role in innate immune response.Citation1-Citation4 IL-1β activation and release arises from the activation of inflammasomes, which are large protein complexes constituting members of the NOD-like receptor (NLRs) or PYHIN protein families.Citation5 Upon sensing microbial or danger-associated molecules, these intracellular receptors recruit the adaptor protein ASC, which engages and activates caspase-1. Activated caspase-1 in turn processes the IL-1β precursor into the active and secreted IL-1β cytokine. Physiologically, inflammasomes activation serves as a natural means to defend against pathogens by initiating innate immune responses. However, several endogenous agents of non-pathogenic origins released upon tissue damage are known to activate the inflammasomes, leading to pathological outcomes.Citation5 For example, the inflammasome activators monosodium urate (MSU) crystals and islet amyloid polypeptides can induce gout disease and Type 2 diabetes, respectively.Citation6,Citation7 Furthermore, genetic defects have also been linked to inflammasome activation-related diseases.Citation8 For example, cryopyrin-associated periodic syndromes (CAPS) arise from a mutation in the CIAS1 gene encoding for cryopyrin/NLRP3, a component of the inflammasome complex that responds to danger signals, resulting in increased inflammasome activity and consequently enhanced IL-1β release. High levels of IL-1β have also been implicated in more common inflammatory and autoimmune diseases, including but not limited to gout, rheumatoid arthritis and diabetes.Citation9,Citation10 As such, IL-1β has been actively pursued as a target for therapeutic antibody development.
To date, three recombinant protein drugs targeting IL-1β signaling have been approved for clinical use. Anakinra, marketed as Kineret®, is a recombinant IL-1 receptor antagonist (IL-1ra) produced in E. coli. Anakinra is the treatment of choice in CAPS, and it is also prescribed to treat rheumatoid arthritis in patients who have failed one or more disease-modifying anti-rheumatic drugs (DMARDs). It can be used alone or in combination with DMARDs other than tumor necrosis factor (TNF)-blocking agents. Rilonacept (Arcalyst®), is a dimeric fusion protein consisting of the ligand-binding domain of the human IL-1 receptor and IL-1 receptor accessory protein, currently in use to treat CAPS. Canakinumab (Ilaris®), an IL-1β neutralizing human monoclonal antibody, is also marketed for the treatment of CAPS in children and adults.
The three approved drugs, along with a number of other IL-1β neutralizing antibodies, are currently in late-stage clinical trials for auto-inflammatory diseases, such as osteoarthritis, gout, juvenile idiopathic arthritis, type 1 and type 2 diabetes. Although targeting the same signaling pathway, each molecule exhibits unique mechanisms of action and displays different pharmacological profiles.Citation11-Citation13
Antibody-based therapeutics that restrain inflammation constitute one of the largest biologic drug families in the biotech/pharmaceutical sectors. For instance, the market for the anti-TNF therapy for rheumatoid arthritis alone is over US$7 billion a year. Likewise, the number of people who could benefit from a novel anti-IL-1-based treatment is very high: 27 millions of patients were estimated to live with osteoarthritis in the US alone in 2005;Citation14 the prevalence of acute gout has doubled between 1990 and 1999 in the population over 65 y of age (data from the US Centers for Disease Control and Prevention), with a prevalence of 8–150/100,000; juvenile idiopathic arthritis is the most common arthritis type among children;Citation15,Citation16 in 2007 in the USA, approximately 24 million people were estimated to suffer from type 1 or type 2 diabetes.Citation17 Although IL-1β therapy has not yet been approved for these indications, the potential need is wide. As such, and similar to anti-TNF biologics, the fact that three anti-IL-1β drugs are already in the market constitutes a proof-of-principle toward the relevance of this approach, but does not preclude the development of new IL-1-targeting drugs that may be superior in certain therapeutic and pharmacological aspects. Here, we present the discovery, engineering and in vitro and in vivo characterization of a novel fully human anti-IL-1β IgG1. The engineered antibody P2D7KK has a higher affinity for IL-1β than the marketed product canakinumab, potently neutralizes human, mouse, and monkey (rhesus macaque) IL-1β, and significantly reduces clinical signs of pathology in various animal disease models.
Results
Antibody isolation from phage display library
Anti-IL-1β antibodies were isolated from a Fab display library.Citation18 This library was derived from 22 anonymous donors’ blood and tissues after informed consent. It consists of 3 × 1010 M13 phagemids, each encoding and presenting one different antibody in a Fab format. After 3 rounds of biopanning and a first ELISA screening, we isolated 78 hits able to bind to human IL-1β. Of these, 22 unique clones were identified by DNA fingerprinting and later confirmed by sequencing. Two clones, 1H and 2H, displayed neutralizing activity, of which clone 2H showed much higher potency. Hence, all subsequent work reported below were derived from clone 2H.
In vitro characterization of clone 2H
After expression as a human IgG1, 2H was tested by ELISA for binding to both human and mouse IL-1β and IL-1α. The results showed that while 2H strongly bound to both human and mouse IL-1β in a dose-response manner, it did not recognize IL-1α of either species (), despite the structural similarity of the two cytokines.Citation19 Its affinity for human and mouse IL-1β as measured by surface plasmon resonance was 127 pM and 239 pM, respectively (). 2H was also tested for its ability to functionally inhibit IL-1β in two cell-based assays. The first assay utilized a cell line (HEK-Blue™ IL-1β) that is engineered such that IL-1R signaling is coupled to the activation of the AP-1 and NF-κB pathways, which drive the expression of the secreted embryonic alkaline phosphatase (SEAP). As such, IL-1β activity can be monitored and quantified by the enzymatic activity of the SEAP produced. In the second assay, IL-1β activity is measured by the production of IL-6 upon exposure of MRC5 cells to IL-1β. The results showed that 2H was able to block the IL-1β induced alkaline phosphatase secretion by HEK-Blue cells () and IL-6 production by MRC5 cells () in a dose-dependent manner. In the MRC5 assay, 2H showed a mean inhibitory concentration 50 (IC50) of 195 pM for the human IL-1β and 1538 pM for its mouse counterpart ().
Figure 1. Binding and neutralization of IL-1β by antibody 2H. (A) ELISA for human IL-1β (closed squares), human IL-1α (closed triangles), mouse IL-1β (open squares) and mouse IL-1α (open triangles), mean ± sem on duplicates. (B) Inhibition of the human (closed squares) or murine (open triangles) IL-1β-induced secreted alkaline phosphatase in HEK-Blue cells by antibody 2H, means ± sem on duplicates. (C) Inhibition of the human (closed squares) or murine (open triangles) IL-1β-induced secretion of IL-6 in MRC5 cells by antibody 2H and an isotype control, means ± sem on duplicates or triplicates.
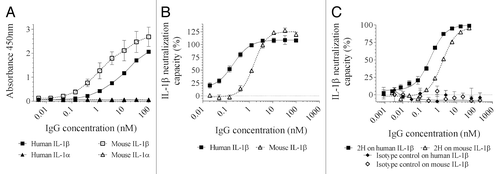
Table 1. Affinity for human and mouse IL-1β
Figure 2. In vitro potency of 2H, its affinity-matured derived antibodies and canakinumab toward human (A) and mouse (B) IL-1β. IC50 extrapolated from the inhibition of IL-1β-induced IL-6 release in MRC5 cells. IC50 was calculated on triplicate wells. Shown are individual data and mean on 2 to 6 independent experiments (n = 6 for P2D7, n = 4 for 2H and canakinumab, n = 2 for other antibodies).
Affinity maturation
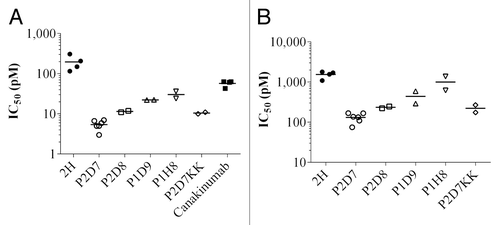
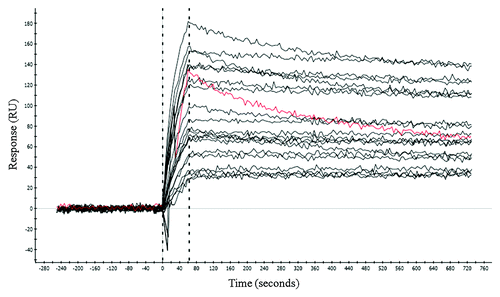
The CDR3 of the light chain (CDR3L) was chosen as the target for affinity improvement by site-directed mutagenesis. The mutagenesis library was constructed using semi-random degenerate codons that encoded the original amino acid plus a small set of residues: Ala, Ser, Tyr, Asp.Citation20 Other amino acids may also be introduced inevitably due to the degenerate codon usage (). The tryptophan residue remained unchanged as its large hydrophobic nature often plays a structural role or contributes to high energy contact with the antigen.Citation21 We employed both thermodynamic (low antigen concentration) and kinetic (long dissociation time) parameters to drive the selection for mutant antibodies with higher affinity and favorable kinetics. A panel of ELISA-positive clones was subjected to off-rate analysis by surface plasmon resonance (SPR) using crude antigen-binding fragment (Fab) extracted from E. coli periplasm. Of the 62 clones analyzed, all showed slower dissociation than clone 2H, with the dissociation rates 1.5 to 30 times slower than the parent antibody (representative clones shown in ). The off-rate improvements observed from this semi-quantitative analysis using crude Fab samples were confirmed by analysis of 15 purified Fabs (data not shown). DNA analysis of these 62 clones revealed 56 unique sequences, with only slight variations between clones. Out of 9 possible residues at each mutated position, only 2–3 amino acids predominated. Interestingly, while other positions can accommodate mutant residues of various chemical natures, we found the glutamic acid at position 8 of this CDR was absolutely conserved among all selected clones (). The conservation of this invariant residue may likely be attributed to a strongly favored electrostatic interaction with a positively charged residue at the antigenic epitope. Four clones, of which the dissociation rates spread across a range of improvement, were chosen to study the affinity-neutralization potency relationship. Affinity measurements by SPR indicated an increase of 21 to 43-fold and 9 to 27-fold for human and mouse IL-1β, respectively, compared with the parent clone 2H (). Neutralization potency of these clones was determined using MRC5 cell-based assays. They inhibited human and mouse IL-1β 6 to 36-fold () and 1.5 to 12-fold (), more potently than 2H, respectively. The improvement in affinity and neutralization showed a parallel trend.
Table 2. CDR3L mutagenesis library design
Figure 3. Dissociation rate analysis by SPR. Dissociation kinetics of crude periplasmic preparations of the affinity matured Fab were analyzed by SPR (see Methods and Materials). Twenty representative clones are shown in black. The parent clone 2H is shown in red.
Among all characterized clones, P2D7 showed high affinity and the highest neutralization efficacy (; ). It had a 4 pM and 14 pM affinity for human and mouse IL-1β, respectively, and neutralized human and mouse IL-1β with IC50 of 5 pM and 132 pM, respectively. Under the same experimental conditions, P2D7 is 11 times more potent than canakinumab in neutralizing human IL-1β in the MRC5 cell assays. Canakinumab does not react to mouse IL-1β. With an affinity of 4 pM for the human IL-1β and an average IC50 of 5 pM in the MRC5 cell assay, P2D7 was clearly a lead among the affinity-matured antibodies derived from 2H. P2D7 was further engineered by site-directed mutagenesis on two amino acids so as to revert it to a germline-like framework (IGHV4–34*01) in order to eliminate potential immunogenicity. The resulting antibody, P2D7KK (R75K/S81K), had an affinity and neutralization potency almost identical to P2D7. Likewise, when converted to a chimera of mouse IgG1 isotype (designated ChP2D7), no loss of activity was observed (data not shown).
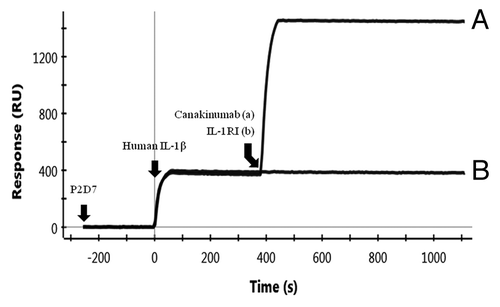
ChP2D7 competes with receptor for IL-1β binding but recognizes a non-overlapping epitope from canakinumab
The binding mode of P2D7 in relation to IL-1RI and canakinumab was investigated by SPR. As shown in , binding of IL-1β to P2D7 (at t = 0) completely abolished its ability to bind IL-1RI (, curve b, no binding at t = 400 s). This competitive binding mechanism is the same as that of canakinumab.Citation22 However, P2D7 and canakinumab bind to non-overlapping epitopes on IL-1β, as revealed by the stepwise P2D7-IL-1β-canakinumab binding (, curve a; binding of IL-1β and P2D7 at t = 0, and subsequent binding of IL-1β and canakinumab at t = 400 s).
P2D7KK treatment abolished collagen antibody-induced arthritic symptoms and reduced MSU-induced peritonitis
P2D7KK was tested in vivo in mouse models of rheumatoid arthritis and gout. The anti-inflammatory effects of P2D7KK were examined in a collagen antibody-induced arthritic (CAIA) model. The inflammation symptoms, e.g., swollen ankles and paw redness, () appeared on day 3–4 and reached plateau on day 7–8. P2D7KK was able to significantly reduce arthritis symptoms in Balb/c mice from day 4 post-arthritis induction and kept arthritic scores low until the end of the experiment (). Massive lymphocyte infiltration and cartilage degradation were observed in mice treated with the isotype control antibody. Notably, P2D7KK treatment completely prevented lymphocyte infiltration in the affected joints and protected the cartilage from erosion ().
Figure 5. Activity of P2D7KK antibody in 3 mouse models: collagen antibody induced arthritis (A and B), monosodium urate crystal induced inflammation (C) and multiple myeloma (D). (A) Representative hind paws and histological analyses of joints from mice injected with the isotype antibody (top) or treated with 5 mg/kg of P2D7KK (bottom). Arrows point to cartilage. (B) Arthritic scores in Balb/c mice after induction of arthritis with anti-collagen antibodies. Shown are mean arthritis score ± sem in mice treated with 5 mg/kg of P2D7KK or injected with the isotype control (n = 8). Comparison between groups was performed with Holm-Sidak-corrected multiple t tests: *P < 0.05, **P < 0.01, ***P < 0.001. (C) Peritoneal infiltration of neutrophils in mice (n = 5) after injection of PBS only or monosodium urate crystals (MSU) followed by administration of PBS, anakinra 30 mg/kg, isotype human antibody 15 mg/kg, P2D7KK 5 mg/kg or P2D7KK 15 mg/kg. Shown are mean ± sem on 3 independent experiments. Data analyzed by ANOVA followed by a multiple test with Bonferroni’s correction: ****P < 0.0001. (D) Survival of mice (n = 10) after inoculation of human myeloma cells. Survival curves were analyzed with a log-rank (Mantel-Cox).
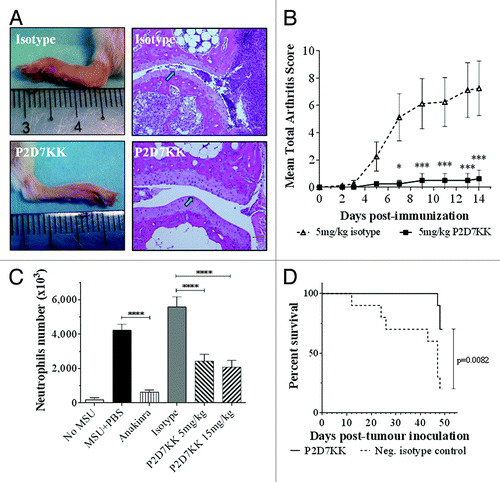
P2D7KK efficacy was also tested in a mouse model of peritonitis induced by MSU crystals, which trigger rapid and robust neutrophils’ recruitment in the peritoneal cavity. This model has been widely used to mimic the acute inflammatory response typical of gout in humans.Citation23 P2D7KK effectively reduced neutrophils’ recruitment in the peritoneum after injection of MSU crystals compared with that in mice receiving the isotype control ().
P2D7KK treatment prevented myeloma cell-induced death in vivo
IL-6 is the central survival and proliferation factor for multiple myeloma and IL-1β appears to be its major inducer in the bone marrow.Citation24,Citation25 Blockade of the IL-1β signaling by the receptor antagonist IL-1ra has shown encouraging results in a clinical study.Citation26 Here we tested the effects of P2D7KK treatment in mice inoculated with human myeloma cells U266B1 that are known to develop tumors in NOD-SCID mice. We found that whereas 80% of the mice receiving isotype control antibody died within 7 wk, 70% of P2D7KK-treated mice survived (). Antibody treatment delayed the first death from day 12 to day 47. Measurement of IL-6 in surviving mice showed that all but one of the animals treated with P2D7KK had lower circulating IL-6 than the mice that received the isotype control (Fig. S1).
Discussion
In this study, we report the isolation of a lead anti-IL-1β antibody from a phage display antibody library, affinity improvement by CDR mutagenesis, in vitro characterization of the neutralization potency, and demonstration of in vivo efficacy in a number of disease models. The initial lead, clone 2H, showed cross-reactivity between human and mouse IL-1β, while no binding activity was observed to the structurally similar ligand, IL-1α. 2H has an affinity and in vitro neutralization potency (IC50) in the 100–200 pM range. Although the serum concentrations of IL-1β may vary from disease to disease and between individuals, we observed that IL-1β can induce strong inflammatory response in epithelial cells at low concentrations <10 pM (data not shown), the effective neutralization of which would require antibodies of higher affinity than 2H. To improve the affinity, we decided to employ a CDR-mutagenesis approach because its efficiency has been demonstrated in many examples, e.g., identification of functional and structural amino-acid residues by parsimonious mutagenesis.Citation27-Citation30 Mutant residues in the CDRs may increase affinity by introducing new contacts and by replacing low affinity or “repulsive” contact residues with more favorable energetics.Citation31 Among the six CDRs, CDR3s, and particularly the heavy chain CDR3, have been shown to be responsible for high energy interaction with antigen.Citation32,Citation33 Hence, we decided to preserve the integrity of the heavy chain, and sought to make improvements by fine-tuning CDR3L for optimal antigen interactions.
The mutagenesis library comprised ~one million diversity and encoded, at each position, the original amino acid and a small set of residues (Ala, Ser, Tyr, Asp) that represents small and large hydrophobic, hydrophilic, and charged side chain chemical diversity.Citation20 Other amino acids may also be introduced due to the codon degeneracy used. Following stringent selection conditions, including low antigen concentration and long duration of dissociation, we found that all the selected clones had slower dissociation rates than the parent clone 2H, demonstrating the effectiveness of the library design and the selection strategy. Surprisingly, almost all of them (56/62) had unique sequences. These results indicate high tolerability of this CDR loop in accommodating side chains of diverse chemical properties, from which a better antibody-antigen complementarity can be achieved through myriad combinations. Improvement in affinity correlated with increase in neutralization potency. Clone P2D7, which showed the highest affinity among all analyzed clones, had five mutations. Reverting each mutant to the parent residue resulted in a 2 to 4-fold loss of affinity (data not shown), indicating that each mutation contributed to affinity improvement.
P2D7 has an in vitro neutralization potency 11 times higher than that of canakinumab, an IL-1β-targeting antibody marketed as a treatment of CAPS and systemic juvenile idiopathic arthritis (). Furthermore, while canakinumab binds only to human IL-1β, P2D7 reacts with human, mouse, and rhesus monkey IL-1β with IC50 of 5 pM, 132 pM, and 4 pM, respectively (data not shown for monkey). This cross-reactivity allows P2D7 to be tested for therapeutic efficacy in mice and for pharmacokinetics and toxicity in monkeys. P2D7KK, the germline-like version of P2D7, showed a remarkable efficacy in a number of inflammatory disease models. In the CAIA mouse model, it prevented inflammatory symptoms such as redness in paws, swollen ankles, and limping. More impressively, it completely abolished lymphocyte infiltration and cartilage erosion that are hallmarks of the disease. In sharp contrast, mice treated with isotype control antibody developed severe arthritic symptoms, had massive lymphocytes infiltration in the joints and degraded cartilage. Similarly, P2D7KK is effective in blocking neutrophil recruitment in a mouse model of gout. Regarding efficacy, the comparison in this model of gout with anakinra is in favor of the receptor antagonist (). This result is not totally unexpected since it has been shown that IL-1α is also involved in neutrophil recruitment in MSU-induced peritonitis,Citation34 and, unlike P2D7KK, anakinra blocks both IL-1β and IL-1α pathways. However, the short half-life of anakinra (4 to 6 h) requires frequent injections of high doses for an effective treatment, e.g., 100 mg daily for rheumatoid arthritis patients, 1–2 mg/kg daily and up to 8 mg/kg daily for CAPS patients. Because P2D7KK is a human IgG, it is expected to have a half-life of ~2 wk like other human antibodies (adalimumab, 10 to 20 d; ipilimumab, ~15 d; nivolumab, up to 25 d; fresolimumab ~14 d). P2D7KK’s longer lasting protection was demonstrated in a prophylactic setting of the gout model in which MSU crystals were injected 18 h post-treatment. P2D7KK was still able to significantly reduce neutrophils influx in the peritoneal cavity 24 h after administration while mice injected with anakinra showed massive infiltrates (Fig. S2). The long-lasting activity of P2D7KK constitutes a potential advantage over anakinra allowing lower doses and fewer injections.
Furthermore, P2D7KK prevented myeloma cell-induced death in a mouse model. Lust et al. have suggested that IL-1β plays a crucial role in the progression of multiple myeloma.Citation24,Citation26 This is based on the observation that IL-1β appeared to be the major cytokine in the bone marrow responsible for the paracrine production of IL-6, the central growth factor for myeloma cells.Citation25 Therefore, blocking IL-1β in patients who have a high risk of evolving from indolent to active myeloma has been proposed as a potentially safe and non-toxic treatment,Citation35 and has shown positive results in a Phase 2 clinical study using IL-1ra.Citation26 However, as IL-1ra clears rapidly due to its small size (∼20 kDa), patients need to receive treatment of 100 mg daily. Thus, therapeutics with much longer half-lives, such as antibodies, would be superior options. In our animal model, P2D7KK treatment conferred protection from myeloma-induced lethality; 70% of P2D7KK-treated mice survived compared with 20% in the isotype group (). Interestingly, the survival rate inversely correlated with the serum level of IL-6 in the mice (Fig. S1), further demonstrating the critical role of IL-1β in the signaling pathway leading to the disease. While the detailed pathology of the mice in this experiment is still under investigation, the encouraging preliminary results suggest that P2D7KK may be effective as a treatment for inflammatory diseases, but may also have potential as an anti-cancer therapeutic.
P2D7 inhibits IL-1β signaling by means of receptor binding site blockade. Upon binding to IL-1β, P2D7 blocked IL-1β from binding to IL-1RI in a direct competition manner as shown by our SPR analysis. This mechanism of inhibition is shared with canakinumab,Citation22 but distinct from gevokizumab (Xoma 052) in that binding of gevokizumab causes an allosteric perturbation in a number of β strands in IL-1β that are compatible with receptor. This results in reduced affinity of the antibody-bound IL-1β to the receptor and hence attenuated signaling capacity.Citation12 Canakinumab and gevokizumab have also been shown to bind to two non-overlapping epitopes on IL-1β.Citation22 Notably, P2D7 and canakinumab also bind to IL-1β at two non-overlapping epitopes, as revealed by the SPR experiments, albeit sharing the same competitive inhibition mechanism. Antibodies against different epitopes of a given target can result in differential biological consequences that are often not fully understood. This has been observed in anti-HER2Citation36 and anti-CD20 antibodiesCitation37,Citation38 and likely many others. Interestingly, P2D7 is 10 times more potent than canakinumab in IL-1β neutralization in the in vitro assays despite the fact that the two antibodies have almost identical affinity () and equivalent efficacy in blocking IL-1β from binding to the receptor IL-1RI (data not shown). Such differences may possibly arise from distinctive epitopes recognized by these two antibodies. This remains to be determined by further studies in the future. Taken together, although three biologics targeting IL-1β signaling pathway have been approved for clinical use in a number of diseases, the unique mode of antigen recognition by P2D7 may have distinctive or even superior therapeutic advantages for certain indications.
In conclusion, we engineered a high affinity anti-IL-1β antibody using phage display technology. The high degree of cross-reactivity among experimental animals would greatly facilitate its preclinical development. We believe that the high potency of P2D7KK, together with its unique binding mode, warrants further investigation of its therapeutic potential.
Materials and Methods
Antibody discovery from phage display library
Anti-IL-1β antibodies were isolated from HX02 human Fab phage display library (Humanyx Pte Ltd) via in vitro selection. We followed the procedures of biopanning, phage amplification, Fab expression and purification described by de Haard et al.Citation39 Briefly, biopanning was performed using human IL-1β (Biolegend) biotinylated using the EZ-Link NHS-PEG4-Biotin labeling kit (Pierce). In the first two rounds of biopanning, IL-1β was immobilized on M280 streptavidin-coated magnetic beads (Life Technologies); in the third round, biotinylated IL-1β was immobilized on the neutravidin-coated microplate in order to avoid isolation of streptavidin magnetic beads binders. 1013 cfu phage in 1 mL casein-PBS blocking buffer was used in the first round, and 1011 cfu phage were used in the second and third rounds. After three rounds of biopanning, the Fab of selected clones was expressed in E. coli TG1 cells (Stratagene) to screen for IL-1β binders by ELISA. Unique clones were identified by DNA fingerprinting technique as previously describedCitation39 and confirmed by DNA sequencing.
Affinity maturation
The CDR3L of clone 2H was chosen as the target for affinity improvement. Semi-random mutations were introduced to the CDR using a PCR primer containing the following degenerate codons to replace the original sequence: BMK KMT TGG KMT DMT DMT DHT KHK (IUB code). Biopanning was performed by incubating the CDR3L mutagenesis library with biotinylated human IL-1β in solution followed by capture of the target-bound phage with streptavidin-coated magnetic beads. The concentrations of the IL-1β decreased from 10 nM in the first two rounds to 0.1 nM in the third round and 0.01 nM in the fourth round. To remove the fast dissociating clones, beads-bound phage were subjected to dissociation in the wash buffer containing non-biotinylated IL-1β (1 μM in round 1 and 2, and 0.5 μM in round 3 and 4), with incubation time of 1, 1.5, and 5 h from round 1 to round 4, respectively. After wash, the beads-bound phage were recovered by porcine trypsin digestion (2 mg/mL in buffer containing 20 mM Tris, 150 mM NaCl, 2 mM CaCl2, at pH 8.0) for 10 min at room temperature. IL-1β positive clones were identified by ELISA as previously described.
IgG expression and purification
Fabs were reformatted into human IgG1 in the pTT5 vector and the antibodies were expressed in HEK293–6E cells following the protocols described by Durocher et al.Citation40 Both the vector and cells were obtained from National Research Council of Canada. Antibodies were purified from the culture supernatant using Protein G resin (Merck Millipore) following standard protocols.
Kinetics and affinity measurements by surface plasmon resonance
Kinetic measurements by SPR were performed on a ProteOn XPR36 system (Bio-Rad). Phosphate-buffered saline running buffer (PBS, pH 7.4, and 0.005% or 0.05% surfactant Tween-20) was used in SPR experiments. To rank the dissociation rates of the selected clones, crude Fab secreted to the periplasm was extracted from 10 mL E. coli cultures.Citation39 The crude preparations were buffer exchanged to PBS by 10 kDa molecular cut-off spin filtration columns (Sartorius) to a final volume of 0.2 mL. IL-1β (Biolegend) was immobilized on a NLC sensor chip. Crude Fab preparations (100 μL) at 1:4 dilution in running buffer were injected over the sensor chip at 100 μL/min. Dissociation was monitored for 10 min. All experiments were performed at 25 °C. To determine the affinity, injections of five concentrations of the human, mouse, and rhesus monkey IL-1β (50, 25, 12.5, 6.25, 3.13 nM) over the antibody-immobilized GLC sensor chip was followed for a dissociation time of 2–3 h. Data were collected at 25 °C. The sensorgrams were double-referenced against reference flow cell (no antibody) and a buffer injection, and fitted globally to a 1:1 binding model from which the dissociation constants (KD) were calculated.
Epitope comparison by surface plasmon resonance
The binding mode of P2D7 in relation to IL-1RI and canakinumab was studied by surface plasmon resonance. P2D7 was immobilized on a GLC sensor chip and IL-1β at 1 μg/mL was injected over the chip followed by injection of either canakinumab or IL-1RI at 2 μg/mL. Experiments were performed at 25 °C.
In vitro neutralization assays
MRC-5 cells (ATCC), human lung fibroblasts, were maintained in MEM supplemented with 10% fetal bovine serum (FBS), 1% sodium pyruvate and 2 mM L-glutamine and used for in vitro neutralization assays as described by Economides et al.Citation13 Briefly, cells were seeded in 96-well plates at 3,000 cells/100 µL/well and incubated overnight at 37 °C in a 5% CO2 humidified incubator. Cells were then treated with 4 pM human IL-1β or 60 pM mouse IL-1β in the presence or absence of various concentrations of test antibodies. Eighteen hours after treatment, IL-1β-induced human IL-6 secretion in the supernatant was measured using the human IL-6 ELISA kit (Biolegend) according to the manufacturer’s protocol. HEK-Blue™ IL-1β cells (InvivoGen) were maintained in DMEM supplemented with 10% FBS, 100 µg/mL Zeocin™ and 200 µg/mL hygromycin B. When used for in vitro neutralization assays, cells were seeded in 96-well plates at 5x104 cells/100 µL/well and incubated overnight at 37 °C in a 5% CO2 humidified incubator. Cells were then treated with 4 pM human IL-1β or 40 pM mouse IL-1β in the presence or absence of various concentrations of test antibodies. Eighteen hours after treatment, IL-1β-induced release of secreted embryonic alkaline phosphatase (SEAP) in the supernatant was assessed using QUANTI-Blue™ (InvivoGen) according to the manufacturer’s protocol.
Animals
C57BL/6 and Balb/c mice were purchased from the Biological Resource Center (BRC), Agency for Science, Technology and Research (A*STAR), Singapore, and bred under specific pathogen-free conditions. All experiments involving animals were performed in accordance with the Institutional Animal Care and Use Committee in compliance with the Law and Guidelines for Animal Experiments of the BRC, Singapore.
Collagen antibody-induced arthritis mouse model
Anti-collagen antibody cocktail was purchased from Chondrex Inc. and used according to the manufacturer’s protocol. Briefly, 7–8 wk old Balb/c mice were injected intraperitoneally (i.p.) with 1.5 mg/kg of anti-collagen antibody cocktail. After 3 d, mice received an i.p. injection of 25 µg of lipopolysaccharide (LPS) to trigger arthritis development. On days 2, 5, and 9, mice were given an i.p. dose of either 5 mg/kg of anti-IL-1β antibody P2D7KK or 5 mg/kg of an isotype control antibody. Mice were scored for clinical arthritis on a scale of 0 to 4 based on signs of swelling and inflammation.Citation41
Monosodium urate (MSU)-mediated peritonitis
8–10-wk old C57BL/6 mice were injected i.p. with anakinra (30 mg/kg, Kineret®, Amgen), P2D7KK (5 mg/kg and 15 mg/kg), isotype control human IgG1 antibody (15 mg/kg), or saline. After 10 min, mice received another i.p. injection of 3 mg MSU crystals (Enzo Life Sciences) in 0.5 mL of saline. Control mice were injected with saline alone. After 6 h, peritoneal exudate cells were collected by lavage with cold medium, centrifuged, and red blood cell lysis was performed using hypotonic ammonium chloride solution for 1 min. Total peritoneal cells were counted, stained with Ly6G PE (1A8, BD PharMingen) and CD11b APC (M1/70, eBioscience), and analyzed by flow cytometry. Neutrophils were identified as Ly6G+CD11b+ cells.
Multiple myeloma model
Human myeloma cells U266B1 were purchased from ATCC and cultured in RPMI-1640 medium supplemented with 10% FBS at 37 °C under 5% CO2 in a humidified incubator. 7–8 wk old female NOD-SCID mice were inoculated with 107 cells via intravenous injections. Antibody treatment started on day 7 and was administered at 10 mg/kg every 4 d for 7 wk.
| Abbreviations: | ||
| CAIA | = | collagen antibody induced arthritis |
| CAPS | = | cryopyrin-associated periodic syndromes |
| ELISA | = | enzyme-linked immunosorbent assay |
| Fab | = | antigen binding fragment |
| IC50 | = | inhibitory concentration 50 |
| Ig | = | immunoglobulin |
| IL | = | interleukin |
| IL-1ra | = | IL-1 receptor antagonist |
| IL-1RI | = | IL-1 receptor type I |
| i.p. | = | intraperitoneal |
| MSU | = | monosodium urate |
| SPR | = | surface plasmon resonance |
| TNF | = | tumor necrosis factor |
Additional material
Download Zip (166.3 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was supported by the core fund of Singapore Immunology Network and Industrial Alignment Fund (IAF311007) from Biomedical Research Council, Agency for Science, Technology and Research (A*STAR), Singapore.
References
- Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol 2007; 179:1178 - 89; PMID: 17617611
- Graves DT, Chen CP, Douville C, Jiang Y. Interleukin-1 receptor signaling rather than that of tumor necrosis factor is critical in protecting the host from the severe consequences of a polymicrobe anaerobic infection. Infect Immun 2000; 68:4746 - 51; http://dx.doi.org/10.1128/IAI.68.8.4746-4751.2000; PMID: 10899881
- Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 2009; 5:487 - 97; http://dx.doi.org/10.1016/j.chom.2009.05.002; PMID: 19454352
- Vonk AG, Netea MG, van Krieken JH, Iwakura Y, van der Meer JW, Kullberg BJ. Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J Infect Dis 2006; 193:1419 - 26; http://dx.doi.org/10.1086/503363; PMID: 16619190
- Zambetti LP, Laudisi F, Licandro G, Ricciardi-Castagnoli P, Mortellaro A. The rhapsody of NLRPs: master players of inflammation...and a lot more. Immunol Res 2012; 53:78 - 90; http://dx.doi.org/10.1007/s12026-012-8272-z; PMID: 22427013
- Kingsbury SR, Conaghan PG, McDermott MF. The role of the NLRP3 inflammasome in gout. J Inflamm Res 2011; 4:39 - 49; http://dx.doi.org/10.2147/JIR.S11330; PMID: 22096368
- Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol 2010; 11:897 - 904; http://dx.doi.org/10.1038/ni.1935; PMID: 20835230
- Conforti-Andreoni C, Ricciardi-Castagnoli P, Mortellaro A. The inflammasomes in health and disease: from genetics to molecular mechanisms of autoinflammation and beyond. Cell Mol Immunol 2011; 8:135 - 45; http://dx.doi.org/10.1038/cmi.2010.81; PMID: 21258359
- Dinarello CA. Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr Opin Pharmacol 2004; 4:378 - 85; http://dx.doi.org/10.1016/j.coph.2004.03.010; PMID: 15251132
- Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med 2005; 201:1355 - 9; http://dx.doi.org/10.1084/jem.20050640; PMID: 15867089
- Mitroulis I, Skendros P, Ritis K. Targeting IL-1beta in disease; the expanding role of NLRP3 inflammasome. Eur J Intern Med 2010; 21:157 - 63; http://dx.doi.org/10.1016/j.ejim.2010.03.005; PMID: 20493414
- Roell MK, Issafras H, Bauer RJ, Michelson KS, Mendoza N, Vanegas SI, Gross LM, Larsen PD, Bedinger DH, Bohmann DJ, et al. Kinetic approach to pathway attenuation using XOMA 052, a regulatory therapeutic antibody that modulates interleukin-1beta activity. J Biol Chem 2010; 285:20607 - 14; http://dx.doi.org/10.1074/jbc.M110.115790; PMID: 20410301
- Economides AN, Carpenter LR, Rudge JS, Wong V, Koehler-Stec EM, Hartnett C, Pyles EA, Xu X, Daly TJ, Young MR, et al. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat Med 2003; 9:47 - 52; http://dx.doi.org/10.1038/nm811; PMID: 12483208
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, et al, National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008; 58:26 - 35; http://dx.doi.org/10.1002/art.23176; PMID: 18163497
- Weiss JE, Ilowite NT. Juvenile idiopathic arthritis. Pediatr Clin North Am 2005; 52:413 - 42, vi; http://dx.doi.org/10.1016/j.pcl.2005.01.007; PMID: 15820374
- Solau-Gervais E, Robin C, Gambert C, Troller S, Danner S, Gombert B, Debiais F, Hankard R. Prevalence and distribution of juvenile idiopathic arthritis in a region of Western France. Joint Bone Spine 2010; 77:47 - 9; http://dx.doi.org/10.1016/j.jbspin.2009.11.002; PMID: 20034832
- CDC National Diabetes Fact Sheet. . General information and national estimates on diabetes in the US. 2007; 2008
- Moreland NJ, Susanto P, Lim E, Tay MY, Rajamanonmani R, Hanson BJ, Vasudevan SG. Phage Display Approaches for the Isolation of Monoclonal Antibodies Against Dengue Virus Envelope Domain III from Human and Mouse Derived Libraries. Int J Mol Sci 2012; 13:2618 - 35; http://dx.doi.org/10.3390/ijms13032618; PMID: 22489114
- Graves BJ, Hatada MH, Hendrickson WA, Miller JK, Madison VS, Satow Y. Structure of interleukin 1 alpha at 2.7-A resolution. Biochemistry 1990; 29:2679 - 84; http://dx.doi.org/10.1021/bi00463a009; PMID: 2346741
- Fellouse FA, Wiesmann C, Sidhu SS. Synthetic antibodies from a four-amino-acid code: a dominant role for tyrosine in antigen recognition. Proc Natl Acad Sci U S A 2004; 101:12467 - 72; http://dx.doi.org/10.1073/pnas.0401786101; PMID: 15306681
- Mian IS, Bradwell AR, Olson AJ. Structure, function and properties of antibody binding sites. J Mol Biol 1991; 217:133 - 51; http://dx.doi.org/10.1016/0022-2836(91)90617-F; PMID: 1988675
- Blech M, Peter D, Fischer P, Bauer MM, Hafner M, Zeeb M, Nar H. One target-two different binding modes: structural insights into gevokizumab and canakinumab interactions to interleukin-1β. J Mol Biol 2013; 425:94 - 111; http://dx.doi.org/10.1016/j.jmb.2012.09.021; PMID: 23041424
- So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther 2007; 9:R28; http://dx.doi.org/10.1186/ar2143; PMID: 17352828
- Lust JA, Donovan KA. The role of interleukin-1 beta in the pathogenesis of multiple myeloma. Hematol Oncol Clin North Am 1999; 13:1117 - 25; http://dx.doi.org/10.1016/S0889-8588(05)70115-5; PMID: 10626139
- Xiong Y, Donovan KA, Kline MP, Gornet MK, Moon-Tasson LL, Lacy MQ, Dispenzieri A, Gertz MA, Greipp PR, Lust JA. Identification of two groups of smoldering multiple myeloma patients who are either high or low producers of interleukin-1. J Interferon Cytokine Res 2006; 26:83 - 95; http://dx.doi.org/10.1089/jir.2006.26.83; PMID: 16487028
- Lust JA, Lacy MQ, Zeldenrust SR, Dispenzieri A, Gertz MA, Witzig TE, Kumar S, Hayman SR, Russell SJ, Buadi FK, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1beta-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc 2009; 84:114 - 22; http://dx.doi.org/10.4065/84.2.114; PMID: 19181644
- Schier R, Balint RF, McCall A, Apell G, Larrick JW, Marks JD. Identification of functional and structural amino-acid residues by parsimonious mutagenesis. Gene 1996; 169:147 - 55; http://dx.doi.org/10.1016/0378-1119(95)00821-7; PMID: 8647439
- Schier R, McCall A, Adams GP, Marshall KW, Merritt H, Yim M, Crawford RS, Weiner LM, Marks C, Marks JD. Isolation of picomolar affinity anti-c-erbB-2 single-chain Fv by molecular evolution of the complementarity determining regions in the center of the antibody binding site. J Mol Biol 1996; 263:551 - 67; http://dx.doi.org/10.1006/jmbi.1996.0598; PMID: 8918938
- Bostrom J, Lee CV, Haber L, Fuh G. Improving antibody binding affinity and specificity for therapeutic development. [xiii.] Methods Mol Biol 2009; 525:353 - 76, xiii; http://dx.doi.org/10.1007/978-1-59745-554-1_19; PMID: 19252851
- Chen Y, Wiesmann C, Fuh G, Li B, Christinger HW, McKay P, de Vos AM, Lowman HB. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol 1999; 293:865 - 81; http://dx.doi.org/10.1006/jmbi.1999.3192; PMID: 10543973
- Novotny J, Bruccoleri RE, Saul FA. On the attribution of binding energy in antigen-antibody complexes McPC 603, D1.3, and HyHEL-5. Biochemistry 1989; 28:4735 - 49; http://dx.doi.org/10.1021/bi00437a034; PMID: 2475171
- Kuroda D, Shirai H, Jacobson MP, Nakamura H. Computer-aided antibody design. Protein Eng Des Sel 2012; 25:507 - 21; http://dx.doi.org/10.1093/protein/gzs024; PMID: 22661385
- Nuttall SD, Irving RA, Hudson PJ. Immunoglobulin VH domains and beyond: design and selection of single-domain binding and targeting reagents. Curr Pharm Biotechnol 2000; 1:253 - 63; http://dx.doi.org/10.2174/1389201003378906; PMID: 11469383
- Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, Quadroni M, Drexler SK, Tschopp J. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 2012; 36:388 - 400; http://dx.doi.org/10.1016/j.immuni.2012.01.018; PMID: 22444631
- Dinarello CA. Targeting the pathogenic role of interleukin 1beta in the progression of smoldering/indolent myeloma to active disease. Mayo Clin Proc 2009; 84:105 - 7; http://dx.doi.org/10.4065/84.2.105; PMID: 19181642
- Fuentes G, Scaltriti M, Baselga J, Verma CS. Synergy between trastuzumab and pertuzumab for human epidermal growth factor 2 (Her2) from colocalization: an in silico based mechanism. Breast Cancer Res 2011; 13:R54; http://dx.doi.org/10.1186/bcr2888; PMID: 21600050
- Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, van Meerten T, Ebeling S, Vink T, Slootstra JW, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol 2006; 177:362 - 71; PMID: 16785532
- Bologna L, Gotti E, Manganini M, Rambaldi A, Intermesoli T, Introna M, Golay J. Mechanism of action of type II, glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J Immunol 2011; 186:3762 - 9; http://dx.doi.org/10.4049/jimmunol.1000303; PMID: 21296976
- de Haard HJ, van Neer N, Reurs A, Hufton SE, Roovers RC, Henderikx P, de Bruïne AP, Arends JW, Hoogenboom HR. A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J Biol Chem 1999; 274:18218 - 30; http://dx.doi.org/10.1074/jbc.274.26.18218; PMID: 10373423
- Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res 2002; 30:E9; http://dx.doi.org/10.1093/nar/30.2.e9; PMID: 11788735
- Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc 2007; 2:1269 - 75; http://dx.doi.org/10.1038/nprot.2007.173; PMID: 17546023